The Anemia Working Group of ERBP in 2010 recommended a target hemoglobin (Hb) level in the range of 11-12g/dL, without intentionally exceeding 13g/dL during the treatment with erythropoiesis stimulating agents (ESAs). This study evaluated if there was a clinical impact of this statement in the anemia management of chronic kidney disease (CKD) patients treated with ESAs not on dialysis in routine clinical practice in Spain.
MethodsThis was an observational and cross-sectional study carried out in CKD patients not on dialysis in Spain who initiated ESA treatment (naïve), or were shifted from a previous ESA to another ESAs (converted) since January 2011.
ResultsOf 441 patients evaluated, 67.6% were naïve and 32.4% were converted. At the study visit, 42.5% of naïve patients achieved the Hb target of 11-12g/dL, with a mean Hb of 11.3±1.3 g/dL (vs 10.1±0.9g/dL at the start of ESA therapy). Only 35.3% of converted patients maintained Hb levels within the recommended target at the study visit. Yet, 8.2% of naïve patients and 7.9% of those converted had Hb levels >13g/dL. Hb levels were similar across subgroups of patients, regardless of the presence of significant comorbidities.
ConclusionsAnemia management in CKD patients treated with ESAs by Spanish nephrologists seems to be aimed at preventing Hb levels <11g/dL, while <50% of patients were within the narrow recommended Hb target range. This, together with the lack of individualization in Hb targets according to patients’ comorbidities show that there is still room for improvement in renal anemia management in the clinical setting.
El grupo de trabajo europeo en anemia-ERBP recomendó en 2010 mantener los niveles de Hb entre 11-12g/dL sin exceder intencionadamente de 13g/dL durante el tratamiento con agentes estimuladores de la eritropoyesis (AEE). Este estudio evaluó si se produjo un impacto clínico de esta declaración en el tratamiento de la anemia en la enfermedad renal crónica (ERC) con AEE en la práctica clínica.
MetodologíaEstudio transversal, observacional y multicéntrico en pacientes con anemia secundaria a ERC y no sometidos a diálisis, que iniciaron tratamiento de la anemia (nuevos) o pasaron de unos AEE a otros (transición de AEE) a partir de enero de 2011.
ResultadosDe los 441 pacientes evaluados, el 67,6% eran nuevos y el 32,4% estaban en situación de transición. En la visita de estudio, el 42,5% de los pacientes nuevos habían alcanzado el rango de Hb de 11-12g/dL (niveles medios de 11,3 ± 1,3g/dL frente a 10,1 ± 0,9 g/dL al inicio del tratamiento con AEE), y el 35,3% de pacientes en situación de transición mantuvieron los niveles de Hb dentro del rango recomendado. A pesar de ello, el 8,2% de los pacientes nuevos y el 7,9% de aquellos en situación de transición tenían niveles de Hb > 13g/dL. Los niveles de Hb fueron similares, independientemente de la presencia o no de comorbilidades significativas.
ConclusionesEn las Unidades de Nefrología de España, el manejo de la anemia en pacientes con ERC no en diálisis en tratamiento con AEE parece dirigido a evitar niveles de Hb < 11 g/dL, aunque menos del 50% de los pacientes se encuentran dentro del estrecho rango recomendado. Ello, junto a la falta de individualización del objetivo de Hb en función de la presencia de comorbilidades, muestra que aún queda margen de mejora en el tratamiento de la anemia en la ERC con AEE en la práctica clínica.
Anemia is a common complication of chronic kidney disease (CKD), that is associated with a reduced quality of life (QoL)1 and is a risk factor for morbidity and mortality. Thus, erythropoiesis-stimulating agents (ESAs) are considered as a key therapy in the management of CKD-related anemia,2–4 considerably reducing the need of blood transfusions and improving the QoL of these patients, among other beneficial effects.
The use of ESAs aiming to normalize hemoglobin (Hb) levels (≥13g/dL), as opposed to the partial correction of anemia (9.0-11.0g/dL) has been associated with minor improvements in QoL (mainly fatigue),3 but with an increased risk of cardiovascular complications. In this regard, contrary to initial observational studies that suggested positive outcomes associated with higher achieved Hb levels,5,6 subsequent large multicentre randomized controlled trials (RCTs) and related meta-analyses7–10 have demonstrated an association between allocation to higher Hb levels (which is also associated with higher ESA doses) and an increased risk of cardiovascular complications without a benefit on mortality.
Treatment of anemia with ESAs in CKD patients has thus experienced a significant shift from using Hb levels as a surrogate end-point, to a more individualized ESA therapy that takes into consideration Hb targets, required ESA doses, and patients’ comorbidities, as well as a recent recommendation of administering the lowest possible ESA dose required to achieve the target Hb levels.11,12 After the publication of the Trial to Reduce Cardiovascular Events with Aranesp® Therapy (TREAT study),8 the Anemia Working Group of the European Renal Best Practice (ERBP) recommended to maintain Hb levels between 11-12g/dL, without intentionally exceeding 13g/dL.13 In this statement, caution was advised when considering Hb targets in diabetic patients, especially those with cardiovascular disease, and in patients with cancer; as well as to consider the dose of ESA required. The Anemia Group of the Spanish Society of Nephrology (S.E.N.) pointed out that ultimately Hb targets should be individualized, taking into account patients’ comorbidities, maximum doses of ESAs and Hb correction speed, as factors to redefine Hb targets.14 Finally, a recent position statement of the ERBP on the management of CKD-related anemia in Europe 12 called for special caution for patients with specific risk factors such as diabetes, cerebrovascular disease or cancer, in which it is advised to target to lower Hb levels (10g/dL) during ESA therapy.
In view of the current statements on anemia management in CKD patients treated with ESA, it is of paramount relevance to analyze the impact of the experts’ recommendations in routine clinical practice. The main objective of our study was to evaluate whether there has been a change in the perception and attitude of nephrologists in the anemia management with ESAs in anemic CKD patients not on dialysis since the recommendations of the Anemia Working Group of ERBP in 2010 were issued.13
Material and methodsStudy designThis was an observational, cross-sectional, multicentre study carried out in nephrology units of 30 Spanish hospitals. The participating centers voluntarily agreed to participate in the study and no previous selection was made. The study was conducted in accordance with the Guidelines for Ethical Review of Epidemiological Studies, Spanish Society of Epidemiology, the principles of the Declaration of Helsinki and its subsequent amendments. The protocol was approved by the Ethics Committee of the Hospital Clinic (Barcelona, Spain), and all patients gave their written informed consent to participate in the study.
Patient population and study proceduresInclusion criteria were patients older than 18 years with anemia secondary to CKD not on dialysis, who started ESA treatment for anemia (naïve patients), or patients who were under ESA treatment previously and were shifted to another ESA according to the investigator criterion (converted patients) after six months since the last recommendations of the Anemia Working Group of ERBP were issued 13 (January 2011). Patients who were in an ESA dose adjustment period or patients who had a functioning kidney transplant were excluded.
Information about the medical history and patients’ characteristics were collected from patients’ medical charts at the study visit. Hb levels, transferrin saturation index (TSAT) and ferritin levels were recorded, as well as other biochemical parameters (serum creatinine, C reactive protein (CRP), albumin and iron). Characteristics of ESA therapy, iron supplementation (route of administration, dose and dosing schedule), and data regarding adverse events, transfusions and the concomitant use of other medications were also collected. Estimated glomerular filtration rate (eGFR) was calculated according to the MDRD formula.15
At the study visit the distribution of ESA in naïve patients was: epoetin alfa (n=5, 1.7%), epoetin beta (n=5, 1.7%), darbepoetin alfa (n=38, 13.1%) and continuous erythropoietin receptor activator (C.E.R.A.) (n=242, 83.4%). Among patients that were treated previously with an ESA and shifted to another (converted patients) the distribution was: epoetin alfa (n=3, 2.1%), epoetin beta (n=6, 4.2%), darbepoetin alfa (n=28, 19.6%) and C.E.R.A. (n=106, 74.1%).
Statistical analysisAs the primary endpoint was the percentage of patients that, at the study visit, maintained Hb levels within the target range of 11-12g/dL (as recommended by the Anemia Working Group of the ERBP in 2010), the sample size was estimated assuming a standard deviation (SD) of Hb values of 0.5g/dL, in the range of SD reported in previous studies for patients achieving mean Hb levels within the target range. With a precision of 0.04 and a type I error rate of 0.05, we calculated that 632 patients would be required, allowing for a percentage of non-evaluable patients not exceeding 5%.
Data were summarized using descriptive statistics. Continuous variables were described using mean, median, standard deviation, and minimum and maximum values and were compared using a t-test or a non-parametric method (Mann-Whitney U test or Wilcoxon test), as appropriate. Absolute frequencies and valid percentages were calculated and compared using chi-squared test or Fisher's exact test, as appropriate. The Charlson comorbidity index (CCI) was calculated in all patients, and a Spearman's correlation was performed to assess the association of CCI with Hb levels, and ESA resistance index (ERI).
In addition to the previously described variables, comparisons between mean Hb levels achieved by subgroups of patients according to their comorbidities were performed: diabetes vs no diabetes, cardiovascular disease vs no cardiovascular disease, cerebrovascular disease vs no cerebrovascular disease, cancer vs no cancer.
Since a significant proportion of patients converted from an ESA to another where shifted from shorter-acting ESA to C.E.R.A. (Mircera®; F. Hoffmann-La Roche Ltd, Basel, Switzerland) in our study, and the scarce data available about this conversion in the clinical setting, especially among CKD patients not on dialyisis, dose conversion factors between the previous ESA and C.E.R.A. were estimated using a lineal regression model (without a constant value), with the dose of the prior ESA as the dependent variable and the dose of C.E.R.A. as the independent variable.
A level of 0.05 was considered to be statistically significant for all analyses. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA).
ResultsPatients and baseline characteristicsA total of 455 patients were enrolled in the study, of which 14 patients were excluded from the analyses due to the following reasons: lack of informed consent form (n=1); not meeting inclusion criteria (n=1); different ESAs at the start and at study visit (n=3); not naïve or converted from a previous ESA, and/or lack of information on either Hb, etiology or stage of CKD, or age (n=9). Thus, the evaluable population included 441 patients, of which 298 (67.6%) were naïve and 143 (32.4%) had been converted from other ESAs.
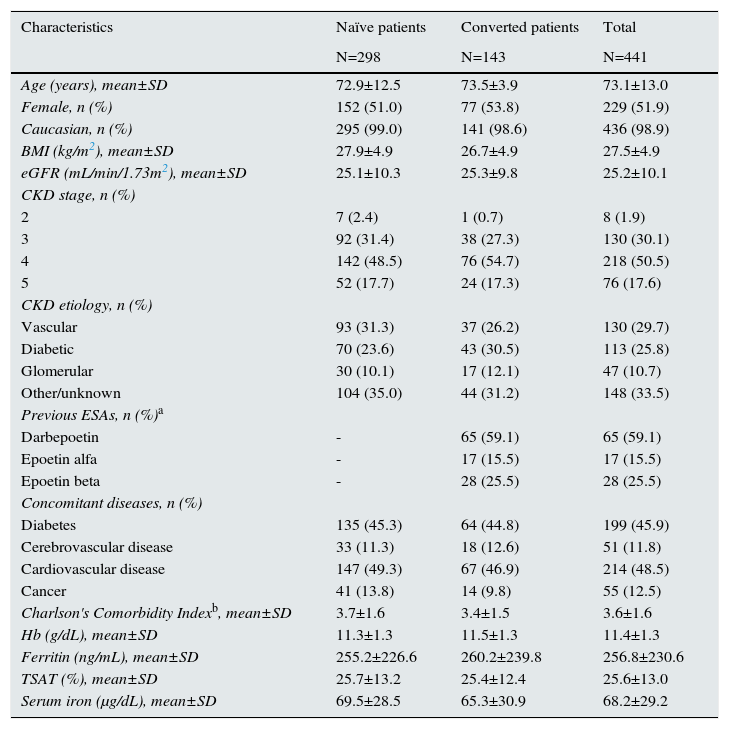
Overall, the mean age was 73.1±13.0 years, and 229 patients (51.9%) were women. Nearly 80% of patients were in CKD stages 3-4, and vascular disease (n=130, 29.7%) and diabetes (n=113, 25.8%) were the predominant etiologies. As for concomitant diseases, both diabetes and cardiovascular disease were reported in nearly 50% of patients. In addition, the mean Charlson comorbidity index revealed a relatively high morbidity in our population (3.6±1.6) (Table 1).
– Baseline characteristics of participating patients.
| Characteristics | Naïve patients | Converted patients | Total |
|---|---|---|---|
| N=298 | N=143 | N=441 | |
| Age (years), mean±SD | 72.9±12.5 | 73.5±3.9 | 73.1±13.0 |
| Female, n (%) | 152 (51.0) | 77 (53.8) | 229 (51.9) |
| Caucasian, n (%) | 295 (99.0) | 141 (98.6) | 436 (98.9) |
| BMI (kg/m2), mean±SD | 27.9±4.9 | 26.7±4.9 | 27.5±4.9 |
| eGFR (mL/min/1.73m2), mean±SD | 25.1±10.3 | 25.3±9.8 | 25.2±10.1 |
| CKD stage, n (%) | |||
| 2 | 7 (2.4) | 1 (0.7) | 8 (1.9) |
| 3 | 92 (31.4) | 38 (27.3) | 130 (30.1) |
| 4 | 142 (48.5) | 76 (54.7) | 218 (50.5) |
| 5 | 52 (17.7) | 24 (17.3) | 76 (17.6) |
| CKD etiology, n (%) | |||
| Vascular | 93 (31.3) | 37 (26.2) | 130 (29.7) |
| Diabetic | 70 (23.6) | 43 (30.5) | 113 (25.8) |
| Glomerular | 30 (10.1) | 17 (12.1) | 47 (10.7) |
| Other/unknown | 104 (35.0) | 44 (31.2) | 148 (33.5) |
| Previous ESAs, n (%)a | |||
| Darbepoetin | - | 65 (59.1) | 65 (59.1) |
| Epoetin alfa | - | 17 (15.5) | 17 (15.5) |
| Epoetin beta | - | 28 (25.5) | 28 (25.5) |
| Concomitant diseases, n (%) | |||
| Diabetes | 135 (45.3) | 64 (44.8) | 199 (45.9) |
| Cerebrovascular disease | 33 (11.3) | 18 (12.6) | 51 (11.8) |
| Cardiovascular disease | 147 (49.3) | 67 (46.9) | 214 (48.5) |
| Cancer | 41 (13.8) | 14 (9.8) | 55 (12.5) |
| Charlson's Comorbidity Indexb, mean±SD | 3.7±1.6 | 3.4±1.5 | 3.6±1.6 |
| Hb (g/dL), mean±SD | 11.3±1.3 | 11.5±1.3 | 11.4±1.3 |
| Ferritin (ng/mL), mean±SD | 255.2±226.6 | 260.2±239.8 | 256.8±230.6 |
| TSAT (%), mean±SD | 25.7±13.2 | 25.4±12.4 | 25.6±13.0 |
| Serum iron (μg/dL), mean±SD | 69.5±28.5 | 65.3±30.9 | 68.2±29.2 |
a p value not significant
b p < 0.05 (naïve vs converted patients)
Except for a minor difference in the mean body mass index (27.9±4.9kg/m2 in naïve vs 26.7±4.9kg/m2 in converted patients; p<0.05), baseline characteristics were not significantly different between both groups, with similar percentages of patients with diabetes, cerebrovascular disease, cardiovascular disease and cancer (Table 1).
There was a negative correlation between CCI and Hb levels (r=–0.185, p<0.001) when the overall population was evaluated. Similar results were obtained for naïve patients (r=–0.234, p<0.001), but not for converted patients. No significant association was found between the CCI and ERI either in the whole population (r=–0.003; p=0.949), or in patients’ subgroups [(naïve patients: r=–0.004; p=0.957; converted patients: (r=0.053; p=0.580)].
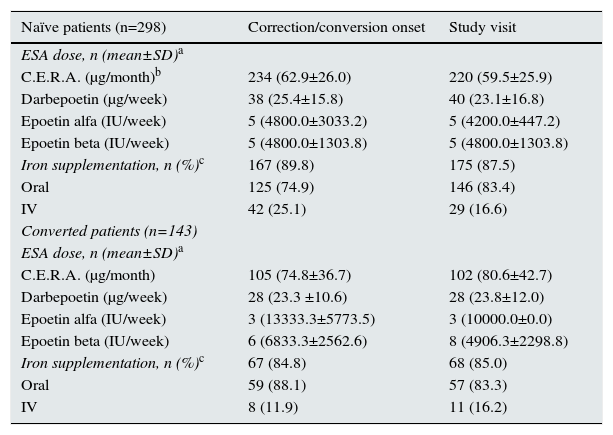
Treatment of anemiaAt correction/conversion onset, 242 (83.4%) naïve patients and 106 (74.1%) of those converted from other ESAs had started C.E.R.A., and the main reason for ESA change in converted patients was less frequent administration of treatment in 51.1% of cases, according to the study investigators. Other ESAs prescribed were: darbepoetin alfa (n=38, 13.1% and n=28, 19.6%, for naïve and converted patients, respectively), epoetin alfa (n=5, 1.7% and n=3, 2.1%, respectively) and epoetin beta (n=5, 1.7% and n=6, 4.2%, respectively).
Regarding iron supplementation, 167 (89.8%) of naive patients and 67 (84.8%) of converted patients received iron supplementation, mainly orally in 74.9% and 88.1% of patients, respectively (Table 2).
– Overall description of anemia treatments used.
| Naïve patients (n=298) | Correction/conversion onset | Study visit |
|---|---|---|
| ESA dose, n (mean±SD)a | ||
| C.E.R.A. (μg/month)b | 234 (62.9±26.0) | 220 (59.5±25.9) |
| Darbepoetin (μg/week) | 38 (25.4±15.8) | 40 (23.1±16.8) |
| Epoetin alfa (IU/week) | 5 (4800.0±3033.2) | 5 (4200.0±447.2) |
| Epoetin beta (IU/week) | 5 (4800.0±1303.8) | 5 (4800.0±1303.8) |
| Iron supplementation, n (%)c | 167 (89.8) | 175 (87.5) |
| Oral | 125 (74.9) | 146 (83.4) |
| IV | 42 (25.1) | 29 (16.6) |
| Converted patients (n=143) | ||
| ESA dose, n (mean±SD)a | ||
| C.E.R.A. (μg/month) | 105 (74.8±36.7) | 102 (80.6±42.7) |
| Darbepoetin (μg/week) | 28 (23.3 ±10.6) | 28 (23.8±12.0) |
| Epoetin alfa (IU/week) | 3 (13333.3±5773.5) | 3 (10000.0±0.0) |
| Epoetin beta (IU/week) | 6 (6833.3±2562.6) | 8 (4906.3±2298.8) |
| Iron supplementation, n (%)c | 67 (84.8) | 68 (85.0) |
| Oral | 59 (88.1) | 57 (83.3) |
| IV | 8 (11.9) | 11 (16.2) |
a not significant
b p< 0.05
c p < 0.01
At the study visit, after a mean of 6.8±3.1 months from the correction/conversion onset, 243 (82.9%) naïve patients and 103 (72.5%) patients on the maintenance phase remained on C.E.R.A. treatment. An overview of the ESA dose and iron therapy is provided in Table 2.
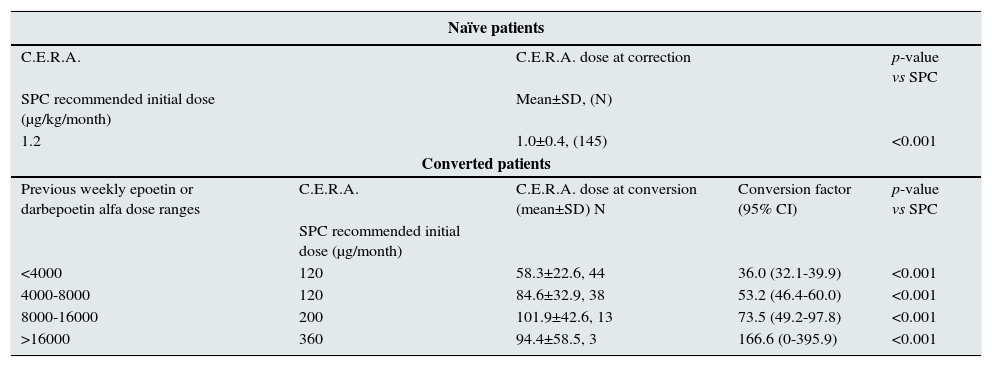
For naïve patients who started treatment with C.E.R.A., the initial mean weight adjusted dose was 1.0±0.4μg/kg/month, which was significantly lower than the dose recommended in the Summary of Product Characteristics (SPC) of 1.2μg/kg/month (p<0.001). In patients converted from other ESAs to C.E.R.A., the estimated dose conversion factor increased as the dose ranges of previous ESA were higher. In these patients, mean starting doses were lower than the recommended initial dose by SPC, based on the total weekly epoetin or darbepoetin alfa dose at the time of conversion. At the time of the study visit, C.E.R.A. doses were similar to the starting doses. Details about C.E.R.A. starting doses in naïve and converted patients are summarized in Table 3.
– C.E.R.A. initial dose at correction/conversion onset.
| Naïve patients | ||||
|---|---|---|---|---|
| C.E.R.A. | C.E.R.A. dose at correction | p-value vs SPC | ||
| SPC recommended initial dose (μg/kg/month) | Mean±SD, (N) | |||
| 1.2 | 1.0±0.4, (145) | <0.001 | ||
| Converted patients | ||||
| Previous weekly epoetin or darbepoetin alfa dose ranges | C.E.R.A. | C.E.R.A. dose at conversion (mean±SD) N | Conversion factor (95% CI) | p-value vs SPC |
| SPC recommended initial dose (μg/month) | ||||
| <4000 | 120 | 58.3±22.6, 44 | 36.0 (32.1-39.9) | <0.001 |
| 4000-8000 | 120 | 84.6±32.9, 38 | 53.2 (46.4-60.0) | <0.001 |
| 8000-16000 | 200 | 101.9±42.6, 13 | 73.5 (49.2-97.8) | <0.001 |
| >16000 | 360 | 94.4±58.5, 3 | 166.6 (0-395.9) | <0.001 |
Analysis of the change in Hb levels between correction/conversion onset and the study visit showed that mean Hb levels increased significantly in naïve patients from 10.1±0.9g/dL to 11.3±1.3g/dL (p<0.001), as expected. For converted patients, after the switch from another ESA, mean Hb levels remained stable (11.4±1.2g/dL vs 11.5±1.3g/dL at the study visit).
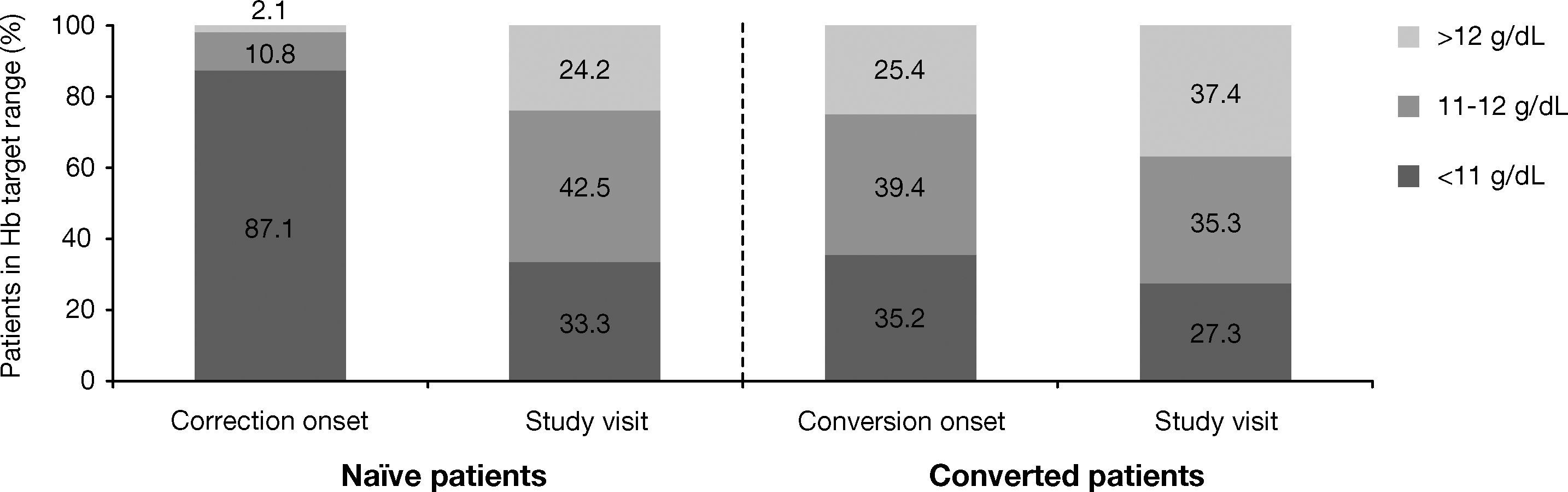
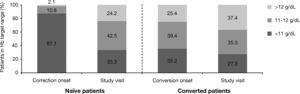
As shown in Figure 1, 12.9% of naïve patients started ESA with Hb levels >11g/dL. When Hb levels were analyzed by ranges (<11g/dL, 11-12g/dL, >12g/dL), the percentage of naïve patients who achieved Hb levels in the target range of 11-12 g/dL increased from 10.8% (n=32) at the start of the correction period to 42.5% (n=125) at the study visit. In converted patients, a similar percentage of patients maintained Hb within the target range after switching from another ESA [39.4% (n=56) vs 35.3% (n=49)] (Fig. 1). Nonetheless, at study visit 24.2% of naïve patients and 37.4% of those converted had Hb levels >12g/dL. Moreover, at correction/conversion onset the percentage of patients with Hb levels >13g/dL was 0.7% (n=2) for naïve and 7.0% (n=10) for converted. At study visit, these percentages increased, particularly in naïve patients, to 8.2% (n=24) and 7.9% (n=11), respectively.
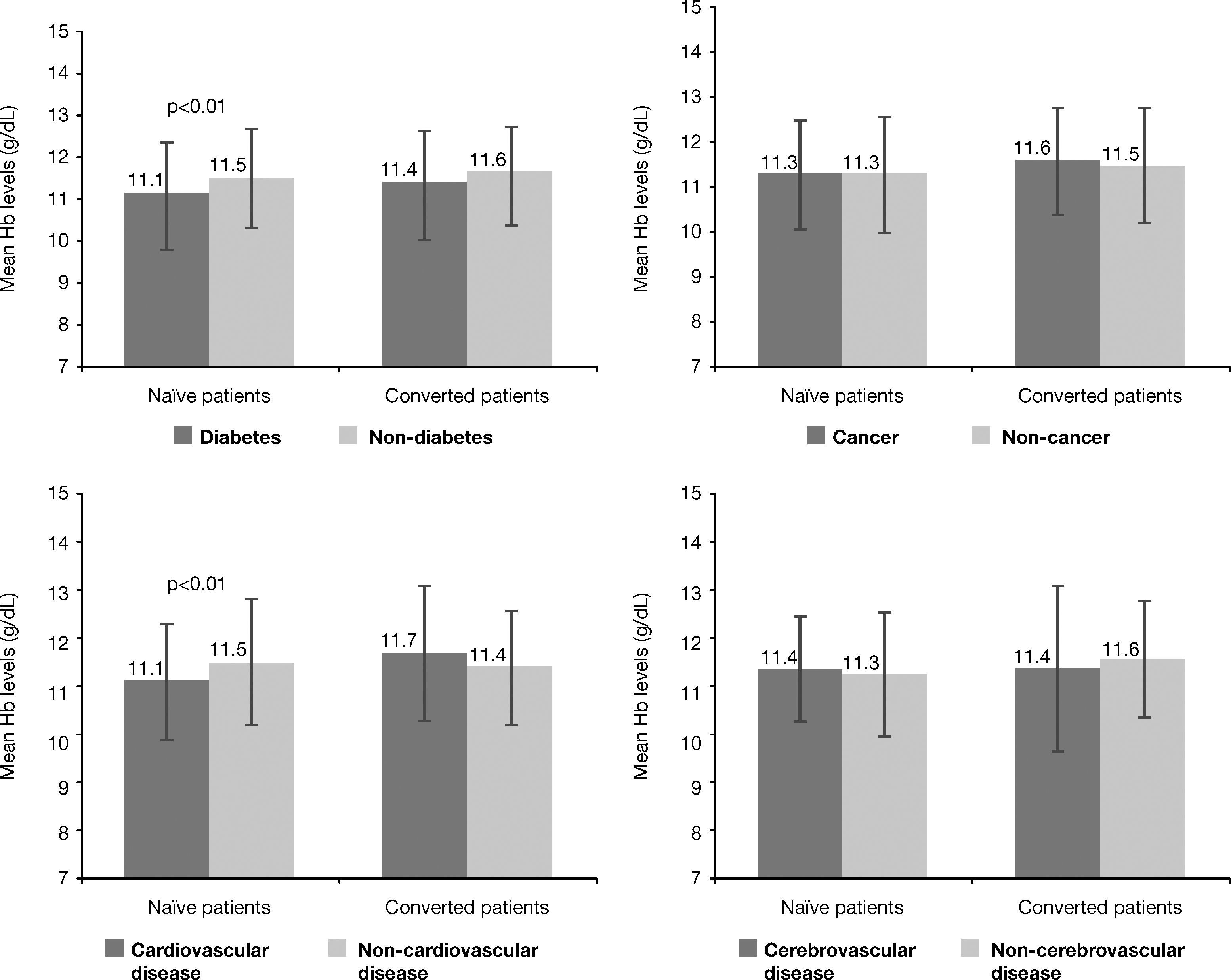
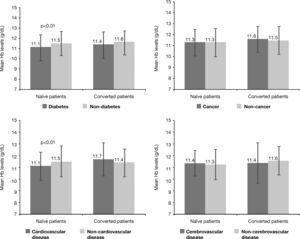
Analysis of Hb levels by patients’ subpopulations according to significant comorbiditiesAn evaluation of mean Hb levels according to the presence or absence of several co-morbidities revealed that naïve patients with diabetes had lower mean Hb levels than naïve patients without diabetes at both correction onset (10.0±1.0g/dL vs 10.2±0.8g/dL, p<0.005) and at the study visit (11.1±1.3g/dL vs 11.5±1.2g/dL, p<0.01). Likewise, naïve patients with cardiovascular disease had lower mean Hb levels than patients without cardiovascular disease at the study visit (11.1±1.2g/dL vs 11.5±1.3g/dL, p<0.01) (Figure 2), but no differences were observed in achieved Hb levels, as reflected by the mean and 95% confidence interval (CI), in naïve patients with cerebrovascular disease (95%CI; 10.6-11.4) vs non cerebrovascular disease (95% CI; 10.8-11.2)] or cancer [95% CI; 10.6-11.3) vs non cancer (95% CI; 10.8-11.1)]. No significant differences in Hb levels based on presence/absence of these comorbidities were observed in converted patients at any of the study times. Specifically, at the study visit the 95% confidence intervals (CI) for the mean of Hb levels shown in Figure 2 were as follows: [diabetes (10.7-11.3) vs non diabetes (10.8-11.2)]; cardiovascular disease (10.7-11.3) vs non cardiovascular disease (10.8-11.2); cerebrovascular disease (10.4-11.5) vs non cerebrovascular disease (10.8-11.2); cancer (10.4-11.6) vs non cancer (10.8-11.2)]. Among diabetic CKD patients with previous cerebrovascular disease, 47.6% were within the range of 11-12g/dL and 38.1% had Hb levels >12g/dL at the study visit.
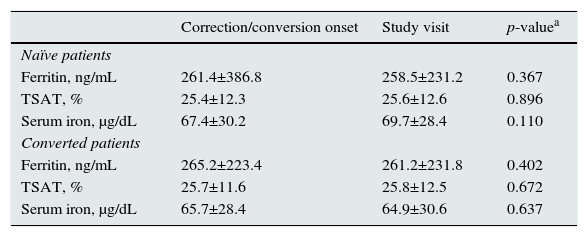
Iron statusMean ferritin and TSAT levels were above the lower recommended levels according to the guidelines. None of the iron-related parameters studied varied from correction/conversion onset until the study visit, and they were maintained within the recommended target ranges for both patients’ groups in both periods (Table 4).
– Levels of iron-related parameters by subgroups of patients.
| Correction/conversion onset | Study visit | p-valuea | |
|---|---|---|---|
| Naïve patients | |||
| Ferritin, ng/mL | 261.4±386.8 | 258.5±231.2 | 0.367 |
| TSAT, % | 25.4±12.3 | 25.6±12.6 | 0.896 |
| Serum iron, μg/dL | 67.4±30.2 | 69.7±28.4 | 0.110 |
| Converted patients | |||
| Ferritin, ng/mL | 265.2±223.4 | 261.2±231.8 | 0.402 |
| TSAT, % | 25.7±11.6 | 25.8±12.5 | 0.672 |
| Serum iron, μg/dL | 65.7±28.4 | 64.9±30.6 | 0.637 |
a significance for p < 0,05
During the study, a total of 8 adverse events (AEs) were reported in 2 naïve (0.7%) and 4 converted patients (2.8%). None of the reported adverse events required specific actions and in all cases patients recovered from the event. Two of the AEs were classified as serious: a hypercalcemic episode experienced by a converted patient, and a case of acute pulmonary edema-congestive heart failure secondary to hypertension, reported in a naïve patient 6 days after beginning treatment with C.E.R.A. This was the only AE considered by the investigators to be related to the study medication. During the correction period, a total of 6 (2.1%) naïve patients required blood transfusions.
DiscussionThe results of the ACERCA study provide an overview of the clinical practice management of anemia with ESA in CKD patients not on dialysis in Spain following the recommendations of the Anemia Working Group of the ERBP in 2010. In recent years, anemia guidelines have been changing according to the new evidences from the results of randomized controlled trials, Accordingly, Hb targets during ESA therapy have been moving towards more conservative goals and to its individualization, according to the patients’ characteristics.
Our results show that less than 50% of naïve or converted patients maintained Hb levels within the range of 11-12g/dL and reflect the difficulties associated with achieving and maintaining such a narrow target range in routine clinical practice also in CKD patients not on dialysis.16–18 This aspect has also been confirmed in a recent study which evaluated the percentage of patients who achieved the 2007 European Medicine Agency (EMA) target range (10-12g/dL), showing that merely one in two patients had an Hb level within this recommended Hb target.19
Around one third of patients, both naïve and converted, had Hb levels below 11g/dL at the study visit despite being stable on ESA therapy. On the other hand, among naïve patients, 12.8% started ESA treatment with Hb levels above 11g/dL, while around one forth of patients had Hb levels >12g/dL, and a small but significant proportion of the overall population (n=35, 8.1%) had Hb >13g/dL at the study visit. These results call for the need to improve anemia management both increasing the percentage of patients within the target range and avoiding high Hb levels and the potential risks associated. The impact of targeting for higher Hb levels, particularly among CKD patients not on dialysis, has been recently evaluated in a systematic review of 24 RCTs including over 10,000 patients.20 CKD patients treated with ESAs allocated to the high Hb group (13g/dL) had an increased risk of hypertension, hospitalization and stroke, as compared with patients allocated to the low Hb target (10g/dL). In addition, a significantly higher risk of mortality (RR 1.18; 95% CI 1.02-1.37) was also observed in the high Hb group. Although the decision of when to start ESA therapy and Hb targets during this treatment in CKD patients remain controversial issues and are continuously revised,12,21 the results of randomized controlled trials and the position statement of the ERBP of 2010 at the time of the study provided enough information for nephrologists to reevaluate, and change accordingly, practice patterns in anemia management in these patients. In this regard, our results indicate that ESA treatment in the clinical setting in Spanish nephrology units seems to be mainly aimed at preventing Hb levels to fall below 11g/dL.
The ACERCA study also evaluated whether Hb targets under ESA therapy in CKD patients were individualized, considering the presence of some frequent comorbidities, such as diabetes, cardiovascular or cerebrovascular disease, or cancer, as prompted by the ERBP statement. It was thus surprising that in our study, mean Hb levels were similar (or with clinically minimal differences) in CKD patients not on dialysis, independent of the presence or absence of significant co-morbidities, suggesting that there might still be some reluctance to individualize Hb targets in patients with significant co-morbidities in the clinical practice setting. Otherwise, we cannot rule out that our study did not capture a delayed or a slow but progressive evolution in the perception and attitude of nephrologists towards anemia management, since the study was performed only 6 months after the appearance of the position statement of the ERBP of 2010 and before the publication of the KDIGO recommendations.21
Another relevant result of our study was the finding that in naïve patients, target Hb levels were achieved requiring a monthly dose of C.E.R.A. (1.0μg/kg), which is lower than the recommended in the Summary of Product Characteristics (SPC) (1.2μg/kg). Similarly, in patients converted from other ESA, the switch to C.E.R.A. required lower doses than those recommended in the SPC to maintain Hb levels. Indeed, our data suggest that the C.E.R.A. doses required to achieve or maintain target Hb levels in CKD patients not on dialysis in Spain in clinical practice are relatively low, which is in agreement with two recent cost-minimization analyses conducted in Spanish hospitals22,23 where C.E.R.A. doses required to achieve target Hb levels in non-dialysis CKD patients were also found to be lower than those recommended by the SPC. Relatively low doses of C.E.R.A. were also required in non-dialysis CKD patients shifted from darbepoetin alfa in an Italian study,24 and similar results of a beneficial dose conversion for C.E.R.A. has been also found in the MICENAS II study in CKD patients nor on dialysis in Spain.25 These findings of a dose-saving effect observed with the use of long-acting ESA, such as C.E.R.A., may have not only economic, but also clinical implications.2,22 In this regard, it must be emphasized that current guidelines indicate that the patient should be prescribed the lowest possible ESA dose to achieve the target Hb.11,26 This is especially relevant in ESA-resistant patients since a recent consensus pointed out that poorly ESA-responsive patients is one of the main challenges in anemia management in CKD.27 These patients, who usually receive higher ESA doses, have a poorer prognosis.11,17,28 Thus, the use of long-acting ESA agents, such as C.E.R.A., is an attractive alternative that may improve safety and cost-effectiveness in this specific subset of patients.
Mean ferritin and TSAT levels were above the minimum targets set by guidelines. In our study, 89.8% of naïve patients and 84.8% of converted patients received iron supplementation. In most patients the oral, as opposed to the intravenous route, was by far the preferred route of iron administration, despite that some studies have shown a higher efficacy of intravenous iron as compared to oral iron in these patients,29–31 the well-established poor gastrointestinal tolerance to oral iron, as well as the reduced intestinal iron absorption in CKD patients,32 that could constrain the efficacy of oral iron supplements and limit the repletion of iron stores. On the other hand, the lack of studies evaluating the long-term safety of intravenous iron, the need to preserve the venous tree for a future vascular accesses, the risk of enhanced oxidative stress, the potential risks of accelerated atherosclerosis, or risk of infections associated with IV iron, together with the need to be administered in the hospital setting, may be barriers to a more liberal use of intravenous iron. The achievement of target levels of ferritin and TSAT might have contributed to the relatively low ESA doses required in our study by allowing an optimization of erythropoiesis, since patients with low TSAT and ferritin levels require higher ESA doses to achieve target Hb levels.22,33
The present study has several limitations including the potential biases inherent to all observational studies. Additionally, our study did not reached the expected sample size, which might have underpowered the statistical analyses conducted. Furthermore, Hb targets have been more recently reevaluated in several guidelines, thus these results may not reflect the current anemia treatment in CKD, but it alerts against a therapeutic inertia, making the results interesting. Finally, in our study the main ESA prescribed was C.E.R.A, both in naïve and in patients that were shifted from another ESA. Although the Nephrology Units involved were selected solely for their interest in participating in the study, and not for the type of ESA prescribed, we cannot extrapolate that the rate of prescription of C.E.R.A in CKD patients not on dialysis at the time of the study in Nephrology Units in Spain was similar
In conclusion, the results of the ACERCA study indicate that anemia management in Spanish nephrology units is mainly aimed at preventing Hb levels below 11g/dL in the clinical setting. The fact that less than 50% of patients had Hb levels within the recommended target range of 11-12g/dL, confirms the difficulty to achieve and maintain such a narrow target range also in non-dialysis CKD patients treated with ESA, as recommended by clinical practice guidelines in the real setting, as well as the reluctance to individualize Hb targets according to patients’ comorbidities. In any case, our study shows that there is still room for improving anemia management in ESA-treated CKD patients not on dialysis, particularly regarding patients with a higher risk to develop ESA-related complications.
Appendix. Investigators of the ACERCA Study GroupIldefonso Varela (Hospital Clínico Virgen de la Victoria, Málaga, Spain); Fernando Vallejo (Hospital Puerto Real, Cádiz, Spain); M Sol García de Vinuesa (Hospital Gregorio Marañón, Madrid, Spain); Carmen Bernis (Hospital La Princesa, Madrid, Spain); Manuel Arias (Hospital Marqués de Valdecilla, Santander, Spain); Antonio Pelegrí (Hospital Universitari Sagrat Cor, Barcelona, Spain); Carmen Vázquez (Complexo Hospitalario Univesitario de Santiago, Santiago de Compostela, Spain); Marisila Molina (Hospital Son Llatzer, Mallorca, Spain); Alba Herreros (Fundació Puigvert, Barcelona); Alfonso Otero (Complexo Hospitalario Universitario de Ourense, Ourense, Spain); M Luisa Méndez (Hospital Universitario Virgen de la Candelaria, Santa Cruz de Tenerife, Spain); José A Herrero (Hospital Clínico San Carlos, Madrid, Spain); Javier Usón (Hospital General Virgen de la Luz, Cuenca, Spain); Joan Fort (Hospital Vall d¿Hebrón, Barcelona, Spain); Xavier Fulladosa (Hospital de Bellvitge, Barcelona, Spain); Enrique Morales (Hospital Universitario 12 de Octubre, Madrid, Spain); Julio Hernández (Hospital La Fe, Valencia, Spain); José R Pons (Hospital General de Castellón, Castellón, Spain); Eduardo Hernández (Hospital Universitario 12 de Octubre, Madrid, Spain); José L Górriz (Hospital Univesitario Dr. Peset; Valencia, Spain).
Conflicts of interestThe ACERCA study was sponsored by the Spanish Society of Nephrology (S.E.N.) with an unrestricted grant from Roche Pharma Spain. AMC declare that has received research support from Amgen, Abbott, Boehringer-Ingelheim and Roche, as well as honoraria for participating in advisory boards from Abbvie, Amgen, Boehringer-Ingelheim, Esteve, Janssen-Cilag, Novartis and Roche. AC has received research support from Amgen and Roche. He has received honoraria for participating in advisory boards from Roche, Amgen, Pfizer, Novartis, Abbott, Astra-Zeneca and received speaker fees from Roche, Amgen, Johnson and Johnson, Bristol Myers Squibb, Novartis, Pfizer, Almirall, Esteve, Astra-Zeneca, Siemens. Others authors declare that they have no competing interests.
The authors would like to acknowledge the Spanish Society of Nephrology (S.E.N.) for its sponsorship and Roche Pharma Spain for the financial support, as well as the rest of investigators of the ACERCA Study Group participating in the study. We would also thank the invaluable help of Beatriz Cuellar.
Medical writing support was provided by Isabel Caballero from Dynamic S.L., during the preparation of this paper. Responsibility for opinions, conclusions and interpretation of data lies with the authors.