Hypervolemia is a major concern in dialysis patients, and is associated with increased cardiovascular risk and death. Cross sectional analysis have previously demonstrated that peritoneal dialysis (PD) patients are not more overhydrated when compared to haemodialysis¿ ones. This study was designed to evaluate longitudinal trends in hydration status and corporal composition in a PD population.
MethodsWe conducted a 2 year prospective observational study of 58 PD patients from a single centre. Incident and prevalent patients were included. Yearly measurements were performed using multifrequency electric bioimpedance. Overhydration (OH) was defined as an extra-cellular water (ECW)/total body water (TBW) over 15%. Clinical and biochemical variables were also explored.
ResultsA total of 30 patients completed evaluation (female 63.3%, mean age 56.9 years, BMI 25.0kg/m2, diabetes 10.0%, APD-50.0%). Median PD vintage was 21.9 months, and 36.7% were anuric. At baseline 6.7% were overhydrated.
On longitudinal analysis no significant changes were found in hydration status, systolic blood pressure, pro-BNP, nor albumin levels. Similar results were found among incident (n=11; APD- 45.5%; anuric- 9.1%) and prevalent (n=19; APD- 52.6%; anuric- 52.6%) patients (p>.05). However, at the second year, prevalent patients were moderately overhydrated compared to incident ones (median 10.2% vs 3.5%; p=.009). Nonetheless, no statistical difference was observed considering adequacy, TBW, or ECW. Moreover, nutritional parameters remained stable.
ConclusionsPeritoneal dialysis maintenance without increasing volume status, nor major deleterious corporal composition trends, is feasible under careful therapy strategies. Longitudinal application of BIA may be a useful clinical tool to evaluate adequacy beyond Kt/V.
La hipervolemia constituye un gran problema en los pacientes de diálisis, y se asocia a un incremento del riesgo cardiovascular y muerte. Los análisis transversales han demostrado previamente que los pacientes de diálisis peritoneal (DP) no sufren de sobrehidratación, en comparación a los pacientes de hemodiálisis. Este estudio fue diseñado para evaluar las tendencias longitudinales de composición corporal e hidratación en una población de pacientes de DP.
MétodosRealizamos un estudio observacional prospectivo de dos años a 58 pacientes de DP de un único centro. Se incluyó pacientes incidentales y prevalentes. Se realizaron mediciones anuales utilizando bioimpedancia eléctrica de multi-frecuencia. La sobrehidratación se definió como el ratio agua extra-celular (ECW)/agua corporal total (ACT) superior al 15%. También se exploraron variables clínicas y bioquímicas.
ResultadosUn total de 30 pacientes completarán el estudio (mujeres: 63,3%, edad media 56,9 años, IMC 25,0kg/m2, diabetes 10,0%, DPA-50,0%). La antigüedad media de DP fue de 21,9 meses, y el 36,7% padecía anuria. Al inicio, el 6,7% padecía sobrehidratación.
En los análisis longitudinales no se hallaron cambios en cuanto a hidratación, presión sanguínea sistólica, pro-BNP, o niveles de albúmina. Se hallaron resultados similares entre los pacientes incidentales (n = 11; APD- 45,5%; anuria- 9,1%) y prevalentes (n = 19; DPA- 52,6%; anuria- 52,6%) (p > 0,05). Sin embargo, al segundo año, los pacientes prevalentes estaban moderadamente sobrehidratados en comparación con los incidentales (media 10,2% frente a 3,5%; p = 0,009). En cambio, no se observó una diferencia estadística en cuanto a adecuación, ACT, o ECW. Además, los parámetros nutricionales permanecieron estables.
ConclusionesLa prevalencia de la diálisis peritoneal sin incremento de volumen ni alteración de los índices de composición corporal es factible si se aplican estrategias terapéuticas prudentes. La aplicación longitudinal de BIA puede constituir una herramienta clínica para evaluar la adecuación por encima de Kt/V.
Hypervolemia is a “traditional” independent risk factor for cardiovascular disease and death among end-stage renal disease patients. It is associated with ventricular hypertrophy,1 nutritional changes,2,3 and inflammation.4–6 Volume overload also promotes endothelial dysfunction7 and nightly non-dipping8 blood pressure in dialysis patients.
The importance of adequacy beyond small solute clearances in the overall patient survival was highlighted by peritoneal dialysis landmark studies such as CANUSA,9 ADEMEX,10 and NECOSAD.11
Residual renal function (RRF) plays a determinant role in the outcome of peritoneal dialysis (PD) patients.11,12 Daily urine output over 250ml represents a 34% increase in survival benefit in peritoneal dialysis patients.9 A reduction in the risk of death,13 volume overload, and left ventricular dysfunction,14 has been observed with increased fluid removal in PD, alongside with sodium restriction. Nevertheless, the ADEMEX study failed to demonstrate mortality differences between anuric patients and the rest, either within and between groups.10
It has been postulated that euvolemia is harder to achieve in PD patients. Nonetheless, in a cross sectional study, Devolder and colleagues15 demonstrated that patients undergoing PD had a similar volume status when compared to equivalent hemodialysis ones.
Determining euvolemia is a challenging task. Reliable evaluation of overhydration can be attained through multifrequency bioimpedance analysis (BIA). We have previously documented that overhydration (OH) in PD patients, defined as extra-cellular water (ECW)/total body water (TBW) over 15%, was associated with age and diabetes, but neither with PD vintage nor anuria.16
This study aimed to evaluate longitudinal changes in hydration status, and identify corporal composition trends in a PD population.
MethodsPopulationWe conducted a 2 year (2009-2011) single centre prospective non-interventional observational study to determine the volume status among incident and prevalent peritoneal dialysis patients. Incident patients were defined as those with less than 6 months on dialysis. All patients over 18 years old were eligible to participate. Contraindication to bioimpedance evaluation – implanted electronic medical device or connection to an external electronic medical device (pacemaker or ICD), any kind of metal implants or metal artificial joints, amputations, pregnancy or in a lactation period, or refusal to participate, were the only exclusion criterion. Patients lost to follow-up were excluded from the analysis.
All PD patients are treated with low glucose degradation product solutions (Balance, Fresenius Medical Care; Physioneal, Baxter Healthcare). Some patients were also on icodextrin due to failure to achieve clinical euvolemia with dietary salt restriction and diuretics. Hypertonic solutions were used only by exception and for a short period of time.
MeasurementsBody composition measurements were performed using multifrequency whole body bioimpedance assessment using the Body Composition Monitor (BCM, Fresenius Medical Care). Patients were in the recumbent position for 5minutes, and all metal accessories removed. Fluid present in the trunk is not measured by bioimpedance spectroscopy and does not influence BIA results.17–19 Therefore, and for logistical reasons measurements were conducted with a full abdomen. Yearly measurements were performed, T0 representing baseline evaluation, T1 the first and T2 the second year determinations, respectively.
Hydration status was evaluated according to parameters provided by the BCM software: 1) total overhydration (in litres), and 2) relative overhydration (rOH - in %) - calculated as the extra-cellular water (ECW) to total body water (TBW) relation.
For statistical analysis, patients were categorized into normohydrated (rOH<6.8%) and severely overhydrated (rOH>15%), according to the definition of Wizemann et al.20 The remaining were assumed to be moderately overhydrated.6,8
Weight was assessed on a calibrated electronic weighing scale, with patients wearing only trousers and a light shirt. Blood pressure was defined as the mean of three measurements in a sitting position, using a standardized electronic device with brachial cuff adjusted to the patient's arm circumference. Blood, urinary, and peritoneal analysis were performed on the day of BIA assessment. Peritoneal ultrafiltration was determined according to patients’ registry (or cyclers’, on APD) on the day previous to BCM assessment, and calculated as the difference between the sum of drained ultrafiltrate and the sum of infused solution in a 24h period.
Statistical analysisContinuous variables are presented as means and standard deviations for parametric, and one-way ANOVA with post hoc Scheffé testing was used for group comparison. Non-parametric variables are presented as median and interquartile range, and group comparison was performed using Kruskal-Wallis analysis. General linear modelling was used for multivariate analysis, including all variables that were deemed relevant and avoiding parameters that were highly correlated with each other.
All statistical analyses were performed using SPSS version 17 (SPSS Inc., Chicago, IL, USA).
ResultsA total of 58 patients were evaluated during the study period. Twenty eight failed to complete 3 measurements, and were lost to follow up: 18 were submitted to transplant, 10 commuted to haemodialysis due to technique failure – 2 with chronic exit site infection, 3 with peritonitis, 2 with ultrafiltration failure, 2 due to abdominal surgical complications, and one patient lost the ability to perform auto-dialysis.
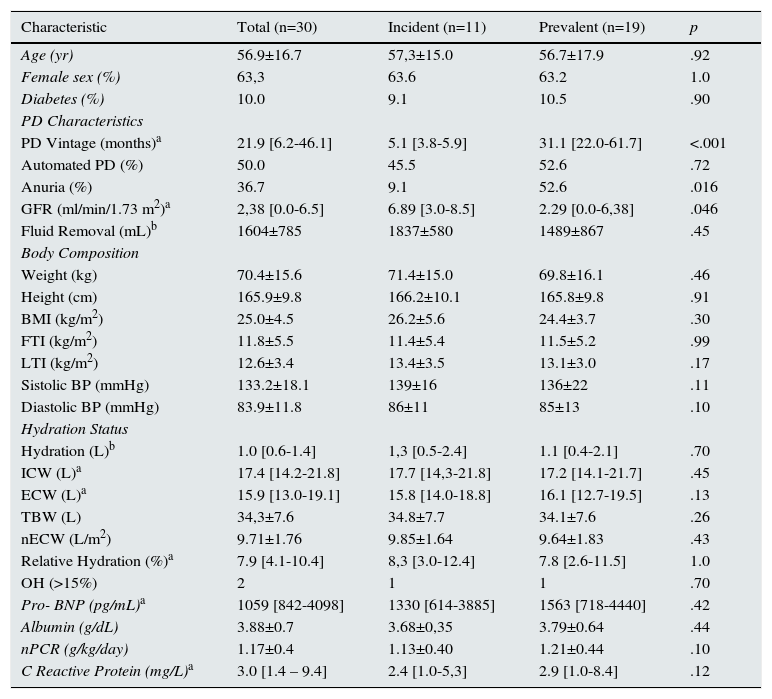
Patients had a mean age of 56.9 years old, 63,3% were female, a PD vintage of 21.9 months, and three (10%) were diabetic. Half were on automated peritoneal dialysis (APD). Eleven were incident patients on PD at the time of the first evaluation. Patient demographics and baseline characteristics can be seen in Table 1.
Baseline characteristics.
| Characteristic | Total (n=30) | Incident (n=11) | Prevalent (n=19) | p |
|---|---|---|---|---|
| Age (yr) | 56.9±16.7 | 57,3±15.0 | 56.7±17.9 | .92 |
| Female sex (%) | 63,3 | 63.6 | 63.2 | 1.0 |
| Diabetes (%) | 10.0 | 9.1 | 10.5 | .90 |
| PD Characteristics | ||||
| PD Vintage (months)a | 21.9 [6.2-46.1] | 5.1 [3.8-5.9] | 31.1 [22.0-61.7] | <.001 |
| Automated PD (%) | 50.0 | 45.5 | 52.6 | .72 |
| Anuria (%) | 36.7 | 9.1 | 52.6 | .016 |
| GFR (ml/min/1.73 m2)a | 2,38 [0.0-6.5] | 6.89 [3.0-8.5] | 2.29 [0.0-6,38] | .046 |
| Fluid Removal (mL)b | 1604±785 | 1837±580 | 1489±867 | .45 |
| Body Composition | ||||
| Weight (kg) | 70.4±15.6 | 71.4±15.0 | 69.8±16.1 | .46 |
| Height (cm) | 165.9±9.8 | 166.2±10.1 | 165.8±9.8 | .91 |
| BMI (kg/m2) | 25.0±4.5 | 26.2±5.6 | 24.4±3.7 | .30 |
| FTI (kg/m2) | 11.8±5.5 | 11.4±5.4 | 11.5±5.2 | .99 |
| LTI (kg/m2) | 12.6±3.4 | 13.4±3.5 | 13.1±3.0 | .17 |
| Sistolic BP (mmHg) | 133.2±18.1 | 139±16 | 136±22 | .11 |
| Diastolic BP (mmHg) | 83.9±11.8 | 86±11 | 85±13 | .10 |
| Hydration Status | ||||
| Hydration (L)b | 1.0 [0.6-1.4] | 1,3 [0.5-2.4] | 1.1 [0.4-2.1] | .70 |
| ICW (L)a | 17.4 [14.2-21.8] | 17.7 [14,3-21.8] | 17.2 [14.1-21.7] | .45 |
| ECW (L)a | 15.9 [13.0-19.1] | 15.8 [14.0-18.8] | 16.1 [12.7-19.5] | .13 |
| TBW (L) | 34,3±7.6 | 34.8±7.7 | 34.1±7.6 | .26 |
| nECW (L/m2) | 9.71±1.76 | 9.85±1.64 | 9.64±1.83 | .43 |
| Relative Hydration (%)a | 7.9 [4.1-10.4] | 8,3 [3.0-12.4] | 7.8 [2.6-11.5] | 1.0 |
| OH (>15%) | 2 | 1 | 1 | .70 |
| Pro- BNP (pg/mL)a | 1059 [842-4098] | 1330 [614-3885] | 1563 [718-4440] | .42 |
| Albumin (g/dL) | 3.88±0.7 | 3.68±0,35 | 3.79±0.64 | .44 |
| nPCR (g/kg/day) | 1.17±0.4 | 1.13±0.40 | 1.21±0.44 | .10 |
| C Reactive Protein (mg/L)a | 3.0 [1.4 – 9.4] | 2.4 [1.0-5,3] | 2.9 [1.0-8.4] | .12 |
BMI – body mass index; BNP – brain natriuretic protein; BP – blood pressure; ECW – extracellular water; FTI - fat tissue index; GFR - Glomerular Filtration Rate; ICW – intracellular water; LTI - lean tissue index; nECW – normalized extracellular water; nPCR – normalized protein catabolism rate; OH –overhydration; PD – peritoneal dialysis; TBW – total body water.
The table summarizes the demographics, clinical, technique, and body composition characteristics of the study population at baseline evaluation. Significance values are regarding incident and prevalent patients’ comparison.
aResults presented as median and interquartile range [IQR 25-75].
bFluid Removal – Diuresis + ultrafiltration.
At baseline, only 40% were normohydrated (fluid overload <6.8%). Only 2 patients were overhydrated (relative OH>15%) - these were older (p<.05) and had higher Pro-BNP levels (p<.05). No differences were found on all the remaining parameters evaluated (p>.05).
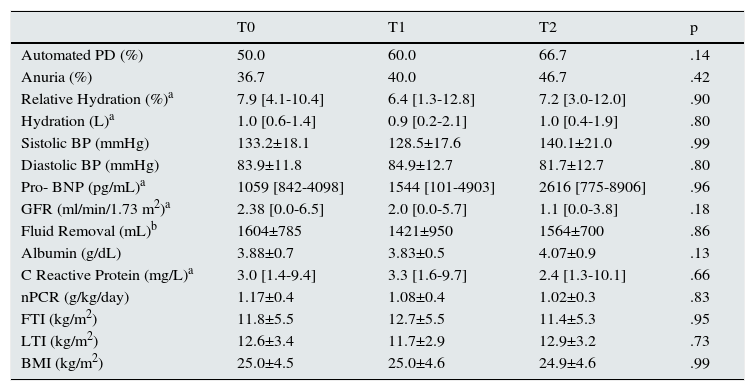
Yearly determinations were performed, with an inter-evaluation time lag of 10,3±3.0 and 12.8±2.7 months between T0-T1 and T1-T2, respectively.
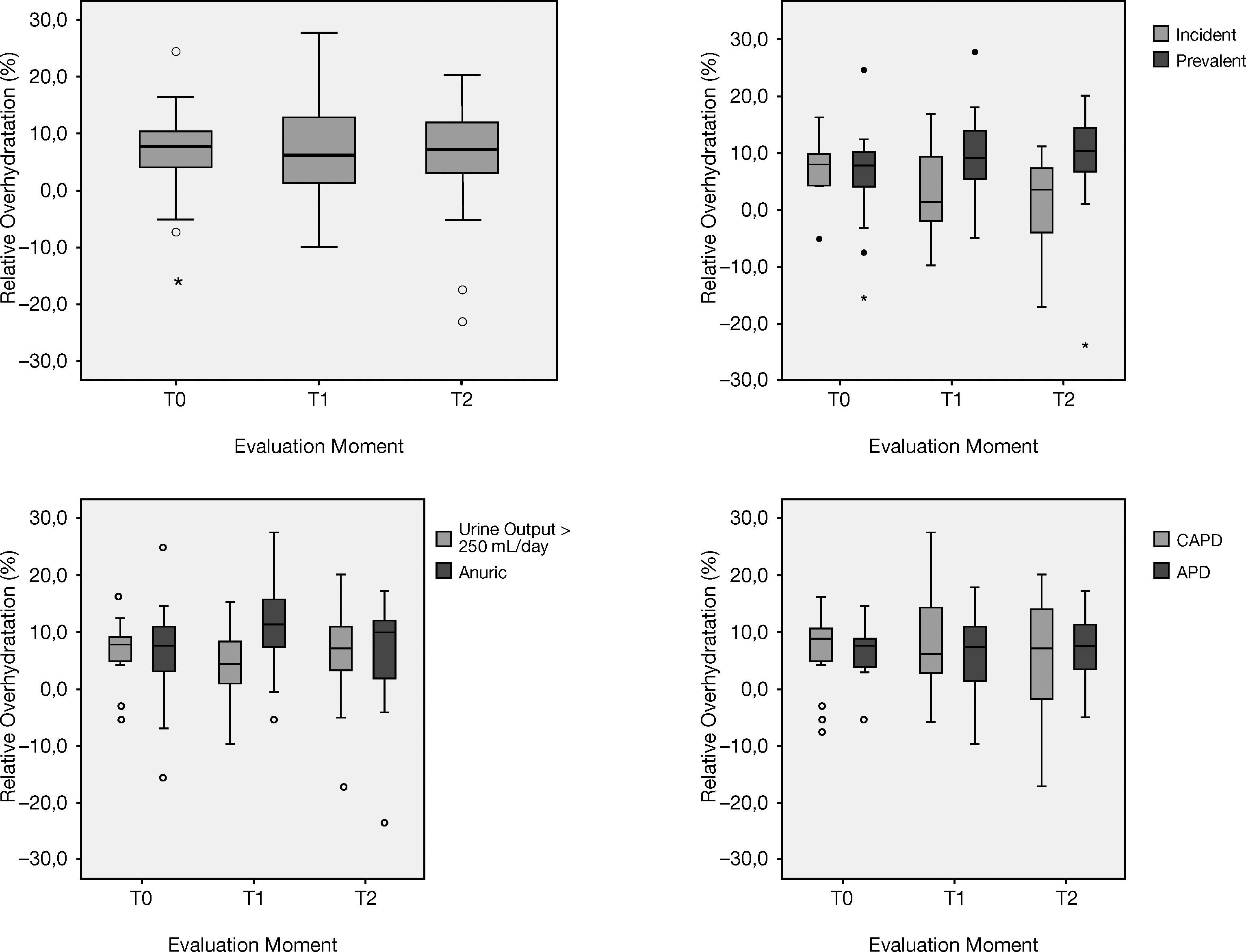
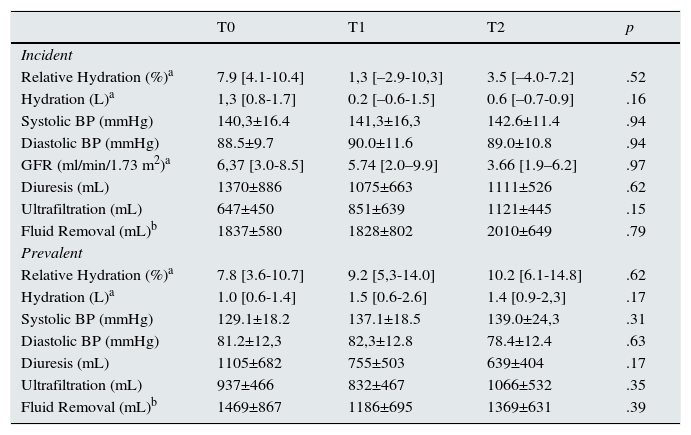
On longitudinal analysis no significant changes were found in bioimpedance hydration status in the global population (Fig. 1). Similar results (p>.05) were found on other hydration parameters, such as systolic blood pressure, and Pro-BNP, even though the latest presented a positive trend – Table 2).
Relative overhydration over 2 years on PD. Hydration status remained fairly stable over the follow-up period, regardless of residual diuresis, PD vintage, or PD modality. The difference found on the first year evaluation (T1) between anuric and patients with diuresis >250ml/day was normalized by the second year (T2).
– Longitudinal changes in overall population.
| T0 | T1 | T2 | p | |
|---|---|---|---|---|
| Automated PD (%) | 50.0 | 60.0 | 66.7 | .14 |
| Anuria (%) | 36.7 | 40.0 | 46.7 | .42 |
| Relative Hydration (%)a | 7.9 [4.1-10.4] | 6.4 [1.3-12.8] | 7.2 [3.0-12.0] | .90 |
| Hydration (L)a | 1.0 [0.6-1.4] | 0.9 [0.2-2.1] | 1.0 [0.4-1.9] | .80 |
| Sistolic BP (mmHg) | 133.2±18.1 | 128.5±17.6 | 140.1±21.0 | .99 |
| Diastolic BP (mmHg) | 83.9±11.8 | 84.9±12.7 | 81.7±12.7 | .80 |
| Pro- BNP (pg/mL)a | 1059 [842-4098] | 1544 [101-4903] | 2616 [775-8906] | .96 |
| GFR (ml/min/1.73 m2)a | 2.38 [0.0-6.5] | 2.0 [0.0-5.7] | 1.1 [0.0-3.8] | .18 |
| Fluid Removal (mL)b | 1604±785 | 1421±950 | 1564±700 | .86 |
| Albumin (g/dL) | 3.88±0.7 | 3.83±0.5 | 4.07±0.9 | .13 |
| C Reactive Protein (mg/L)a | 3.0 [1.4-9.4] | 3.3 [1.6-9.7] | 2.4 [1.3-10.1] | .66 |
| nPCR (g/kg/day) | 1.17±0.4 | 1.08±0.4 | 1.02±0.3 | .83 |
| FTI (kg/m2) | 11.8±5.5 | 12.7±5.5 | 11.4±5.3 | .95 |
| LTI (kg/m2) | 12.6±3.4 | 11.7±2.9 | 12.9±3.2 | .73 |
| BMI (kg/m2) | 25.0±4.5 | 25.0±4.6 | 24.9±4.6 | .99 |
BMI, body mass index; BNP, brain natriuretic protein; BP, blood pressure; FTI, fat tissue index; GFR, Glomerular Filtration Rate; LTI, lean tissue index; nPCR, normalized protein catabolism rate; OH, overhydration; PD, peritoneal dialysis.
Longitudinal changes encountered in the overall population during the follow-up period.
aResults presented as median and interquartile range [IQR 25-75].
bFluid Removal–Diuresis+ultrafiltration.
Incident patients presented a non-significant reduction in overall hydration status from T0 to T1 and T2 (p>.05), and a trend toward a decline in GFR (6.4 vs 5.7 vs 3.7ml/min, p>.05) – Table 3.
– Longitudinal hydration status among incident and prevalent patients’.
| T0 | T1 | T2 | p | |
|---|---|---|---|---|
| Incident | ||||
| Relative Hydration (%)a | 7.9 [4.1-10.4] | 1,3 [–2.9-10,3] | 3.5 [–4.0-7.2] | .52 |
| Hydration (L)a | 1,3 [0.8-1.7] | 0.2 [–0.6-1.5] | 0.6 [–0.7-0.9] | .16 |
| Systolic BP (mmHg) | 140,3±16.4 | 141,3±16,3 | 142.6±11.4 | .94 |
| Diastolic BP (mmHg) | 88.5±9.7 | 90.0±11.6 | 89.0±10.8 | .94 |
| GFR (ml/min/1.73 m2)a | 6,37 [3.0-8.5] | 5.74 [2.0–9.9] | 3.66 [1.9–6.2] | .97 |
| Diuresis (mL) | 1370±886 | 1075±663 | 1111±526 | .62 |
| Ultrafiltration (mL) | 647±450 | 851±639 | 1121±445 | .15 |
| Fluid Removal (mL)b | 1837±580 | 1828±802 | 2010±649 | .79 |
| Prevalent | ||||
| Relative Hydration (%)a | 7.8 [3.6-10.7] | 9.2 [5,3-14.0] | 10.2 [6.1-14.8] | .62 |
| Hydration (L)a | 1.0 [0.6-1.4] | 1.5 [0.6-2.6] | 1.4 [0.9-2,3] | .17 |
| Systolic BP (mmHg) | 129.1±18.2 | 137.1±18.5 | 139.0±24,3 | .31 |
| Diastolic BP (mmHg) | 81.2±12,3 | 82,3±12.8 | 78.4±12.4 | .63 |
| Diuresis (mL) | 1105±682 | 755±503 | 639±404 | .17 |
| Ultrafiltration (mL) | 937±466 | 832±467 | 1066±532 | .35 |
| Fluid Removal (mL)b | 1469±867 | 1186±695 | 1369±631 | .39 |
Longitudinal analysis of hydration status makers evaluated.
aResults presented as median and interquartile range [IQR 25-75].
bFluid Removal – Diuresis + ultrafiltration.
On the other hand, an inverse trend was found among prevalent patients, presenting an increase in the relative hydration status (7.8 vs 9.2 vs 10.2%, p>.05), regardless of maintaining stable total daily fluid removal (Table 2).
Anuric patients (n=11, 82% on APD) presented at baseline a similar hydration status when compared to those with urine output >250ml/day. Regardless of the significant difference on year 1 evaluation (p=.03), no differences were found on the 2nd year determination (p>.05) – Figure 1. Five of those patients (45%) performed a daily dwell with icodextrin at baseline, which increased to 7 patients (63%) two years later.
Similar hydration status was found between APD and CAPD patients in every evaluations (p>.05) – Figure 1.
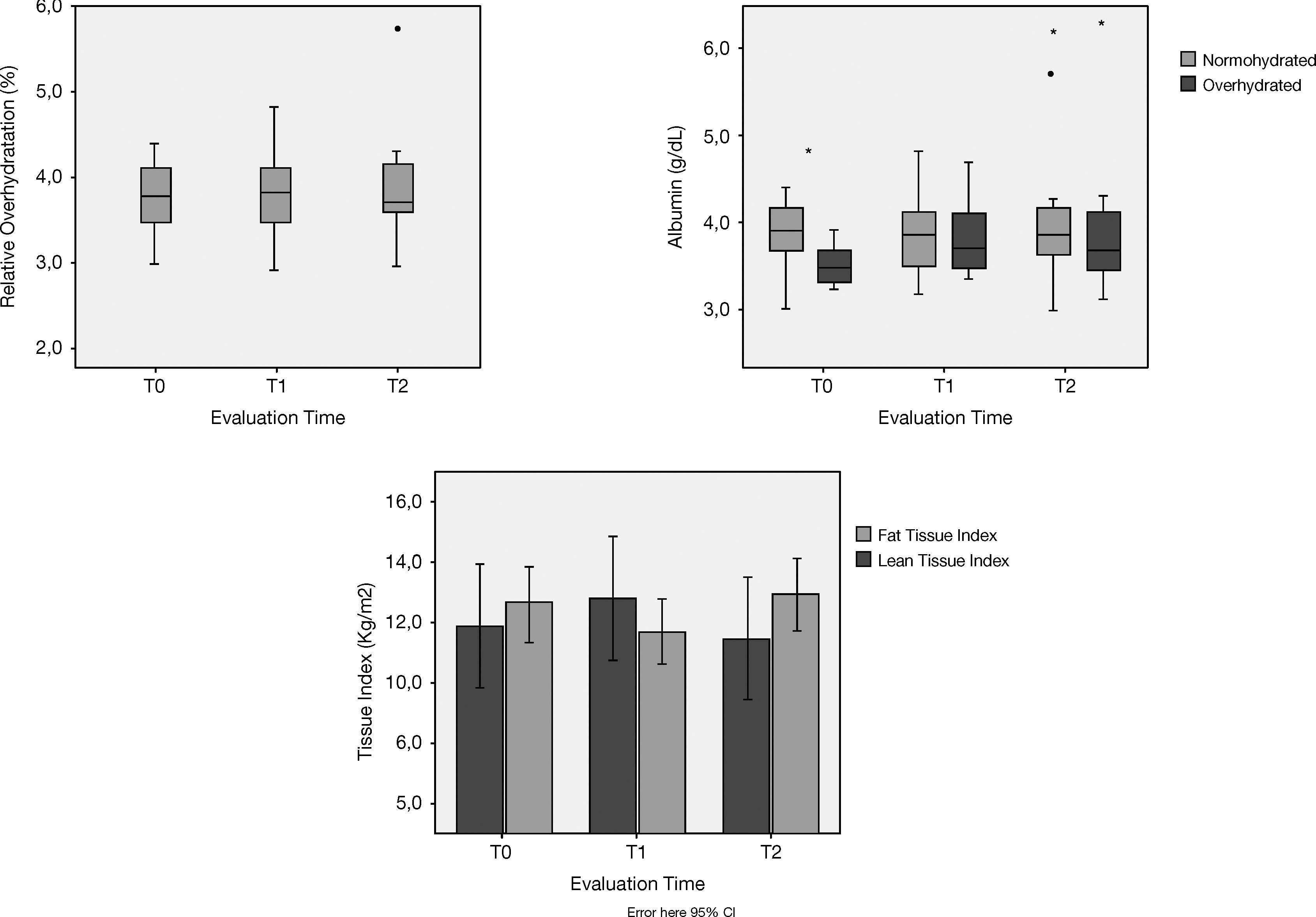
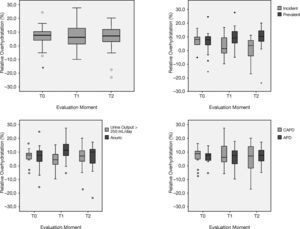
Regarding nutritional parameters, clinical (BMI), biochemical (normalized protein catabolic rate (nPCR) and albumin), and BCM (lean tissue mass, fat tissue mass, and ICW) remained stable throughout the evaluation period (Fig. 2). Albumin, a marker of both nutrition2 and hydration status,21 was negatively correlated to hydration status at baseline, but failed to achieve statistical significance (p>.05). No differences were found in the posterior evaluations as well (p>.05).
DiscussionHerein we present a longitudinal analysis on the hydration status of 30 PD patients, including prevalent and incident patients - at baseline only 6.7% patients were overhydrated and, more relevant, no significant changes were observed in the same population over a 2 year evaluation period.
It is generally held belief that volume status is difficult to maintain in peritoneal dialysis (PD), and that these patients are persistently overhydrated - although data to support this view are scarce. Determining euvolemia has hampered either on expensive, invasive, and unpractical techniques, such as gold-standard dextran determination, or under imprecise clinical, biochemical, and ultrasound determinations. Clinical tools such as changes in body weight and blood pressure are unreliable for small differences. Multifrequency bioimpedance analysis (BIA) provides an objective determination of hydration,1,14,22 and nutritional status.2,23,24
Previous studies have demonstrated that decreasing ultrafiltration volumes negatively influences outcomes in PD, independent of the levels of small solute clearance.13 In the presented study, no correlation was found between ultrafiltration nor total fluid removal and overhydration at baseline determination. Additionally, on the longitudinal analysis, although there was a reduction in the daily urine output and a trend towards reduction in glomerular filtration rate, total fluid removal maintained stable. Added to the lack of increase in overhydration among the overall population, it reinforces the belief that individualization of PD strategies, with different solutions, dwells, and PD techniques (CAPD vs APD), enables PD patients to remain normohydrated.
Regardless of these evidences, fear exist in the management of PD patients with daily urine output under 250ml. Also, these patients have shorter technique survival.13 Though phosphorus and small solutes removal were not analysed here, anuric patients were not more overhydrated over time, nor did the ones who lost residual renal function during the study period. Interestingly, one should observe the inverted tendency observed among both incident and prevalent: in incident patients rOH decreased dispite decreasing diuresis, while it increased in prevalent ones. A possible explanation may rely on the regular use of icodextrin in the long dwells (as in night dwells on CAPD patients) at our centre, but may also reflect a patient's better volume control and assimilation of the need to salt restriction. These results reinforce the need to continuously educate patients to maintain an adequate fluid balance.
Devolder et al.15 have previously demonstrated that hydration status was not worse among PD patients when compared to HD ones, though it was a transversal single centre analysis. The results presented here add yet evidence that PD is able to prevent overhydration after 2 years of follow-up, regardless of urine output, age, BMI, or diabetes. Inclusion of prevalent and incident (<6 months on PD) in the same analysis conferred a false positive PD vintage. Though incident patients were included, PD vintage of prevalent patients at baseline was 2.5 years, and still no long-term changes regarding hydration status were found.
Pro-brain natriuretic peptide (BNP) was once a promising marker of overhydration in dialysis patients and related to cardiovascular mortality.22,25,26 Though it failed to achieve statistical significance, we also found a positive correlation between BNP levels and hydration status. One of the main obstacles to BNP use in hydration status characterization is the lack of standardization and cut-off values. Still, it may be useful as a marker for volume variations in asymptomatic patients.
Dialysis is associated with a catabolic state, and nutritional changes occur in these patients.2,27,28 PD vintage has been associated with increasing fat mass27 and protein-energy wasting due to chronic inflammation of the peritoneal membrane, abdominal distension, gastroesophageal reflux, and inadequate dialysis dose.2
Female gender, age, and diabetes, well established risk factors in the general population, have been described as risk factors for increasing fat tissue mass in PD patients, as determined by BIA.15 These results suggest that the measurements done with multifrequency bioimpedance can be reliable.
A major finding from our study is the stable nutritional status observed during the 2 year evaluation period, regardless of the parameter evaluated (lean tissue index, fat tissue index, albumin, or nPCR). One may postulate this may be due to a short follow-up period (2 years), and a lower percentage of diabetics (10.5%) when compared to major survival studies.10,12 Nonetheless, an interquartile range PD vintage of 22.0-61.7months can barely be assumed as short.
To the authors’ belief, more than the overall nutritional status, the failure to find relevant changes over time reveals that an adequate PD program may prevent malnourishment. Some explanations seem plausible. First, and likely most relevant, patients are regularly evaluated from a nutritionist. Also, low-AGE's solutions, and focus on preservation of RRF are common practice at our centre.
There are some limitations to this study. The major is the small sample size, underrating the analysis between incident and prevalent patients. Also, BIA determinations were performed with a full abdomen, which interference with results were believed to be marginal at the study initiation. Finally, the study represents the current practices and results from a single centre, and caution must be taken in when making further generalizations.
Nevertheless, this is, to the authors, knowledge, the longer and larger longitudinal BIA analysis in a PD population, and the first to analyse incident and prevalent patients separately, highlighting that the results found are not likely to represent a positive selection bias.
In conclusion, this study demonstrates that thorough attention to hydration status and current prescription strategies are able to prevent relevant overhydration in PD patients - at time of the first evaluation only 6.7% were overhydrated - regardless of RRF and urine output. Driving volume overload in PD patients may be related to centre practice patterns, and may explain differences in the overhydration percentages among studies. Hopefully, the results of the EuroBCM19 and de ongoing multicentre IPOD study (NCT01285726) will either refute or corroborate the ones presented here.
FundingPedro Ventura Aguiar, by Abbott laboratory for conferences presentations.
Conflict of interestsThe authors declare that they have no conflict of interests.
The results of this study have been presented at the 49th ERA-EDTA congress, on the 26th of May 2012.