Dear Editor,
We present a case of kidney transplant recipient who developed a rare, life threatening, lower GI bleeding six months after the transplantation, possibly associated with MMF treatment.
The most common side effects of MMF are gastrointestinal (GI) disturbances (nausea, vomiting, abdominal discomfort, diarrhea or constipation), hematological disorders, hepatic dysfunction etc.1,2 GI bleeding requiring hospitalization has been observed in approximately 3% of renal transplant patients, GI perforations have rarely been observed.1
A 73-year-old Caucasian male kidney transplant patient was hospitalized due to profuse haematochezia.
Six months prior to admission the patient received a renal transplant from a deceased donor due to an end stage renal disease. For induction therapy the patient received mycophenolate mofetil 1g and daclizumab 75mg preoperatively. Prednisone in a dose of 500mg was administrated intraoperatively. On the second post transplant day tacrolimus was introduced. The patient was discharged from the hospital with immunosuppressive regimen consisting of mycophenolate mofetil 750mg bid, tacrolimus 3mg bid and prednisone 20mg plus pantoprazole 40mg.
At admission, laboratory findings showed severe posthemoragic anemia (hemoglobin level 62g/L), slightly elevated kidney function parameters (BUN 13,9mmol/L, creatinine 137µmol/L) and clinical signs of hipovolemic shock. Coagulation parameters were within normal ranges.
Because of severe haematochezia the patient was admitted to gastroenterology intensive care unit where erythrocyte transfusions and crystalloid infusions were given for initial stabilization. His immunosuppressive therapy was continued and consisted of mycophenolate mofetil 750mg bid, tacrolimus 3mg bid and prednisone 20mg plus pantoprazole 20mg qd.
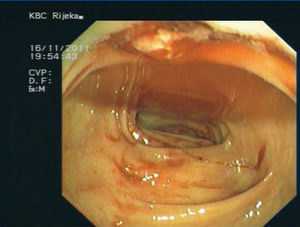
Two days upon admission a new episode of profuse haematochezia occurred and after basic bowel preparation, urgent colonoscopy was performed. Diverticular disease of left colon was found with intense bleeding in several diverticules which disabled local haemostatic therapy (Figure 1). After rinsing left colon with saline and epinephrine solution an octreotide therapy was administered (500mL saline with 0.6µg octreotide, 40mL/hour). Repeated erythrocyte transfusions were given. In total, the patient received eight erythrocyte units during a period of three days. In consultation with nephrologists, MMF dose was reduced to 250mg bid, and prednisone to 8mg while the dose of tacrolimus remained unchanged, 3mg bid.
The following day the bleeding had stopped with slow recovery in hemoglobin level. An ultrasound of kidney graft revealed normal kidney parenchyma with slightly increased arterial resistance index of 0.75 at the level of interlobar arteries.
At a discharge, on the 9th day the laboratory findings were satisfactory (hemoglobin level 133g/L, BUN 7,9mmol/L and creatinine 126µmol/L). Suggested maintenance immunosuppressive therapy consisted of mycophenolate mofetil 250mg bid, tacrolimus 2mg bid and prednisone 8mg daily. The patient is well without symptoms of GI toxicity or rejection for 5 months.
MMF is used in treatment regimens as an immunosuppressive agent in both solid organ and bone marrow/peripheral blood stem cell transplantation, as well as in treatment of autoimmune disorders, such as lupus.3 Drug effects are mediated via the active metabolite, MPA which seems to be responsible for GI toxic effects. GI adverse events are common following renal transplantation and all immunosuppressive regimens have been associated with such events. They are the most frequent problems associated with MMF therapy occurring in up to 20%4 or, in some studies up to 40%5 of renal transplant recipients. Such adverse events can extend along the entire GI tract, and can vary in severity from those which are mild (nausea, discomfort, appetite loss) and do not require altering immunosuppressive regimen to those which are more severe or even life threatening (severe diarrhea, GI tract ulcerations, hemorrhage and perforations).4,6
The etiology of GI disorders following transplantation is not well understood. Because of enterocyte dependency for de novo purine synthesis MMF exposure could thus restrict the ability of intestinal epithelial cells to maintain normal barrier function, or decrease their capacity to recover from damage.7
Our patient has experienced a life threatening, severe lower GI bleeding which reoccurred within 2 days upon initial stabilization while on a stable immunosuppressive regimen. Upon dose reduction, the bleeding had stopped, indicating the possible adverse effect of MMF.
A database from the United States Food and Drug Administration’s (US FDA) Adverse Event Reporting System (AERS), containing more than 4,000,000 adverse events reported between 2004 and 2011, has a record of 9 cases of haematochezia (0.02%) associated with MMF treatment (www.drugcite.com; accessed Feb 1, 2012).
We have reported this case to the Croatian National Drug Agency and in feedback letter have been informed that it is a serious, unexpected adverse drug reaction, possibly associated with MMF treatment. A total of 16 cases have been reported to the WHO Adverse Drug Reaction Monitoring Center with two fatal outcomes (WHO, UMC VigiBase, 29th November 2011).
Clinicians should be aware of possible, rare, but life threatening, lower GI bleeding associated with MMF treatment in renal transplant patients. Special caution should be given to patients with digestive system disease even if asymptomatic.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Figure 1.