Studies analyzing non-antibiotic alternatives in kidney transplant UTI's are lacking. d-Mannose, a simple sugar, inhibits bacterial attachment to the urothelium, as does Proanthocyanidins; both could act as a synergic strategy preventing UTI; nonetheless their efficacy and safety have not been evaluated in kidney transplant population yet.
MethodsThis is a pilot prospective, double-blind randomized trial. Sixty de novo kidney transplant recipients were randomized (1:1) to receive a prophylactic strategy based on a 24-h prolonged release formulation of d-Mannose plus Proanthocyanidins vs. Proanthocyanidins (PAC) alone. The supplements were taken for the first 3 months after kidney transplant and then followed up for 3 months as well. The main objective of the study was to search if the addition of Mannose to PAC alone reduced the incidence of UTI and/or asymptomatic bacteriuria in the first 6 months post-transplantation.
Results27% of patients experienced one UTI episode (cystitis or pyelonephritis) while asymptomatic bacteriuria was very common (57%). Incidences according UTI type or AB were: 7% vs. 4% for cystitis episode (p 0.3), 4% vs. 5% for pyelonephritis (p 0.5) and 17% vs. 14% for asymptomatic bacteriuria (p 0.4) for patients in the Mannose+PAC group vs. PAC group respectively. The most frequent bacteria isolated in both groups was Escherichia coli (28% of all episodes), UTI or AB due to E. coli was not different according to study group (30% vs. 23% for Mannose+PAC vs. PAC alone p 0.37).
ConclusionsNon-antibiotic therapy is an unmet need to prevent UTI after kidney transplantation; however, the use of d-Mannose plus PAC does not seem capable to prevent it.
Faltan estudios que analicen alternativas no antibióticas para tratar las infecciones del tracto urinario (ITU) en los pacientes trasplantados renales. La D-Manosa, un azúcar simple, inhibe la adhesión bacteriana al urotelio, al igual que las Proantocianidinas; ambas moléculas podrían actuar como una estrategia sinérgica para prevenir las ITUs; pero su eficacia y seguridad aún no se han evaluado en la población trasplantada renal.
MétodosEste es un ensayo piloto prospectivo y doble ciego. Sesenta receptores de trasplante renal de novo fueron asignados al azar (1:1) para recibir una estrategia profiláctica basada en una formulación de liberación prolongada de 24 horas de D-Manosa más Proantocianidinas (PAC), frente a solo Proantocianidinas (PAC). Los suplementos se tomaron durante los primeros 3 meses después del trasplante renal y luego se realizó un seguimiento durante otros 3 meses. El objetivo principal del estudio fue determinar si la adición de D-Manosa a PAC reducía la incidencia de ITU y/o bacteriuria asintomática en los primeros 6 meses después del trasplante.
ResultadosEl 27% de los pacientes experimentó un episodio de ITU (cistitis o pielonefritis), mientras que la bacteriuria asintomática fue muy común (57%). Las incidencias según el tipo de ITU o bacteriuria asintomática fueron: 7% frente a 4% para episodio de cistitis (p 0.3), 4% frente a 5% para pielonefritis (p 0.5) y 17% frente a 14% para bacteriuria asintomática (p 0.4) en el grupo de manosa + PAC frente al grupo PAC, respectivamente. La bacteria más frecuente en ambos grupos fue Escherichia coli (28% de todos los episodios), sin embargo las ITU o bacteriuria asintomática debidas a E. coli no fueron diferentes según el grupo de estudio (30% frente a 23% para Manosa + PAC frente a PAC solo, p.0.37).
ConclusionesLa terapia no antibiótica es una necesidad para prevenir las ITU después del trasplante renal; sin embargo, el uso de D-Manosa más PAC no parece ser capaz de prevenir las ITU en este grupo especial de pacientes.
UTI is by far the most common infection after kidney transplantation (KTX). Its incidence is highly variable, ranging between 23 and 75% in the first year. This wide variability may be due to different screening strategies, definitions, population characteristics, and prophylactic strategies.1 It increases the morbidity and mortality in kidney allograft recipients.2–4 On the other hand, antibiotic treatment and prophylaxis for UTI increase antibiotic resistance as well as Clostridioides difficile infection. Therefore, searching for prevention strategies aimed at limiting antibiotic use and reducing the UTI incidence should be pursued.
Although non-antimicrobial therapies have already proven some benefit in clinical trials in general population, there is few evidence in SOT. Methenamine, probiotics and bacterial vaccines are well tolerated, but data are drawn from small case series and non-randomized clinical trials.5–7 The role of cranberry juice is still controversial: even though several studies suggest it is useful for kidney transplants with recurrent UTI, a recent Cochrane systematic review and meta-analysis found that, compared with placebo, cranberry juice did not significantly reduce the incidence of UTI.8d-Mannose, a simple sugar, could play a role in the prevention of UTI by inhibiting the attachment of bacterial type 1 fimbriae to the urothelium.9 In vitro data suggest that Proanthocyanidins (PAC) inhibits the adherence of Escherichia coli by a similar mechanism.10 Studies in murine models of UTI with different mannoside fimbriae antagonist led to a reduction in the number of UFC/ml in the urine.11 Both d-Mannose and PAC have also shown promising results in reducing the risk of recurrent UTI in healthy women,12 but their effect on UTI infection after kidney transplantation has not been evaluated yet.
The purpose of this clinical trial is to test the efficacy and safety of a 24-h prolonged release formulation of d-Mannose plus PAC vs. PAC alone, administered on UTI and/or AB episodes throughout the first 3 months after kidney transplantation, pursuit by a follow-up period of 3 months.
Materials and methodsStudy designThis is a pilot, randomized, double-blind parallel study group (1:1 ratio) clinical trial, comparing the efficacy of daily intake of d-Mannose plus PAC vs. PAC alone to prevent UTI and AB after kidney transplantation. Since the mechanism of the mannose in experimental models has led to a reduction in CFU/ml in urine,11 we decided to screen for asymptomatic bacteriuria throughout the study to detect if its addition to PAC reduces the bacterial colonization and/or its correlation with UTI.
UC were screen with the following frequency: once a week after the first month of KTX, twice a month until the 3rd month and once a month until the 6th month post-transplant; also, at each time that the patient experiences any urinary symptom. AB was treated just in the first 3 weeks after transplantation. A TMP/SMX prophylaxis was given following the local protocol during the first 3 months after transplantation.
During an episode of UTI or AB that required antibiotic treatment, patients were educated to continue study supplements intake.
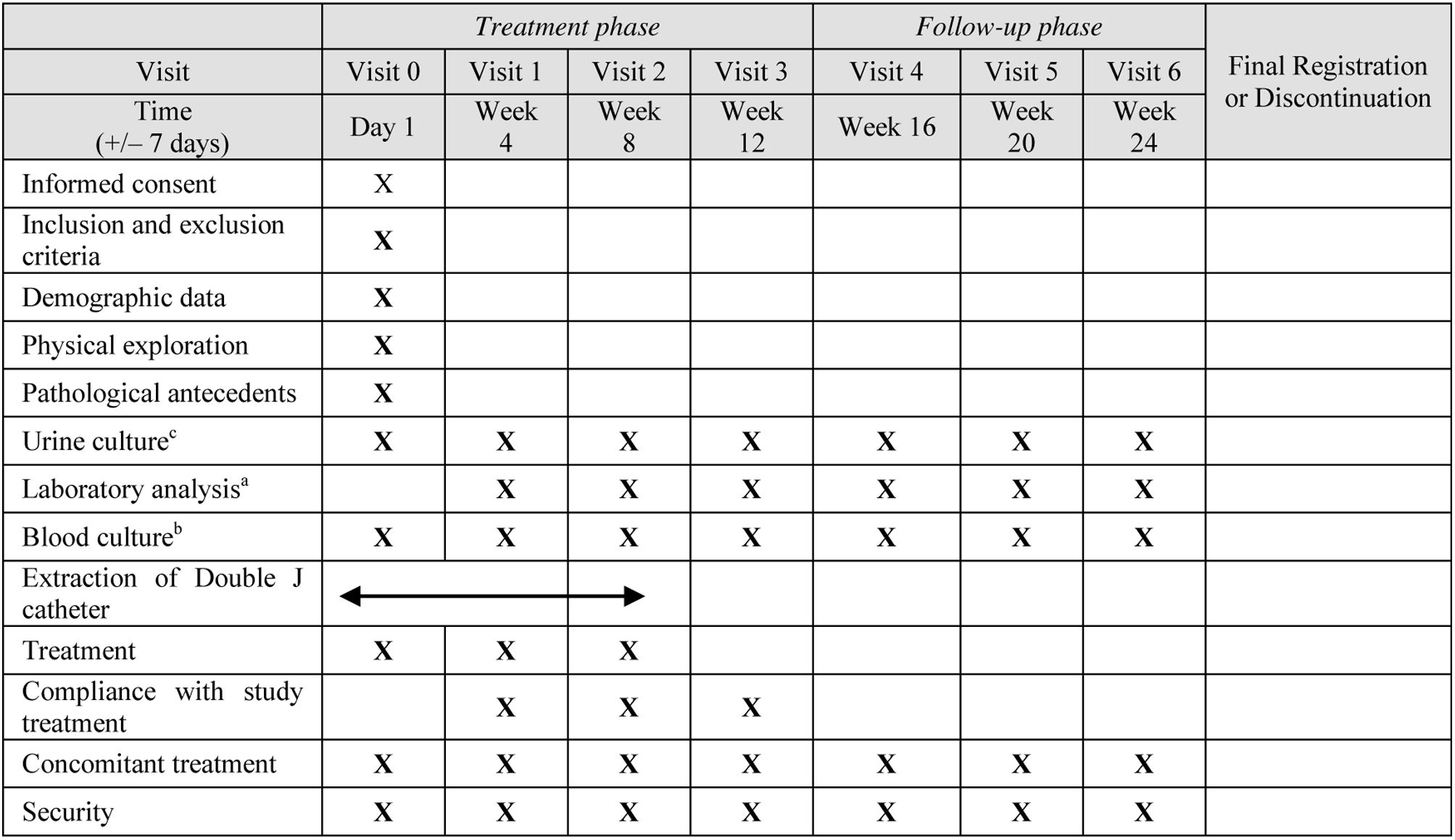
Table 1 shows the study protocol. This clinical trial was registered on clinicaltrials.gov (NCT05109455) and sponsored by Arafarma S.A. Entry of data into the study database and analysis of the results were done independently by academic authors without the involvement of the sponsor. Since both formulations are dietary supplements and the dosage used was proven to be safe, the study was considered as a low-intervention clinical trial by the Spanish Regulatory Agency (AEMPS).
The internal ethical review board of our institution approved the trial (PR421/18).
MedicationThe product is a supplement consisting of d-Mannose, PAC and Ursodeoxycholic acid, as well as several vitamins. Supplements were provided by the Sponsor of the study. See Supplementary Table 1 for more information about the composition.
PopulationEligible patients were those who had received a kidney transplant at Bellvitge University Hospital between April 2019 and September 2020. Exclusion criteria were: age below 18 years, recipients of an organ transplant other than kidney, patients with Bricker or Studer neobladders, and those participating in another clinical study in which the sponsor had already established the treatment for UTI or whose immunosuppressive protocols differed from our local practice.
DefinitionsAsymptomatic bacteriuria was defined as a culture yielding significant growth of urinary tract pathogens (>105colony forming units/ml) in the absence of symptoms attributable to the infection. UTI was defined as the presence of a positive urine culture (bacteriuria count>105colony forming units/ml) in the presence of urinary symptoms. The type of UTI was defined according to the international guidelines13: Episodes of urinary frequency augmentation, dysuria, or suprapubic pain without fever were categorized as cystitis. Patients having fever and a positive culture as defined above, with or without flank pain, were diagnosed as having acute graft pyelonephritis (AGP). Acute bacterial Prostatitis was defined as the presence of discomfort referred to the lower urogenital and perineal area associated to fever and chills. Recurrent UTI was defined as the occurrence of at least two episodes of UTI within 6 months. Contaminated cultures, considered as those where more than 2 microorganisms were isolated from a single specimen with less than 105colony forming units/ml without symptoms, were excluded from the study analysis. A multi-resistant bacterium is one that exhibits resistance to a minimum of 3 antibiotic groups or to a specific group of antibiotics (extended-spectrum beta-lactamases, methicillin-resistant Staphylococcus aureus, and carbapenemases).14
More details on microbiological technique analytics, and ureteric stent manipulation are depicted in Supplementary Box 1.
OutcomesThe primary outcome was a composite endpoint of the incidence (first episode) of UTI and/or AB within the first 6 months after kidney transplantation. For analysis purpose, we showed outcomes also according to 3 time periods: period 1, from first day after kidney transplantation to double J removal (21–27 days post-transplantation); period 2, from double J removal to the last day of supplements intake (meaning 3 months after KTX); and period 3, from the third month to the end of study follow-up (6 months).
The secondary outcomes were: global incidence of UTI and/or AB, analysis of types of UTIs, microbiological characteristics, incidence of double J colonization, incidence of delayed graft function (DGF), rejection rates, kidney allograft function, incidence and type of adverse events, and patient and kidney survival.
We followed the Consort 2010 checklist (Consolidated Standards of Reporting Trials) in reporting the results of this trial.
Randomization and medication administrationAfter randomization, patients received the first dose of either Mannose plus PAC or PAC alone within the first 24h from transplantation. In case of paralytic ileus, the introduction of study product or of the comparator could be delayed until 72h. After this time, as well as in case of immediate transplant removal due to thrombosis, patients were considered screening failure. The study was double-blinded. For more details on the protocol, see Supplementary Fig. 1 and Supplementary Box 2.
StatisticsThis is a pilot and exploratory trial, and a sample-size was estimated. Continuous variables were expressed as mean±standard deviation (SD) or median and interquartile range (IQR), and categorical variables as number of total (n) and percentage (%). Comparison between groups was performed using Pearson’ χ2 test for categorical data, and the Fisher-exact test was applied when the number of cases was less than five. One-way analysis of variance and t-tests were used for normally continuous distributed data, and nonparametric Kruskal–Wallis test and Mann–Whitney U-test for non-normally distributed variables. Free survival from UTI was analyzed through Kaplan–Meier analysis. All p-values were two-tailed and statistical significance level was fixed at p<0.05. SPSS 20.0 software (SPSS Inc., Chicago, IL) and GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA) were used for data management and analysis.
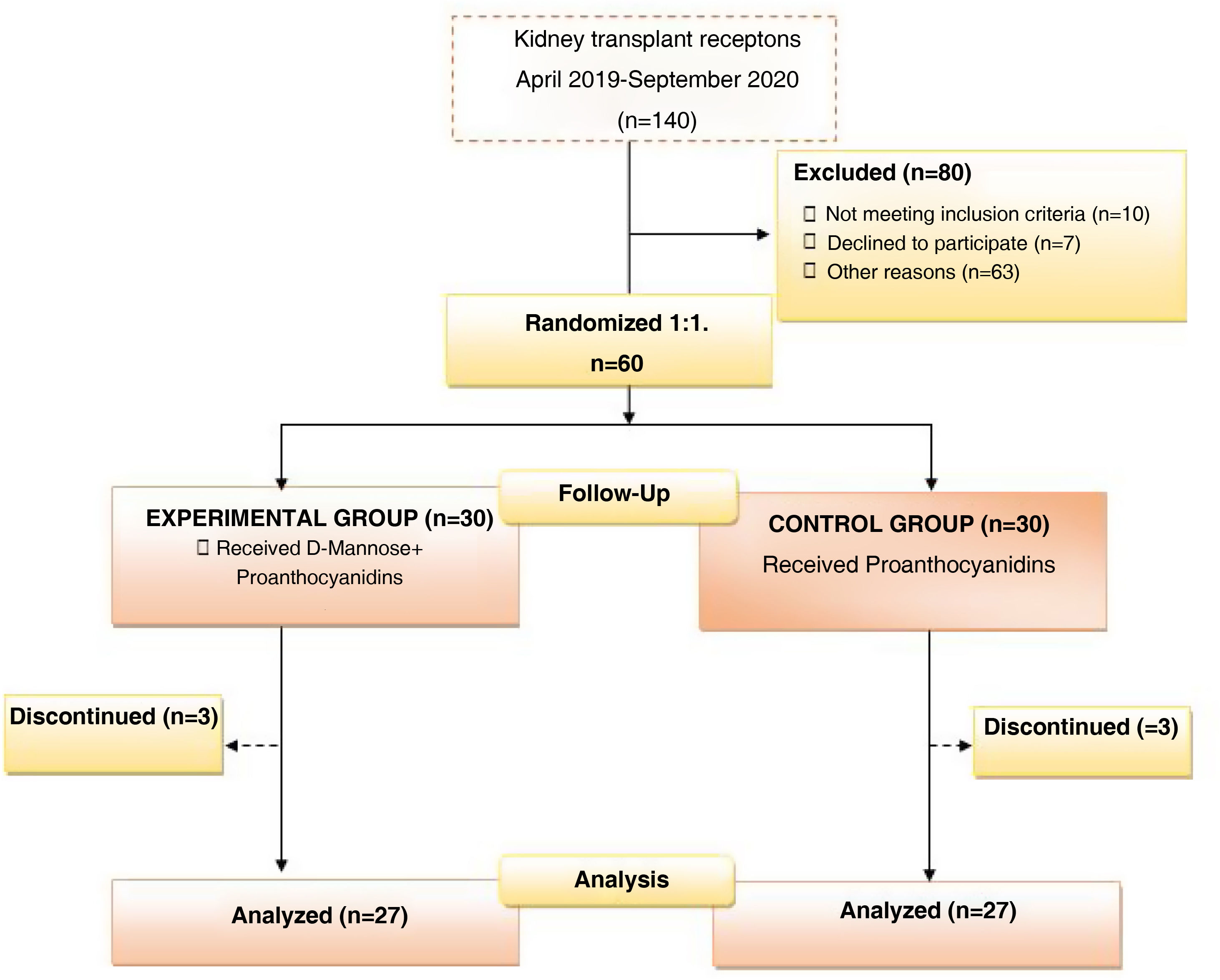
ResultsBetween April 2019 and September 2020, 140 kidney transplants were performed at our center. Ten patients did not meet the study inclusion criteria, seven patients were not willing to participate, and 63 patients already participated in other clinical studies. Overall, 60 patients were included in the study and randomized in two groups: a control group (PAC alone), and an experimental group using Mannose plus PAC. Three patients dropped out from each group. In the experimental group, two patients dropped out due to study protocol deviations (taking treatment during only the first week after transplantation, then withdrawing their consent to stay in the study), and a third one died after COVID-19 infection. In the control group, one patient requested to drop out of the study; one patient had a never-functioning graft; and one patient died after COVID-19 infection. Fig. 1 shows the flowchart of the study.
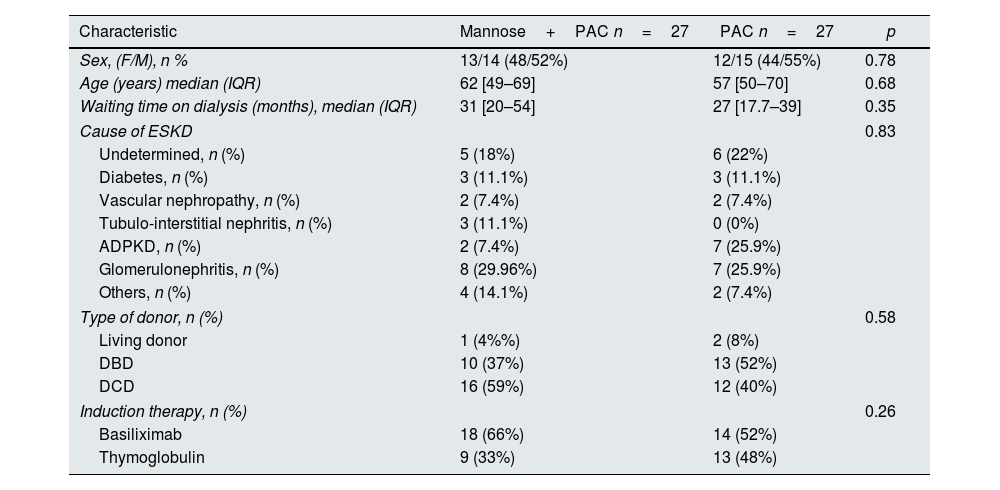
As shown in Table 2, no statistically significant differences were observed between the two treatment groups regarding the baseline characteristics.
Baseline demographics and clinical characteristics depending on treatment group.
| Characteristic | Mannose+PAC n=27 | PAC n=27 | p |
|---|---|---|---|
| Sex, (F/M), n % | 13/14 (48/52%) | 12/15 (44/55%) | 0.78 |
| Age (years) median (IQR) | 62 [49–69] | 57 [50–70] | 0.68 |
| Waiting time on dialysis (months), median (IQR) | 31 [20–54] | 27 [17.7–39] | 0.35 |
| Cause of ESKD | 0.83 | ||
| Undetermined, n (%) | 5 (18%) | 6 (22%) | |
| Diabetes, n (%) | 3 (11.1%) | 3 (11.1%) | |
| Vascular nephropathy, n (%) | 2 (7.4%) | 2 (7.4%) | |
| Tubulo-interstitial nephritis, n (%) | 3 (11.1%) | 0 (0%) | |
| ADPKD, n (%) | 2 (7.4%) | 7 (25.9%) | |
| Glomerulonephritis, n (%) | 8 (29.96%) | 7 (25.9%) | |
| Others, n (%) | 4 (14.1%) | 2 (7.4%) | |
| Type of donor, n (%) | 0.58 | ||
| Living donor | 1 (4%%) | 2 (8%) | |
| DBD | 10 (37%) | 13 (52%) | |
| DCD | 16 (59%) | 12 (40%) | |
| Induction therapy, n (%) | 0.26 | ||
| Basiliximab | 18 (66%) | 14 (52%) | |
| Thymoglobulin | 9 (33%) | 13 (48%) | |
PAC, Proanthocyanidins; ESKD, end stage kidney decease; ADPKD, autosomic dominant polycystic kidney decease; CMV, cytomegalovirus.
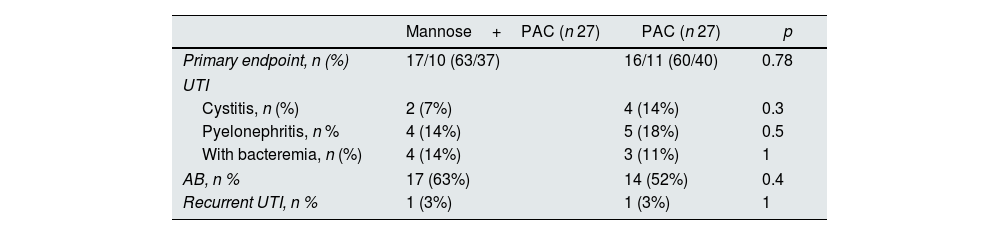
There were 94 UTI and AB episodes in the whole cohort (3.4 UTI and AB episodes for patient/year – see Supplementary Table 2). Table 3 summarizes the results, showing no differences in the outcomes per study group. 31 patients had at least one episode of AB, 17 (63%) in the Mannose+PAC group vs. 14 (52%) in the control group (p 0.4); six had cystitis, 2 (7%) vs. 4 (14%) p 0.3, and nine had acute pyelonephritis 4 (14%) vs. 5 (18%) p 0.5. Six pyelonephritis episodes appeared after urinary manipulation (two episodes occurred after the double J ureteral stent removal, three episodes after the bladder stent removal, and one after nephrostomy placement). Two patients had recurrent UTI (one for each group). Also, the total number of UTI and AB episodes was similar among groups (3.1 UTI and AB/patient/year vs. 3.9 UTI and AB/patient/year for experimental vs. control group respectively, p 0.7).
Primary endpoint, UTI, AB and recurrent UTI (expressed as number of patients with at least one episode) by treatment group.
| Mannose+PAC (n 27) | PAC (n 27) | p | |
|---|---|---|---|
| Primary endpoint, n (%) | 17/10 (63/37) | 16/11 (60/40) | 0.78 |
| UTI | |||
| Cystitis, n (%) | 2 (7%) | 4 (14%) | 0.3 |
| Pyelonephritis, n % | 4 (14%) | 5 (18%) | 0.5 |
| With bacteremia, n (%) | 4 (14%) | 3 (11%) | 1 |
| AB, n % | 17 (63%) | 14 (52%) | 0.4 |
| Recurrent UTI, n % | 1 (3%) | 1 (3%) | 1 |
UTI, urinary Tract infections; AB, asymptomatic bacteriuria; PAC, Proanthocyanidin.
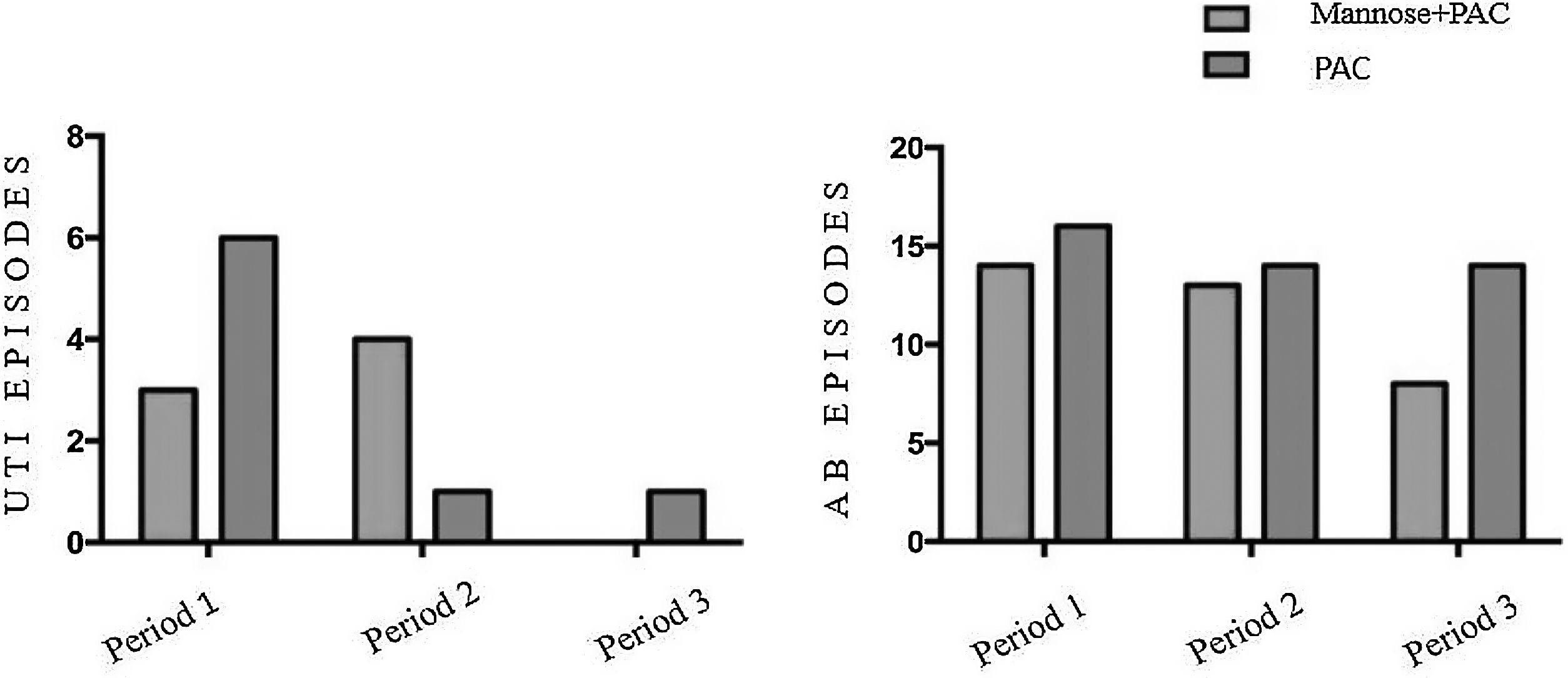
We analyzed UTI and AB episodes and incidence according to three different periods (see Fig. 2) and, again, we did not observe significant differences between the two groups. Also, when comparing groups after removal of double J, we did not observe differences in patients having at least one UTI and AB episode (49% Mannose+PAC vs. 59% PAC alone, p 0.41). As expected, UTIs were more frequent in the first three months (periods 1 and 2) compared with the following three month-period (median UTI episodes 1 [0–5] vs. 0 [0–3], p 0.002) in the whole cohort.
Median time to first UTI and AB episode was similar in both study groups (14 IQ 6–23 days vs. 22 IQ 7–29 days, p 0.3). Also, the time of the first occurrence of UTI and/or AB was similar (log Rank p 0.4 see Supplementary Fig. 2).
Patients with urological complications had a higher UTI and AB incidence (83% vs. 50%, p 0.034). Correlation with AB and pyelonephritis was statistically significant (r 0.3, p 0.02). In 5 patients, pyelonephritis was caused by the same bacteria as previously identified in an AB culture.
Females tended to have a higher UTI incidence, although this was statistically significant only for asymptomatic bacteriuria (72% vs. 44% for female vs. male patients, p 0.04).
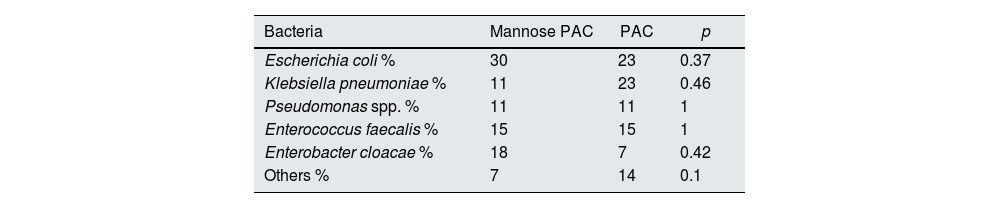
No differences in frequency of isolated bacteria culture type were observed among study groups (Table 4). Of note, we did not observe significant differences regarding UTI and AB due to E. coli between groups, neither when excluding the period prior to the double J removal (24% vs. 17% for Mannose plus PAC vs. PAC alone respectively, p 0.2). E. coli was the most frequent pathogen, observed in 28% of UTI and AB episodes.
% of patient with UTI or AB, according to bacteria.
| Bacteria | Mannose PAC | PAC | p |
|---|---|---|---|
| Escherichia coli % | 30 | 23 | 0.37 |
| Klebsiella pneumoniae % | 11 | 23 | 0.46 |
| Pseudomonas spp. % | 11 | 11 | 1 |
| Enterococcus faecalis % | 15 | 15 | 1 |
| Enterobacter cloacae % | 18 | 7 | 0.42 |
| Others % | 7 | 14 | 0.1 |
UTI, urinary tract infections; PAC, Proanthocyanidins; Others (Candida albicans, Proteus mirabilis, Morganella morganii, Staphylococcus aureus).
Multidrug-resistant microorganism UTI and/or BA episodes were detected in 7 of 17 patients in the Mannose plus PAC group as opposed to 4 of 16 patients in the PAC alone group (41% vs. 27%, p 0.472).
Regarding the length of antibiotics course, there was no statistical significant difference (median 7 IQ 1–14 vs. 12 days IQ 1–15, for Mannose plus PAC vs. PAC alone, p 0.44).
The frequency of uropathogens isolated in the whole cohort is showed in Supplementary Fig. 3.
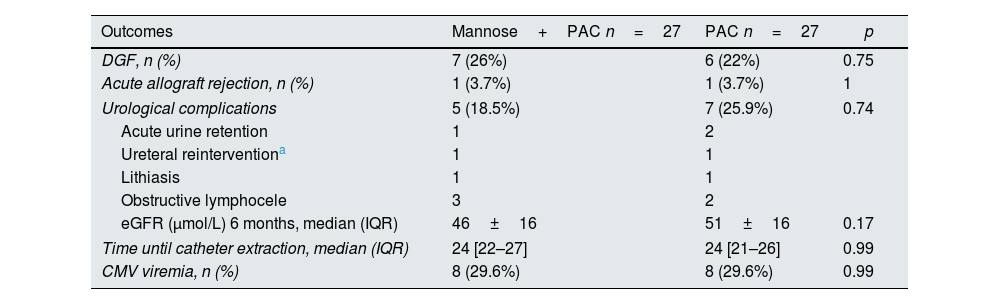
Kidney transplant-related outcomesTable 5 shows the main graft and urological-related outcomes depending on treatment group. No major differences were observed. 26% of patients in the experimental group and 22% of patients in the control group had DGF. One patient from each group developed an acute allograft rejection. The eGFR at 6 months post-transplantation was also similar. There was 1 episode of lithiasis in each group, in both cases kidney stones were transferred from the donor.
Graft and transplant-related outcomes depending on treatment group.
| Outcomes | Mannose+PAC n=27 | PAC n=27 | p |
|---|---|---|---|
| DGF, n (%) | 7 (26%) | 6 (22%) | 0.75 |
| Acute allograft rejection, n (%) | 1 (3.7%) | 1 (3.7%) | 1 |
| Urological complications | 5 (18.5%) | 7 (25.9%) | 0.74 |
| Acute urine retention | 1 | 2 | |
| Ureteral reinterventiona | 1 | 1 | |
| Lithiasis | 1 | 1 | |
| Obstructive lymphocele | 3 | 2 | |
| eGFR (μmol/L) 6 months, median (IQR) | 46±16 | 51±16 | 0.17 |
| Time until catheter extraction, median (IQR) | 24 [22–27] | 24 [21–26] | 0.99 |
| CMV viremia, n (%) | 8 (29.6%) | 8 (29.6%) | 0.99 |
All double J catheters except one were cultured, the one exception being due to accidental contamination during the removal procedure. Out of the 53 cultured catheters, 40% were colonized: 13% had typical uropathogens, 22% had non-uropathogens, and 4% had both type of bacteria. Colonization of double J was similar between groups (48% vs. 31% for Mannose plus PAC vs. PAC alone, p 0.19). The presence of uropathogens in double J culture was higher in patients with UTIs (89% vs. 55%, p 0.04) and in patients with asymptomatic bacteriuria (89 vs. 51%, p 0.03).
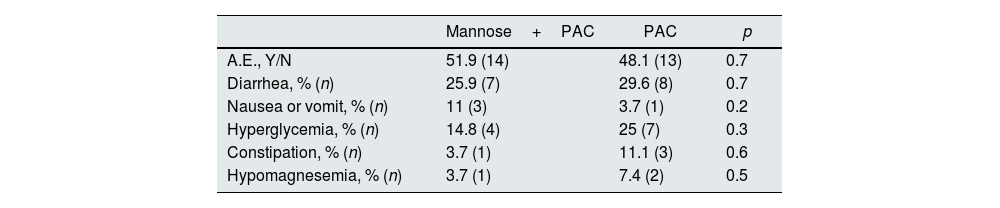
SafetyThe adverse effects reported were minor. In total, 50% of patients presented adverse effects; being 51.9% in those treated with PAC alone and 48.1% in those treated with Mannose (p 0.7). Diarrhea was the most frequently reported adverse event in both groups (see Table 6).
Adverse effect according to treatment group.
| Mannose+PAC | PAC | p | |
|---|---|---|---|
| A.E., Y/N | 51.9 (14) | 48.1 (13) | 0.7 |
| Diarrhea, % (n) | 25.9 (7) | 29.6 (8) | 0.7 |
| Nausea or vomit, % (n) | 11 (3) | 3.7 (1) | 0.2 |
| Hyperglycemia, % (n) | 14.8 (4) | 25 (7) | 0.3 |
| Constipation, % (n) | 3.7 (1) | 11.1 (3) | 0.6 |
| Hypomagnesemia, % (n) | 3.7 (1) | 7.4 (2) | 0.5 |
PAC, Proanthocyanidins; A.E., adverse effects.
The present study explores the feasibility of d-Mannose plus PAC use in preventing UTI after kidney transplantation. The main finding is it does not reduce UTI incidence in the first three months after kidney transplantation compared with PAC alone.
The rationale for the combination is the potentially synergic effect. d-Mannose avoids the infection by targeting a specific type of fimbriae: The FimH or the mannose-sensitive (type 1), whereas PAC contains other lectin-mediated inhibitor that targets the Fim P which are mannose resistant.10,11
A combination of non-antibiotic measures might lead to superior results to monotherapy, as shown in a small pilot study in 33 premenopausal women; with a combination of d-Mannose with cranberry extract and lactobacilli; to successfully treat acute uncomplicated cystitis and symptoms of UTI.15
Vasileiou et al. suggested that other cranberry components such as ursolic acid, have a synergistic role with PAC in the antiad-hesion process by causing differential gene expression in E. coli, resulting in inhibition of biofilm formation.16 That's why it was added to the treatment powder in the experimental group as shown in Supplementary Table 1.
Some studies in general population suggest that non-antibiotic prevention strategies are useful. There is one multicenter-randomized trial from Spain including 93 non-transplant women with recurrent non-complicated UTI, randomized to receive Mannose+PAC vs. PAC, and, in contrast with our results, the percentage of UTI in the group treated with Mannose+PAC was 24%, vs. 45% in the PAC group (p<0.05).12 More recently, Lenger et all found, in a meta-analysis comparing d-Mannose vs. other agents for recurrent UTI prevention in adult women, that d-Mannose appears to be as effective as antibiotics.17
In the pathogenesis of UTIs, not only the attachment of uropathogens to the urothelium is important, but also their ability to produce biofilm; composed of bacteria and self-produced mucopolysaccharides. Biofilm can be formed at the surface of any inert structure such as catheters. Mannose+PAC acts by preventing bacterial adhesion, but their efficacy against the biofilm is probably limited. Therefore, the negative results from our study could be explained by the biofilm persistence (present, for example, in double J catheters).18 To support this hypothesis, our study analyzed, by a special sonication technique, the presence of biofilm at the ureteral (double J) catheter and found that the uropathogens attached to the stent are common (40% were colonized 13% of them with typical uropathogens) and found that the presence of uropathogens in double J culture was higher in patients with UTIs (89% Mannose+PAC vs. 55% PAC alone, p 0.04) and in patients with asymptomatic bacteriuria (89 vs. 51%, p 0.03) respectively.
AB remains a controversial issue in kidney transplantation; while there is consensus to avoid the screening after two months post-surgery; guidelines still consider evaluating the short course of antibiotic if AB is detected in the first months after kidney transplantation.19 The low number of cystitis reported in our study and the high number of AB are probably due to inconsistency of symptoms (especially dysuria) that could be often underreported or related to double J catheter irritation. This could also explain the low rate of UTI recurrence in our study. Moreover, although AB is not considered UTI, in the first days after transplantation, when a vesical catheter or a double J is inserted, the impact of AB is unknown. In our study, 55% of pyelonephritis was anticipated by AB, sharing the same bacteria previously detected in the screening cultures; thus, suggesting that, at least at an initial phase, screening for AB could be useful.
In terms of tolerance, the reported adverse events were minor and mainly gastrointestinal (GI). Therefore, the addition of Mannose was not associated with a higher number of adverse events. However, our numbers are higher than those reported by Casado et al. in their study in non-transplanted women.12 Indeed, in transplant population GI-adverse events are very common, occurring in up to 20% of patients,20 especially in those treated with MPA,21 as recently showed in the TRANSFORM study.22 Thus, the reported adverse events seem to be related to the kidney transplant procedure and to the immunosuppressants, rather than associated with the study medication.
The present study has some limitations. Due to its exploratory design, the statistical power is limited by the small number of patients included and by a low number of UTI compared to AB. Also, the follow-up period is quite short, nonetheless most UTIs in kidney transplant recipients occur early after surgery and their incidence decreases with time. Finally, although the lack of a comparator group with placebo is another limitation, our study was designed to provide evidence that the synergist effect of d-Mannose plus PAC could be capable to reduce incidence of UTI or AB rather than PAC alone. On the other hand, our study has some strengths, such as the design of the clinical trial (randomized, double-blind, prospective), the high number of microbiological culture and the precise technique used to analyze the double J. For all these reason we believe that the negative result of our feasibility study recommends avoiding designing larger studies with d-Mannose or PAC in KT.
ConclusionsWith the present study, we have provided valuable information on a prophylactic strategy that has proven to be effective in non-transplanted population. Unfortunately, the use of d-Mannose plus PAC in the early post-transplant period, even though safe and well tolerated, does not seem to add any protective effect, confirming once again the complexity of pathogenesis in the kidney transplant population and the unmet need for preventive strategies for UTI.
Author's contributionsM.R., E.M., A.S., J.C. helped recruiting data, prepared the draft and performed the statistical analysis of the study. N.S., S.M., A.C., C.A. helped with the microbiological analysis of the catheters. M.F., L.R., B.E. helped with the catheter's extraction and correct transportation. M.D. performed the follow-up and support to any patients concerned. All authors critically reviewed and approved the manuscript draft.
FundingThis study was supported by Instituto de Salud Carlos III (ISCIII), RICORS 2040 RD21/0005/0021. Financed by the “European Union – NextGenerationEU”, Mecanismo para la Recuperación y la Resiliencia (MRR) and by “Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias (CB06/06/0037)” an initiative of the Instituto de Salud Carlos III, Madrid, Spain co-funded by the European Regional Development Fund/European Social Fund (ERDF/ESF, “Investing in your future”). We thank CERCA Program/Generalitat de Catalunya for institutional support. SM was supported by Miguel Servet contract “CP19/00096” (ISCIII).
Conflict of interestThe authors of this manuscript have no conflicts of interest to disclose.
We thank all the transplant professionals at the Bellvitge Hospital for their everyday hard work at the Transplant Unit.