The shortage of organs is a major hurdle in kidney transplantation, and one solution to the problem is to extend the age of the donor. However, organs from older donors are often discarded due to the macroscopic appearance of the parenchyma or major vessels. On the other hand, a large number of elderly patients are potential candidates for kidney transplantation, while many kidneys from elderly deceased donors are discarded due to a lack of age-matched recipients. In addition, a large number are often discarded due to the lack of compatible recipients among elderly patients undergoing chronic dialysis. A possible solution to avoid this wastage of kidneys potentially suitable for transplantation could be the performance of preemptive kidney transplantation (PKT) in carefully selected elderly patients. PKT improves graft and patient survival compared to other renal replacement therapy options. There is no information about PKT in elderly patients receiving kidneys from elderly deceased donors.
MethodsFrom 2007 to 2012, we performed a prospective observational study comparing 26 elderly patients receiving PKT with a control group of 26 elderly patients receiving a first transplant after prior dialysis.
ResultsMean age of recipients was 74.3±2.9 years and mean age of donors was 73.8±4.1 years. Induction immunosuppression was similar in both groups. Death-censored graft survival was 96% in the PKT group and 68% in the control group (p=0.02), at 5 years after transplantation. Immediate and delayed graft function occurred in 92% and 3.8%, respectively, of patients in the PKT group and 53% and 34.6% of patients in the control group (p=0.005). Acute rejection was significantly more frequent in PKT patients (23.1% vs 3.8%, p=0.043). At the end of follow-up time 35.5±20.1 months, the glomerular filtration rate was similar in both groups (42.2±11.7 vs 41.7±11.2ml/min, p-value=0.72). Patient survival was similar in the two groups.
ConclusionsElderly patients with end stage of renal disease non-dialysis may benefit from PKT elderly deceased donors whose kidneys were to be discarded for there are not patients in the waiting list.
La escasez de órganos constituye una importante dificultad para los trasplantes renales, y una posible solución del problema está en ampliar el margen de edad aceptado para los donantes. Sin embargo, los órganos de donantes de edad avanzada se desechan con frecuencia debido al aspecto macroscópico del parénquima o de los vasos sanguíneos principales. Por otro lado, hay un gran número de pacientes ancianos que son posibles candidatos a un trasplante renal, mientras que muchos riñones de donantes ancianos fallecidos se desechan porque no hay receptores de una edad similar. Además, a menudo se desecha un gran número de órganos a causa de la falta de receptores compatibles entre los pacientes ancianos en diálisis crónica. Una posible solución para evitar que se desperdicien estos riñones que pueden ser apropiados para un trasplante sería llevar a cabo un trasplante renal prediálisis (TRP) en pacientes ancianos cuidadosamente seleccionados. El TRP mejora la supervivencia del órgano trasplantado y del paciente, en comparación con otras opciones de terapia sustitutiva renal. No disponemos de información acerca del TRP en pacientes ancianos a los que se trasplantan riñones de donantes ancianos fallecidos.
MétodosDe 2007 a 2012 llevamos a cabo un estudio prospectivo observacional en el que se compararon 26 pacientes ancianos que recibieron un TRP con un grupo de control formado por 26 pacientes ancianos a los que se practicó un primer trasplante después de una diálisis previa.
ResultadosLa media de edad de los receptores fue de 74,3±2,9 años y la de los donantes de 73,8±4,1 años. La inmunosupresión de inducción fue similar en los dos grupos de tratamiento. La supervivencia del órgano, con censura para el análisis estadístico a la muerte del paciente, fue del 96% en el grupo de TRP y del 68% en el grupo de control (p=0,02), 5 años después del trasplante. Hubo una función inmediata y tardía del riñón trasplantado en el 92% y 3,8% de los pacientes, respectivamente, en el grupo de TRP, y en el 53% y 34,6% de los pacientes en el grupo de control (p=0,005). El rechazo agudo fue significativamente más frecuente en los pacientes a los que se practicó un TRP (23,1% frente a 3,8%, p=0,043). Al final del periodo de seguimiento de 35,5±20,1 meses, la filtración glomerular fue similar en ambos grupos (42,2±11,7 frente a 41,7±11,2ml/min, valor de p=0,72). La supervivencia de los pacientes fue también similar en los dos grupos.
ConclusionesEn los pacientes ancianos con una enfermedad renal terminal no dializados, puede resultar beneficioso un TRP de donantes ancianos fallecidos cuyos riñones se desecharían si no hubiera pacientes en lista de espera.
In recent years, the number of patients older than 65 years with end stage renal disease (ESRD) has increased1 and is now the fastest growing patient population requiring renal replacement therapy in both Europe and the United States. Kidney transplantation improves life expectancy and quality of life in patients of all ages with ESRD2,3 and is an attractive option for elderly patients, in whom it confers lower mortality rates and improved quality of life compared to dialysis.4,5 However, a vast majority of kidney transplants in elderly patients are performed after a period of chronic dialysis, and in those elderly patients in whom a preemptive kidney transplant (PKT) is performed the organ comes from a living donor. No experience about PKT using kidneys from elderly deceased donors has so far been reported. This lack of information is important because PKT is the ideal treatment for patients with advanced chronic kidney disease (CKD). Not only does PKT obviate the need for vascular access or the placement of a peritoneal catheter, thus reducing the cost of dialysis and improving the quality of life of the patient,6 but more importantly, it also extends the survival of patients compared to other therapeutic options and attains superior outcomes in terms of both graft and patient survival than transplantation after prior dialysis.7,8 In fact, time spent on dialysis prior to transplantation has been shown to be one of the most significant factors affecting the outcome of kidney transplantation.9,10 On the other hand, the onset of chronic dialysis (building of a successful vascular access for hemodialysis, or peritoneal catheter placement) is more challenging in elderly patients, thus reinforcing the theoretical advantages of PKT in these patients.
The shortage of organs is a limiting factor for kidney transplantation in all age ranges and has led to the use of expanded criteria donors, including those aged over 60 years.4,11 In Spain, 50% of donors are older than 60 and up to 25% are older than 70. Kidney transplantation with expanded criteria donor organs has been associated with longer survival than dialysis,3 but data are limited on elderly patients. Although graft survival is generally poorer with older than with younger donors, when kidneys are selected based on renal function, macroscopic examination and histological information, excellent results can still be attained.12 In spite of the shortage of organs, a significant number of kidneys from older deceased donors are not used because of donor hypertension, diabetes or renal failure, macroscopic or microscopic alterations in the kidney, or importantly, the lack of a suitable recipient. In a previous study, we found that more than 50% of kidneys from older donors were discarded mainly because there was no potentially compatible recipient on the waiting list.13
In order to avoid the wasting of kidneys from deceased elderly donors due to the lack of compatible recipients among elderly patients undergoing chronic dialysis, we created in January 2007 a waiting list of predialysis elderly patients to receive these kidneys. Here we report our experience with this cohort of elderly patients receiving PKTs from elderly deceased donors and compare it with that of a cohort of elderly patients who received a kidney transplant after the onset of chronic dialysis.
Patients and methodsPatients and study designThis is a longitudinal, prospective, observational study of patients undergoing PKT at a single center. From January 2007 to December 2012, we consecutively enrolled 26 patients undergoing PKT at the Department of Nephrology and Transplantation in the Hospital 12 de Octubre, Madrid, Spain. Inclusion criteria were age >65 years, advanced CKD, GFR according to the four-variable MDRD<15ml/min, progression of renal failure (25% decrease in GFR over the past 12 months), no prior kidney transplant, and non-hyperimmunized status. Exclusion criteria were stable renal function (<25% decrease in GFR), active tumor, multiple=“multiple” aortoiliac calcifications, and medical and/or surgical contraindications. The study was approved by the Institutional Review Board of the Hospital 12 de Octubre, and all patients gave their signed informed consent.
The control group comprised an additional 26 patients aged >65 years who received a first kidney transplant after the onset of chronic dialysis. Thirteen (50%) of the controls received paired kidneys from the same donors as the PKT patients; the remaining recipients were selected as controls because their transplants were performed immediately before or after the PKT patients.
The following donor variables were recorded: age, gender, ABO blood group, BMI, history of hypertension or diabetes mellitus, cytomegalovirus (CMV) immunization, viral hepatic infections, sepsis, cause of death, serum creatinine (Scr) levels at procurement, estimated GFR according to the four-variable MDRD, cold ischemia time, warm ischemia time, kidney anatomy, and perfusion solution. The following recipient characteristics were recorded: age, underlying renal disease, time on dialysis, serology, immunological data, BMI, arterial hypertension, hyperlipidemia, diabetes, pre-transplant cardiovascular disease, waiting time for kidney transplant, type of dialysis (hemodialysis or peritoneal dialysis), panel-reactive antibodies, HLA-A, HLA-B, and HLA-DR mismatches, and baseline immunosuppression. Post-surgical complications, infections, tumors, modification of immunosuppression, and length of hospital stay were also recorded. Renal function and proteinuria were recorded weekly during the first month, every three months during the first year, and annually thereafter.
Main outcomes were graft survival and patient survival. Secondary outcomes were immediate graft function, delayed graft function (defined as the need for at least one hemodialysis session during the first week post-transplantation), and biopsy-proven acute rejection (BPAR). Causes of graft loss and causes of death were also recorded. Graft survival was calculated from the date of transplantation until death, return to dialysis, or the end of the study period. Death-censored graft survival was calculated when death occurred with functioning graft.
StatisticsContinuous variables were expressed as mean and standard deviation or as median and range, as appropriate. Categorical variables were expressed as frequencies or percentages. Student's t test or the Mann–Whitney U test was used to compare continuous variables. Qualitative variables were analyzed with the chi-squared test with Yates’ correction or Fisher's exact test. Graft and patient survival were calculated using the Kaplan–Meier method and compared with the log-rank test. Univariate and multivariate regression analyses (with a backward stepwise procedure) were performed with the Cox proportional hazards model to obtain hazard ratios (HRs) with their 95% confidence intervals (CIs). All factors with p<0.05 in the univariate analysis and all clinically relevant factors with p<0.2 in the univariate analysis were included in the multivariate model. Significance was set at p<0.05. All statistical analyses were performed with IBM SPSS Statistics for Windows version 15.0.
ResultsDonor and recipient characteristicsFifty-two patients were enrolled in the study: 26 (11 women, 15 men) PKTs and 26 (13 women, 13 men) controls. Table 1 shows the baseline characteristics of the transplant recipients. Mean age was 74.3±2.9 years (range 68–81 years) in the PKT group and 73.4±4.1 years (range 65–79 years) in the control group. All 26 patients in the PKT group had hypertension, compared to only 18 (69.2%) in the control group (p=0.02). There were no other significant differences in baseline characteristics between the two groups (Table 1). In the control group, the interval between onset of chronic dialysis and kidney transplantation was 15±14 months (range 3–62). Twenty-five recipients (96.2%) in each group received quadruple immunosuppressive therapy with interleukin-2 receptor antagonists, low doses of calcineurin inhibitors, mycophenolate mofetil, and corticosteroids. Induction treatment consisted of basiliximab, an anti-intcrleukin-2 receptor monoclonal antibody, in 50 patients (96%) and of timoglobulin in two (4%).
Baseline recipient and donor characteristics.
| Recipient characteristics | PKT group(N=26)N (%) | Control group(N=26)N (%) | p-Value |
|---|---|---|---|
| Age, yrs | |||
| Mean (SD) | 74.3 (2.9) | 73.4 (4.1) | 0.34 |
| Gender | |||
| Male | 15 (57.7) | 13 (50) | 0.56 |
| Female | 11 (42.3) | 13 (50) | |
| Cause of ESRD | |||
| Hypertensive nephropathy | 10 (38.5) | 6 (23.1) | 0.23 |
| Diabetes | 4 (15.4) | 6 (23.1) | 0.48 |
| Chronic glomerulonephritis | 3 (11.5) | 5 (19.2) | 0.44 |
| Hypertension | 26 (100) | 18 (69.2) | 0.02 |
| Diabetes | 9 (34.6) | 10 (38.5) | 0.77 |
| Dyslipemia | 8 (30.8) | 8 (30.8) | 1.0 |
| Ischemic heart disease | 5 (19.2) | 3 (11.5) | 0.44 |
| Peripheral arterial diseases | 5 (19.2) | 1 (3.8) | 0.08 |
| Cerebrovascular accident | 1 (3.8) | 1 (3.8) | 1.0 |
| Neoplasia | 1 (3.8) | 4 (15.4) | 0.16 |
| Hepatitis C virus infection | 2 (7.7) | 2 (7.7) | 1.0 |
| Donor characteristics | PKT group(N=26)N (%) | Control group(N=26)N (%) | p-Value |
|---|---|---|---|
| Age, yrs | |||
| Mean (SD) | 73.8 (4.1) | 74.4 (5.1) | 0.63 |
| Gender | |||
| Male | 14 (53.8) | 17 (65.4) | 0.39 |
| Female | 12 (46.2) | 9 (34.6) | |
| Cause of death | |||
| Acute cerebrovascular accident | 20 (76.9) | 21 (80.8) | 0.73 |
| Cranioencephalic trauma | 4 (15.4) | 3 (11.5) | 0.68 |
| Serum creatinine (mg/dl)a | 0.7 (0.1) | 0.7 (0.1) | 0.98 |
| GFR (MDRD) (ml/min/1.73m2)a | 90.4 (19.6) | 94.2 (24.3) | 0.54 |
| Donor renal biopsy | 16 (61.5) | 20 (76.9) | 0.23 |
| Glomerulosclerosis same donor (N=10)a | 6.9 (3.7) | 9.6 (5.2) | 0.20 |
| Glomerulosclerosis different donor (N=6 y 10)a | 7.4 (7.6) | 5.9 (7.9) | 0.71 |
| HLA mismatches ≥3 | 5 (19.2) | 4 (15.4) | 0.71 |
| Cold ischemia time (hours)a | 21.4 (5.3) | 21.6 (4) | 0.88 |
| Baseline immunosuppression with basiliximab+tacrolimus+mycophenolate mofetil+steroids | 25 (96.2) | 25 (96.2) | 1.0 |
Table 1 displays the baseline characteristics of the donors. Median donor age was 73.8±4.1 years (range, 65–80 years) in the PKT group and 74.4±5.1 years (range 65–84 years) in the control group. There were no significant differences in baseline characteristics between the two groups. The median Scr level at the time of procurement was 0.7mg/dl (range 0.5–1.1mg/dl) in both groups. Median estimated GFR was 90.4±19.6ml/min/1.73m2 in the PKT group and 94.2±24.3ml/min/1.73m2 in the control group. Kidney biopsy was performed in 16 donors in the PKT group and in 20 in the control group, and the percentage of sclerotic glomeruli was 6.3% (range 4.2–10%) and 5.8% (4–15%), respectively.
Median cold ischemia time was 21.4±5.3h (range 7–33.3) in the PKT group and 21.6±4h (range 8.2–26.3) in the control group (Fig. 1).
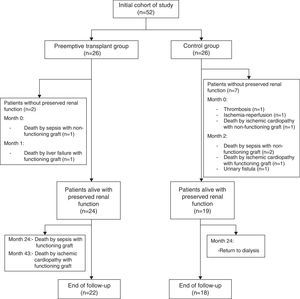
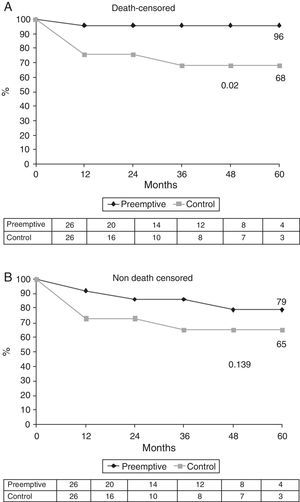
Main outcomesThe actuarial 1-, 3-, and 5-year graft survival rates (death-censored) were 96%, 96% and 96% in the PKT group and 76%, 68% and 68% in the control group (p=0.02) (Fig. 2). Primary non-function was observed in one patient (arterial thrombosis) in the PKT group and in three in the control group (two cases of arterial thrombosis and one of venous thrombosis) (Table 2). Early graft loss (less than three months) occurred in one patient in the PKT group (death from liver failure with functioning graft) and in four patients in the control group (one death with graft thrombosis, one death from sepsis, one death from heart attack, and one urinary fistula).
Causes of graft loss.
| Cause | PKT group(N=26)N (%) | Control group(N=26)N (%) | p-Value |
|---|---|---|---|
| Primary non-function | 1 (3.8)a | 3 (11.5)b | 0.29 |
| Delayed graft function | 1 (3.8) | 9 (34.6) | 0.005 |
| Early graft loss (≤3 months) | 1 (3.8)c | 4 (15.4)d | 0.15 |
| Acute rejection | 6 (23.1) | 1 (3.8) | 0.043 |
| Cellular | 4 (66.7) | 1 (100) | |
| Humoral | 2 (33.3) | 0 (0) | |
| Exitus with a functioning graft | 3 (75) | 1 (25) | 0.15 |
| Total number of deaths | 4 (15.4)e | 4 (15.4)f | 1.0 |
| Return to dialysis | 0 (0) | 4 (15.4) | 0.03 |
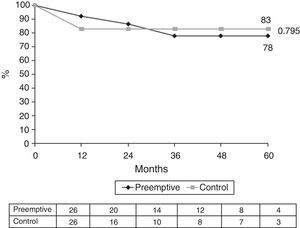
The actuarial 1-, 3-, and 5-year patient survival rates were 92%, 78% and 78% in the PKT group and 83%, 83% and 83% in the control group (Fig. 3). One patient in each group died with a functioning graft. Four patients died in each group. Causes of death in the PKT group were sepsis/multi-organ failure (2), acute liver failure (1) and unclear (1). Causes of death in the control group were sepsis/multi-organ failure (2) and cardiac failure (2) (Table 2).
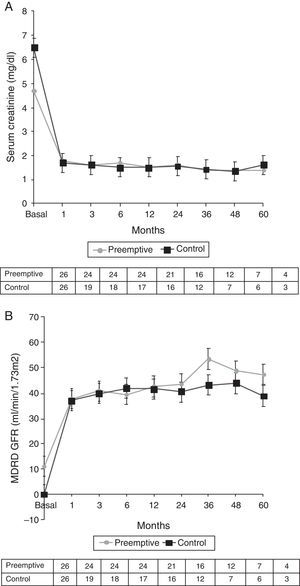
Secondary outcomesDelayed graft function was observed in one patient (3.8%) in the PKT group and in nine (34.6%) in the control group (p=0.005). Six patients (23.1%) in the PKT group experienced BPAR: four with acute cellular rejection and two with acute humoral rejection. One patient (3.8%) in the control group experienced acute cellular rejection (p=0.043) (Table 3). After a median follow-up of 34 months, 24 patients in the PKT group and 19 in the control group had preserved renal function without the need for dialysis (Fig. 1). At the end of the study, renal function was similar in the two groups (PKT: Scr 1.7±0.8mg/dl and GFR-MDRD 42.2±18.3ml/min/1.73m2; control: Scr 1.6±0.9mg/dl and GFR-MDRD 40.4±13.8ml/min/1.73m2). Proteinuria was 0.6g/24h in the PKT group and 0.3g/24h in the control group (p=0.08) (Table 3 and Fig. 4). No patients in the PKT group and four in the control group returned to dialysis (p=0.03).
Evolution of renal function and proteinuria.
| Variable | PKT group(N=24) | Control group(N=19) | p-Value |
|---|---|---|---|
| Serum creatinine (mg/dl) | |||
| 6 months (PKT=24/CG=19 patients) | 1.7±0.7 (0.9–3.9) | 1.5±0.4 (0.8–2.5) | 0.23 |
| 12 months (PKT=24/CG=19 patients) | 1.5±0.4 (1–2.8) | 1.5±0.5 (0.9–3.1) | 0.86 |
| End of follow up (PKT=22/CG=18 patients) | 1.7±0.8 (0.7–4.2) | 1.6±0.9 (0.8–5) | 0.78 |
| GFR (MDRD) (ml/min/1.73m2) | |||
| 6 months (PKT=24/CG=19 patients) | 39.5±14.6 (15.2–73) | 41.9±11.9 (19.2–72.1) | 0.58 |
| 12 months (PKT=24/CG=19 patients) | 42.7±11.7 (21.9–65) | 41.7±11.2 (14.7–58) | 0.80 |
| End of follow up (PKT=22/CG=18 patients) | 42.2±18.3 (13.7–87) | 40.4±13.8 (8.4–72.1) | 0.72 |
| Decline in GFR (6 months – end of follow-up) (ml/min/1.73m2per year) | +0.9±0.5 (−5.9 to 12.8)1.2 (−2.9 to 3.8) | +0.1±3.4 (−6.9 to 5.3)0 (−2.1 to 2.5) | 0.52 |
| Proteinuria (g/24h) | |||
| 6 months (PKT=24/CG=19 patients) | 0.4 (0.2–0.9) | 0.4 (0.2–0.6) | 0.31 |
| 12 months (PKT=24/CG=19 patients) | 0.5 (0.2–1) | 0.3 (0.2–0.5) | 0.09 |
| End of follow-up (PKT=22/CG=18 patients) | 0.6 (0.3–1) | 0.3 (0.2–0.4) | 0.08 |
| Follow-up (months) | 35.5±20.1 (5–67) | 32±20.9 (2–66) | 0.58 |
During follow-up, immunosuppression consisted of a combination of tacrolimus, mycophenolic acid and steroids in 67% of all patients. Other immunosuppressive regimens used were similar in the two groups. Tacrolimus levels at the end of follow-up were 6.9±2.3 in the PKT group and 7.5±2.6ng/ml in the control group (p=0.43).
CMV infections and urinary tract infections were similar in the two groups. Urological complications and tumor incidences were also similar in the two groups, while a slightly higher number of cardiovascular complications and peripheral arterial diseases were observed in the PKT group (p=0.06) (Table 4).
Major surgical and medical complications.
| Complication | PKT group(N=26)N (%) | Control group(N=26)N (%) | p-Value |
|---|---|---|---|
| Infection | 10 (41.7) | 9 (47.4) | 0.70 |
| Urinary tract | 3 (30) | 4 (44.4) | 0.45 |
| CMV | 3 (30) | 4 (44.4) | 0.45 |
| Urological problem | 10 (38.5) | 10 (38.5) | 1.0 |
| Urinary fistula | 2 | 4 | |
| Wound dehiscence | 1 | 1 | |
| Seroma | 3 | 2 | |
| Arterial stenosis | 2 | 2 | |
| Ureter stenosis | 2 | 1 | |
| Tumor | 2 (8.3)a | 2 (10.5)b | 0.80 |
| Cardiovascular disease | |||
| Hypertension | 22 (91.7) | 17 (89.5) | 0.80 |
| Diabetes | 10 (41.7) | 7 (36.8) | 0.75 |
| Dyslipemia | 11 (45.8) | 11 (57.9) | 0.43 |
| Ischemic heart disease | 7 (29.2) | 3 (15.8) | 0.30 |
| Cerebrovascular accident | 1 (4.2) | 0 | 0.36 |
| Chronic limb ischemia | 4 (16.7) | 0 | 0.06 |
In the univariate analysis including age, time on dialysis, delayed graft function, HLA incompatibility, belonging to PKT group, diabetes mellitus, serum creatinine at month 6, proteinuria at month 6, and the difference in GFR between month 6 and end of study, only those belonging to PKT group were associated with improved outcome (p=0.049). Multivariate logistic regression analysys was performed and the factor belonging to PKT group did not reach statistical significance (HR=0.18; 95% CI, 0.03–1.02; p=0.053) (Table 5).
Risk factors for graft survival.
| Factor | Univariate analysisHR (95% CI) | p-Value | Multivariate analysisHR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.1 (0.90–1.40) | 0.27 | 1.1 (0.94–1.46) | 0.14 |
| Delayed graft function | 2.1 (0.33–13.7) | 0.42 | ||
| HLA incompatibilities | 1.5 (0.73–3.27) | 0.25 | 1.5 (0.64–3.60) | 0.33 |
| PKT group | 0.2 (0.03–0.99) | 0.049 | 0.18 (0.03–1.02) | 0.053 |
| Acute rejection | 0.9 (0.39–2.06) | 0.90 | ||
| Serum creatinine at 6 months | 4.1 (0.47–35.8) | 0.39 | ||
| Proteinuria at 6 months | 1.5 (0.6–3.8) | 0.42 |
The shortage of organs is a major hurdle in kidney transplantation, and one solution to the problem is to extend the age of the donor. However, organs from older donors are often discarded due to the macroscopic appearance of the parenchyma or major vessels. In addition, a large number are often discarded due to the lack of compatible recipients among elderly patients undergoing chronic dialysis.13 A possible solution to avoid this wastage of kidneys potentially suitable for transplantation could be the performance of PKT in carefully selected elderly patients. Patient and graft survival are longer for patients undergoing PKT than for those transplanted when receiving dialysis, and time spent on dialysis prior to transplantation significantly affects graft and patient outcome.8,9 In a study of 1849 kidney recipients, including 385 PKTs, patient survival at 5 years was higher in PKT than in non-PKT patients. With deceased donors, patient survival was 92.6% and 76.6%, respectively (p=0.001), and with living donors, patient survival was 93.3% and 89.5%, respectively (p=0.02). Graft survival was also higher among the PKT patients.14 Several other studies have also shown better results when performing PKT as compared to transplantation after the onset of chronic dialysis.
Nevertheless, no studies have analyzed the performance of PKT in elderly patients using kidneys from elderly deceased donors. This policy would gather the benefits linked to PKT and the use of valid organs that otherwise would be discarded. Our pilot study demonstrates that PKT in very elderly patients (mean age 74.3±2.9 years) using kidneys from very elderly deceased donors (mean age 73.8±4.1 years) offers excellent results. Graft survival was significantly better in PKT patients as compared to that of a control group of elderly patients who received kidneys from elderly deceased donors after having started chronic dialysis. There were no differences between both groups regarding patient survival. By univariate analysis, belonging to PKT group was the only factor associated with an improved outcome, although it did not reach statistical significance by multivariate analysis (Table 5).
The reason for improved graft survival with PKT is not completely clear. Several studies have shown a negative impact of waiting time on dialysis, with a directly proportional relationship to the risk of graft loss and shorter patient survival.15 The time on dialysis also influences chronic allograft nephropathy.8 The exact reasons for this negative effect remain unclear, but several potential explanations have been postulated, such as an increase of persistent pro-inflammatory and pro-atherogenic molecules, malnutrition, immune system disorders and inadequate clearance of toxic metabolites.16,17 The preservation of residual renal function may influence the improvement in survival. Recent studies have found no clear evidence on this factor. On the other hand, differences in the rate of immediate or delayed graft function, or in the rate of acute rejection, could influence better outcomes of PKT. We found that our elderly PKT patients had a significantly higher rate of immediate graft function (92% vs 53%) and a significantly lower rate of delayed graft function (3.8% vs 34.6%), as compared to control patients. On the contrary, we found a significantly higher rate of BPAR among our PKT patients (23.1% vs 3.8% in the control group). Some studies have shown a significantly lower rate of BPAR within the first six months post-transplantation in PKT patients.18,19 In contrast, in another study of 1463 kidney transplants more patients experienced an acute rejection episode in the PKT group,20 which the authors tentatively attributed to poor drug compliance or to the absence of the immunosuppressive effect of the uremic state.6 Although age was associated with a decrease in the function of the immune system and therefore a decrease in the rate of acute rejection, however the impact of acute rejection on graft loss has been reported to be more pronounced in elderly patients, independent of graft quality.21 However, in all these studies graft survival was higher in the PKT group.20 Although we should not forget that the information in these studies is biased toward patients who received a kidney transplant from living donor. There is little information in the literature on the incidence of acute rejection and its evolution in older patients who receive the anticipated renal graft from cadaver or living. Recent findings from our group provide new information in the field of immunology (imbalance of the different lymphocyte populations, immunoglobulins and complement) in dialysis patients and its influence on the development of infections.22,23 These findings should lead to new lines of research aimed at knowledge and behavior of lymphocyte subsets in patients with advanced chronic kidney disease and dialysis population and its possible influence on acute rejection. Taken together, these findings suggest that PKT may have a long-term advantage regardless of acute rejection episodes.
Since our study is the first to analyze PKT in very elderly patients using very elderly donors, more studies are required to confirm our results and to gain insights into the influence of uremia and chronic dialysis in the risk of acute rejection in this population.
Our study has several limitations, including a relatively small sample size. It was conducted at a single center, and our criteria for including patients – GRF<15ml/min and 25% progression in renal failure over the past year24 – may rule out extrapolating our findings to other centers using different criteria. Furthermore, we did not evaluate residual renal function, which may confer a survival benefit and which merits investigation in future studies. Nevertheless, although deceased donor organs are, for ethical reasons, reserved for patients on dialysis, our experience has shown that PKT can be an option for patients with non-dialysis ESRD with no detriment to dialysis patients on the waiting list. This policy may both provide non-dialysis elderly patients with the opportunity for a PKT; with the promise of excellent results, and also help overcome the problem of organ shortages by using kidneys destined to be discarded due to a lack of elderly recipients. The focus of clinicians should be on shortening transplant waiting times for elderly patients using preemptive transplantation whenever possible. The kidneys from deceased very old donors can be successfully transplanted in ESRD non-dialysis elderly recipient when there are not other very old candidates in the waiting list.
Conflicts of interestThe authors declare no conflicts of interest.
This study has been supported by grants from REDINREN (RD012/0021) and AITER (Asociación para la Investigación y Tratamiento de la Enfermedad Renal).