Chronic kidney disease (CKD) is a risk factor for the development of acute kidney injury (AKI). Recent studies have revealed numerous biomarkers eligible for AKI prediction. However, the expression and performance of AKI biomarkers in acute injury superimposed on preexisting CKD (AonC) remain elusive. The aim of this study was to evaluate whether biomarkers which robustly expressed in acute kidney injury could predict acute injury based on CKD.
Materials and methodsMice were classified into cohorts: AKI, CKD, AonC and sham. The AonC model mice were subjected to renal bilateral ischemia/reperfusion (I/R) injury fourteen days after intraperitoneally administrated with 20mg/kg aristolochic acid. Severity of acute ischemic injury was stratified by clamping the dissected bilateral renal arteries with non-traumatic microvascular clips for 20 or 35min. The AKI mice were induced with renal bilateral I/R injury and CKD mice were crafted with 20mg/kg aristolochic acid administrated intraperitoneally. Histology, genetic and protein expression of biomarkers were measured in three cohorts.
ResultsWe found that serum creatinine dramatically increased in severe (sAonC) but not in moderate (mAonC) injury mice. Upregulation of Kidney injury molecule-1 (KIM-1) mRNA, tissue inhibitor of metalloproteinase-2 (TIMP-2), Syndecan-1 (SDC-1) mRNA and insulin-like growth factor binding protein-7 (IGFBP7) protein indicated the onset of mAonC. An increase in neutrophil gelatinase-associated lipocalin (NGAL), rhomboid-like protein 2 (RHBDL2), Syndecan-1 (SDC-1) mRNA and protein, and a decrease in IGFBP7 protein were associated with sAonC.
ConclusionsOur study revealed the variational expression of AKI biomarkers in AonC kidneys, and uncovered IGFBP7 protein can be used as a sensitive biomarker to predict and differentiate AonC severity. The performance of RHBDL2 and SDC-1 in predicting severe AonC was promising, providing new biomarkers for predicting AonC.
La enfermedad renal crónica (ERC) es un factor de riesgo para el desarrollo de una lesión renal aguda (LRA). Estudios recientes han revelado numerosos biomarcadores para la predicción de LRA. No obstante, la expresión y el rendimiento de los biomarcadores de LRA en lesiones agudas superpuestas a una ERC preexistente (AonC, en inglés) siguen siendo imprecisos. El objetivo de este estudio fue evaluar si los biomarcadores que se encuentran muy expresados en la lesión renal aguda podrían predecir una lesión aguda superpuesta a una ERC.
Materiales y métodosSe dividieron ratones en cohortes (LRA, ERC, AonC y grupo de referencia). A los ratones del modelo de AonC se les indujo una lesión renal bilateral por isquemia/reperfusión (I/R) 14días después de la administración intraperitoneal de 20mg/kg de ácido artistolóquico. La gravedad de la lesión isquémica aguda se estratificó pinzando las arterias renales bilaterales diseccionadas con horquillas microvasculares no traumáticas durante 20 o 35min. A los ratones de LRA se les indujo una lesión renal bilateral por I/R y a los ratones de ERC se les administraron 20mg/kg de ácido artistolóquico por vía intraperitoneal. Se determinaron la histología, la genética y la expresión de proteínas en las tres cohortes.
ResultadosObservamos que la creatinina sérica aumentaba drásticamente en los ratones con lesiones graves (AonCg) pero no en aquellos con lesiones moderadas (AonCm) El aumento del ARNm de la molécula1 de lesión renal (KIM-1), del inhibidor tisular de metaloproteinasas2 (TIMP-2), del ARNm de sindecán1 (SDC-1) y de la proteína de unión al factor de crecimiento insulinoide7 (IGFBP7) fue indicativo de aparición de AonCm. El incremento de la lipocalina asociada a la gelatinasa de neutrófilos (NGAL), la proteína romboidal2 (RHBDL2) y el ARNm y la proteína de sindecán1 (SDC-1), así como la reducción de la proteína IGFBP7, se asociaron a una AonCg.
ConclusionesNuestro estudio mostró la variación de la expresión de los biomarcadores de LRA en riñones con AonC y descubrió que la proteína IGFBP7 puede emplearse como biomarcador sensible para la predicción y la diferenciación de la gravedad de la AonC. El rendimiento de RHBDL2 y SDC-1 en la predicción de AonC graves resultó prometedor, lo que ofrece nuevos biomarcadores para la predicción de AonC.
Kidney dysfunction can be classified into two types: acute kidney injury (AKI) and chronic kidney disease (CKD). Due to the increasing prevalence of hypertension and diabetes, the global prevalence of both sorts of kidney dysfunction rises unceasingly.1 Moreover, studies have shown causal interrelationship between AKI and CKD. CKD is reported in approximately 30% of AKI patients and AKI without remission transitions to CKD in turn.2,3 Since both kidney diseases are associated with high cardiovascular events and mortality, early diagnosis has received considerable attention.
The current definition of AKI is based upon serum creatine and urine output.4 Serum creatine is influenced by muscle mass, fluid overload and certain medications.5 Moreover, it takes several hours to days for these indicators to manifest adequately for reaching the diagnosis criteria, while the kidney is suffering from injury constantly.6 Lately, biomarkers were discovered for predicting AKI at an earlier stage effectively.5–10 Despite the development of biomarkers in predicting AKI, there has been little discussion about the early diagnosis of AKI superimposed on CKD (AonC). Furthermore, previous studies failed to specify whether preexisting kidney disease confounds the predictive role of biomarkers in AonC.
There are two primary aims in this study: (1) to investigate the difference of biomarkers among isolated AKI/CKD and AonC; (2) to identify eligible biomarkers for predicting different severity AonC.
Materials and methodsAnimalsAll animal experiments were performed in strict accordance with the animal use protocol approved by the Institutional Animal Care and Use Committee of Fudan University and complied with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Eight-weeks-old male C57BL/6 mice were housed under standard conditions (specific pathogen-free) and kept in an environment with constant temperature and humidity under a 12-h phase light–dark cycle. Drinking water (ozone-treated and acidified) and food were freely available. The mice were randomly assigned to six groups: (1) sham (n=3), (2) CKD (n=4), (3) moderate AonC (I/R 20mins) (n=4), (4) severe AonC (I/R 35mins) (n=4), (5) moderate AKI (I/R 20mins) (n=3), (6) severe AKI (I/R 35mins) (n=3). All CKD model mice were intraperitoneally injected with 20mg/kg aristolochic acid (Sigma, A9451). After 14-days, AonC groups were subjected to renal bilateral I/R injury, moderate or severe ischemia was induced by clamping the dissected bilateral renal arteries with non-traumatic microvascular clips for 20 or 35minutes. During the surgical period, body temperature was maintained between 35°C and 37.5°C using a temperature-controlled heating system. Pre-warmed saline (1ml; 37°C) was instilled intraperitoneally as volume supplement before the abdomen was closed in two layers. Every effort was made to minimize animal suffering. Animals were sacrificed by an overdose of isoflurane or by exsanguination. Mice were anaesthetized at the indicated times after ischemia occurred and were sacrificed at 24h (n=4). The kidneys were quickly removed and processed for histological evaluation, protein extraction or RNA extraction. Sham operation groups were set at 24h (n=3).
Serum creatinine measurementA blood sample from each animal was extracted from mice eyeball after the mice were anaesthetized. Serum blood creatinine levels were determined with a QuantiChrom™ Creatinine Assay Kit following the manufacturer's instructions.
Histologic analysisKidneys were fixed in 10% neutral formalin. Hemisected kidneys were embedded in paraffin wax and cut into 2-μm-thick sections for Periodic acid-Schiff (PAS) staining. Masson's trichrome stains were used to assess collagen.
RNA isolation and qPCRRNA was isolated using Trizol agents (Sigma, T9424). 0.5 ug RNA was reverse transcribed using a cDNA archival kit (Vazyme, R323-01), and quantitative real-time PCR was conducted in the QuantStudio5 (Life Technology) machine using SYBR qPCR Master Mix (Vazyme, Q511-02). The data were normalized and analyzed using the delta/delta CT method. Primers used are listed in Table 1.
Primer list.
| Name | Sequence 5′–3′ |
|---|---|
| Kim-1 | FOR: AGGCGCTGTGGATTCTTATG |
| REV: AAGCAGAAGATGGGCATTGC | |
| NGAL | TGGCCCTGAGTGTCATGTG |
| CTCTTGTAGCTCATAGATGGTGC | |
| IGFBP7 | TAACCTGCGAATCCATGAGC |
| AGAGAAGTGTGTCAGGCAAGAG | |
| TIMP-2 | ATTGCAGGAAAGGCAGAAGG |
| AGTGATCTTGCACTCACAGC | |
| RHBDL2 | ATGGCTGTTGCTCACGAGATG |
| GCTCCTGGGGAAGTCTTTACC | |
| SDC-1 | AACGGGCCTCAACAGTCAG |
| CCGTGCGGATGAGATGTGA | |
| ACTIN | GGCTGTATTCCCCTCCATCG |
| CCAGTTGGTAACAATGCCATGT |
KIM-1: Kidney injury molecule-1; NGAL: neutrophil gelatinase-associated lipocalin; IGFBP7: insulin-like growth factor binding protein-7; TIMP-2: tissue inhibitor of metalloproteinase-2; RHBDL2: Rhomboid-like protein 2; SDC-1: Syndecan-1.
Renal tissue was lysed and denatured at 70°C for 10min in a SDS buffer (Tanon, Biofuraw™) and separated by 10% PAGE gels. The proteins were transferred on to a PVDF membrane, blocked for 1h with 5% (w/v) dried non-fat skimmed milk powder in TBST (TBS containing 0.1% Tween 20) and probed with the indicated antibodies (Kim-1, NGAL, IGFBP7, TIMP-2, RHBDL2 and SDC-1) overnight at 4°C. Horseradish peroxidase-conjugated secondary antibodies were applied, and ECL (Tanon, 180-5001B) was conducted to detect proteins. An ACTIN-specific antibody (GTX, 109639) was used for loading controls on stripped membranes. The gray values were measured using ImageJ software (NIH) and normalized to ACTIN.
Statistical analysesThe two-tailed, unpaired t-tests were used to analyze differences between two groups, and ANOVA was used to analyze intergroup differences. p<0.05 was considered significant. The analysis was performed using GraphPad Prism 7 (GraphPad software, Inc., San Diego, CA, USA).
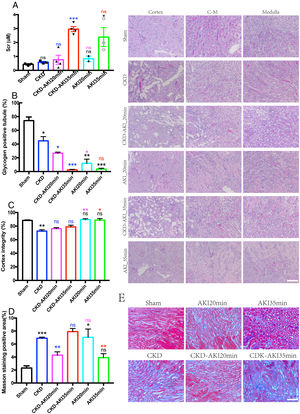
ResultsSerum creatine and histological characteristics of AonC miceTo investigate changes of AKI biomarkers in AonC setting, we used both ischemic AKI and nephrotoxic CKD models induced by ischemia–reperfusion (IR) and aristolochic acid (AA), which represent distinct etiologies leading to AKI and CKD in the clinical setting. We constructed two mouse models of I/R AKI by changing the time of renal ischemia from 20 to 35min to investigate the effects of moderate and severe renal injury. During reperfusion, changes in renal function and morphological characteristics were monitored over time (Fig. 1A and B). Both mice, AonC and AKI, with severe renal injury (I/R 35min) showed a more significant increase in serum blood creatinine than those with moderate injury (I/R 20min; Fig. 1A).
Serum creatinine and renal histopathological changes after acute kidney injury based on CKD. A: Serum creatinine levels can reflect acute kidney injury in both normal and CKD kidneys. Serum creatinine dramatically increased in severe acute kidney injured CKD (sAonC) mice, but not in moderate acute kidney injured CKD (mAonC) mice. B–D: CKD kidneys exhibited more severe morphological injury in the cortex region, but with fewer injuries in corticomedullary junction. Acute injury caused the severe injury in corticomedullary junction in both AKI and AonC kidneys Scale bar=100μm. E: Masson's trichrome staining was performed to visualize fibrosis in kidneys. Scale bar=100μm. F: Quantification of Masson's trichrome stained fibrosis in kidneys. The data are shown as the mean±SEM, *, p<0.05; **, p<0.01; ***, p<0.001; n=3–4. * Color means compared with the corresponding column.
CKD kidneys exhibited more severe morphological injury, particularly in the cortex region, characterized by loss of brush border and tubular cell death, whereas with normal morphology in the cortico-medullary junction (Fig. 1B, D). The injury in AonC cortex was significantly more severe than AKI kidneys (Fig. 1B, D). I/R induced severe acute injury in the cortico-medullary junction in both AonC and AKI mice (Fig. 1B, C). The injury in moderate AonC cortico-medullary junction was more severe than CKD, but less than moderate AKI, whereas no significance was found between severe AonC and severe AKI cortico-medullary junction (Fig. 1B, C). In contrast to CKD, AKI mice displayed much more normal morphology in the cortex region (Fig. 1B, D). Meanwhile, CKD mice kidneys displayed considerably more Masson staining, whereas staining was dramatically decreased in kidneys of mAonC mice, as illustrated in Fig. 1(E, F).
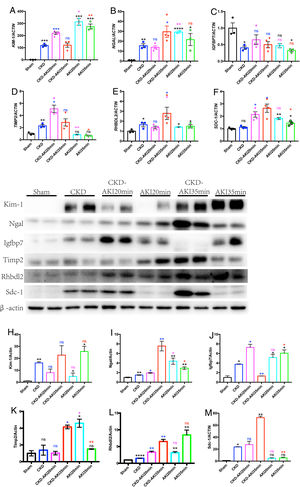
Various expression of classical AKI biomarkers in AonC kidneysTo investigate the performance of existing AKI biomarkers for predicting acute injury on CKD, we evaluated the expression of Kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein-7 (IGFBP7) through mRNA and protein analysis. We found that the mRNA level of KIM-1 dramatically increased in mAonC mice kidneys, but no difference was found between sAonC and CKD kidneys (Fig. 2A). In AKI kidneys, the mRNA level of KIM-1 robustly elevated in both sAKI and mAKI kidneys (Fig. 2A). As in protein levels, there was no difference among CKD, mAonC and sAonC kidneys, but all higher than Sham kidneys (Fig. 2G, H). These results suggested that moderate acute injury might occur when the KIM-1 mRNA level elevated in CKD kidney.
Variation of AKI biomarkers’ expression in AonC and AKI kidneys. A–F: mRNA levels of KIM-1, NGAL, IGFBP7, TIMP-2, RHBDL2 and SDC-1 in AonC and CKD kidneys. G: protein levels of KIM-1, NGAL, IGFBP7, TIMP-2, RHBDL2 and SDC-1 in AonC and CKD kidneys. H–M: Quantification of KIM-1, NGAL, IGFBP7, TIMP-2, RHBDL2 and SDC-1 protein expression in AonC and CKD kidneys. The data are shown as the mean±SEM, *, p<0.05; **, p<0.01; ***; n=3–4. * Color means compared with the corresponding column.
Both NGAL mRNA and protein were dramatically elevated in sAonC mice kidneys when compared with CKD kidneys (Fig. 2B, G, I). In contrast, there is no difference between mAonC and CKD kidneys in the mRNA level (Fig. 2B). Thus, a severe acute injury might occur if both NGAL mRNA and protein levels increased in CKD kidneys.
The combination of urinary TIMP-2 and IGFBP7 has been shown to be an excellent predictor of AKI.11 In this study, IGFBP7 mRNA dramatically dropped in CKD kidneys, while TIMP-2 mRNA significantly increased in CKD kidneys when compared with Sham controls (Fig. 2C, D). There is no difference among CKD, mAonC and sAonC kidneys in IGFBP7 mRNA levels. Interestingly, the magnitude of TIMP-2 mRNA in mAonC kidney was higher than that in CKD and sAonC kidneys (Fig. 2D). Compared with the Sham kidneys, the IGFBP7 protein in CKD kidneys increased, and that increased further in the mAonC kidneys but significantly decreased in sAonC kidneys (Fig. 2G, J). In contrast, TIMP-2 protein in CKD kidneys has no difference compared with Sham kidneys (Fig. 2G, K). Moderate AonC doesn’t influence TIMP-2 protein level, while sAonC dramatically increased TIMP-2 protein (Fig. 2G, K). TIMP-2 protein in mAKI kidneys was significantly increased, whereas with no change in sAKI kidneys (Fig. 2G, K). Those results indicated that a higher TIMP-2 mRNA level in CKD kidney predicts mAonC whereas a decrease in the level of IGFBP7 protein and an increase in TIMP-2 level means sAonC onset.
New biomarkers could reflect AonC at different degreesRhomboid-like protein 2 (RHBDL2), similarly to rhomboids in Drosophila, has a role in the generation of bioactive EGF ligands and triggering activation of the EGFR in mammal.12 The expression of rhomboids was controlled by E-cadherin. In Drosophila intestine, individual apoptotic enterocytes promote divisions by loss of E-cadherin, which releases cadherin-associated β-catenin (Armadillo in Drosophila) and p120-catenin to induce rhomboid. Thus, an increase in RHBDL2 indicates apoptosis in epithelial cells.13 In this study, RHBDL2 mRNA increased in CKD kidneys and further increased in sAonC kidneys (Fig. 2E). RHBDL2 protein dramatically elevated in CKD kidneys, and increased further in both mAonC and sAonC kidneys compared with Sham control (Fig. 2G, L). It means that an increase in RHBDL2 protein could reflect AonC.
Syndecan-1 (SDC-1) is a cell surface heparan sulfate proteoglycan that is predominately expressed by epithelial cells.14 In our previous study, SDC-1 robustly expressed in kidneys after acute injury and shed from renal tubular cells surface into urine.15 We found that chronic renal injury has no influence on the expression of SDC-1 mRNA. Whereas, SDC-1 mRNA significantly increased in mAonC and sAonC kidneys compared with CKD kidney (Fig. 2F). CKD kidneys have higher levels of SDC-1 protein than Sham kidneys. Moreover, SDC-1 protein dramatically increased in sAonC kidneys (Fig. 2G, M). Thus, an increased SDC-1 mRNA level in CKD kidney was associated with AonC, and a dramatically elevated SDC-1 protein indicated sAonC occurrence.
DiscussionAKI superimposed on CKD is prevalent as CKD patients are susceptible to AKI risk factors like nephrotoxic drugs and major surgeries, which lead to intrinsic and hypovolemic AKI.16,17 Early identification of AonC is exceptionally crucial as AKI develops faster in vulnerable CKD patients. During the past decades, much more information has become available on biomarkers for early predicting AKI.5,7–10,18 However, in recent years, there has been an increasing amount of literature on the different pathomechanism between isolated AKI and AonC.2,3,19,20 Therefore, the performance of AKI biomarkers for predicting AonC raises our concern.
To our best knowledge, this is the first study in which the performance of AKI biomarkers were evaluated for predicting AonC. In our study, the expression of mRNA and protein of AKI biomarkers were analyzed and comparison was performed between different severity kidney dysfunction. Moreover, we evaluated potential new biomarkers for predicting AonC, based on prevalent understanding of AonC pathophysiological mechanism.
Renal function and histological characteristics in AonCWe started with the most accessible and affordable kidney function indicator, serum creatine (SCr), which was identified as the criteria for AKI diagnosis in last 50 years. Including delaying identify kidney injury, many more limitations of SCr were revealed.6,21 Though SCr is an indirect marker for kidney dysfunction, it remains the golden standard in clinical practice. In the current study, the increasing Scr indicated the development of severe AKI and AonC, while no significance found between moderate acute injury and CKD. Hence SCr is not sensitive enough for early detecting moderate AKI or AonC.
With a histological evaluation, we found that the injury location of isolated CKD and AKI were renal cortex and cortico-medullary junction, respectively. The injury of renal cortex in AonC mice was not distinguished from CKD, but more severe than AKI mice (Fig. 1D). The predominant damage of AonC located in the cortico-medullary junction and the magnitude of severity was between CKD and isolated AKI (Fig. 1B, C). This histological finding illustrated that the injury of AonC comprehended both cortex and cortico-medullary junction, derived from CKD and AKI, respectively.
It's interesting to note that fibrosis was found mitigated in the mice with moderate AonC, one possible reason was that the preexisting renal mass reduction didn’t impact the recovery capacity of tubule from moderate acute ischemic injury. However, prominent tubulointerstitial fibrosis was found in severe AonC mice, indicating the failure of recovering due to severe injury was similar to CKD (Fig. 1E, F).22 Further experimental studies are needed to demonstrate its implication.
AKI biomarkers in AonCReportedly, KIM-1, NGAL, IGFBP7 and TIMP-2 were identified as potential biomarkers for predicting AKI effectively.5,7–9,23,24 Although extensive studies have been carried out on biomarkers for AKI, no single study exists which adequately evaluates the performance of biomarkers predicting AonC. In the current study, their mRNA and protein were observed separately between diverse severity AonC settings and compared with isolated AKI/CKD.
KIM-1 is a 90kDa type 1 transmembrane glycoprotein and significantly expressed in kidneys, specifically in proximal tubular cells. The upregulation of KIM-1 in the AKI is reported participating in renal repair.25 The significant increasing magnitude of KIM-1 mRNA in moderate AonC suggested that underlying repairment was initiated whereas in severe AonC, a similar attempt didn’t manifest. In sAonC histology, majority of tubule was damaged and fibrosis was prominent. Nevertheless, KIM-1 remained as a novel biomarker for AKI prediction, regardless of the severity of I/R.
TIMP-2 and IGFBP7 are mediators of cell-cycle arrest and block the effect of cyclin-dependent protein kinase complexes and cause short periods of G1 cell-cycle arrest,5 which was recognized as a protective mechanism of the body in response to AKI. To avoid duplication of damaged DNA, renal tubular epithelial cells enter cell cycle arrest. Markers of cell cycle arrest, such as IGFBP7 and TIMP-2, emerge and respond to the imminent AKI.26 However, sustained cell cycle arrest may result in cell senescence and maladaptive repair. In our study, the mRNA of TIMP-2 and protein of IGFBP7 expressed significantly in moderate AonC, indicating that cell-cycle arrest initiated with the purpose of early protection and avoidance of further maladaptive repair, which was found attenuated in moderate AonC biopsy. Conversely, in severe AonC mice, the magnitude of IGFBP7 protein was low while TIMP-2 protein increased drastically, suggesting that TIMP-2 has replaced IGFBP7 to maintain cell cycle arrest on the purpose of preventing severe I/R injury. The product of the two protein concentrations ([TIMP-2]·[IGFBP7]) in urine has been approved by FDA to predict AKI (Nephrocheck).8 Likewise, whether the combination of TIMP-2 and IGFBP7 was able to predict AonC requires further validation. Nonetheless, we found that the magnitude of IGFBP7 has potential capacity of predicting the severity of AonC. However, prolonged cell-cycle arrest led to fibrosis as well as the failure of recovery from renal tubular cell injury.
NGAL is a 25-kDa protein belonging to the lipocalin superfamily and is expressed in various tissues in the body, such as the lung, gastrointestinal tract, liver, and kidney.24 It is markedly induced in injured epithelial cells in response to injury, inflammation, and neoplastic transformation. Both genetic and protein expression of NGAL in our severe AonC mice were significantly prominent. A study revealed that NGAL was produced in injured distal nephron epithelium when the kidney was injured.27 Hence, continuous and chronic injury in the CKD setting elevates the baseline NGAL level and delays the kinetic change of NGAL. The NGAL level in CKD mice was higher than sham, but lower than both m/sAonC. A previous clinical study revealed preoperative plasma NGAL in AonC patients who underwent cardiac surgery was higher than preoperative CKD/post-operative AKI patients.28 Consistently, plasma NGAL concentration in 0h (after cardiac surgery) decreased in AonC patients, regardless of adjustment with intraoperative fluid accumulation. In our study, NGAL was revealed adequate for predicting both m/sAonC and m/sAKI. Further studies shall be warranted to investigate the performance of NGAL in the AonC model, particularly crafted with direct tubular damage.
Potential predictive biomarkers in AonCNumerous reports have reported E-cadherin, which is mainly expressed in epithelial cells, plays a key role in establishing stable cell-cell contacts through controlling cell signaling cascades driving cell proliferation.13,29 Among existing E-cadherin mediating pathways, the expression of RHBDL2 was controlled by E-cadherin and the up-regulation of RHBDL2 indicates apoptosis in epithelial cells.13 In the current study, severe tubular epithelial cells apoptosis and loss were found in AonC histology while a strong association between RHBDL2 and AonC was revealed. In reviewing the recent literatures,16,30 E-cadherin was found significantly down-regulated in the early stage of AKI. Hence, the underlying E-cadherin processing mechanism mediated by RHBDL2 was possible in the AonC setting. Compared with isolated CKD mice, RHBDL2 expressed significantly more remarkable in both m/sAonC models, indicating RHBDL2 to be a new and notable biomarker for predicting AonC.
Syndecan-1 belongs to the endothelial glycocalyx, a protective layer covering the endothelium luminally.31 The glycocalyx damage and dysfunction are related to the pathophysiologic development of AKI and contribute to increased morbidity and mortality.32,33 The serum syndecan-1 magnitude was reported as a biomarker of endothelial glycocalyx damage.34 Despite rarely reported in the AonC scenario, SDC-1 mainly emerges associating endothelial damage in AKI clinical and experimental literatures.33,35 The mRNA and protein expression of SDC-1 in the AonC rodent model was measured in our study. Specially, the mRNA of SDC-1 showed higher discriminatory capacity in predicting both moderate and severe AonC, whereas the increment of SDC-1 protein expression was associated with the onset of severe AKI. Meanwhile, the level of SDC-1 protein in isolated AKI was not significantly high. SDC-1 protein robustly shedding from renal epithelial and endothelial cells after AKI,15 and clean away with urine. While in CKD kidneys, eGFR and urine were extremely low, SDC-1 was accumulated in the kidney.
Given the identity of the pilot study, there are several limitations. First, it was performed in a single-center, with sample heterogeneity considered and excluded. Second, the present study aimed to identify potential biomarkers for predicting AonC, the underlying mechanism between these predictors and AonC remains unclear, which requires further investigation to elucidate.
ConclusionsIn this study, numerous biomarkers were investigated and their predictive capacity for acute kidney injury superimposed on chronic kidney disease were evaluated. AonC injury mainly located in cortico-medullary junction. Severe AonC was associated with increased serum creatinine, NGAL, RHBDL2 and SDC-1 mRNA and protein, TIMP-2 protein and decreased IGFBP7 protein. While elevated KIM-1 and TIMP-2 mRNA and IGFBP7 protein indicate moderate AonC occurrence. The magnitude of IGFBP-7 protein can identify severity of AonC.
FundingThis work was supported by the National Natural Science Foundation of China (81800596), National Key Research and Development Program (2016YFC1305500), Shanghai Most Important Clinical Medical Center and Key Discipline Construction Program (2017ZZ01015), Youth Fundation of Zhongshan Hospital (No.2020ZSQN44) and Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-07-E00009).
Conflict of interestThe authors declare that they have no competing interests.