Mucociliary clearance (MC) is a critical defense mechanism for the protection of the entire respiratory system. Nasal colonization of some pathogens and chronical nasal infections are important risk factors for peritonitis. Any disturbance in the MC causes stasis of secretions and secondary infections.
ObjectiveThe aim of the study was to evaluate the patients with chronic kidney disease (CKD) receiving continuous ambulatory peritoneal dialysis (CAPD) in terms of nasal MC. More specifically, the goal is to investigate the possible correlation between the nasal MC and peritonitis.
MethodsForty CAPD patients and 39 healthy volunteers were involved in the study. The nasal MC was evaluated with the saccharin test, in which a 1mm diameter saccharin particle was carefully placed on the antero-medial surface of inferior nasal concha. The time taken by the subjects from the placement of particle to the perception of the sweet taste was taken as mucociliary clearance time (MCT). The groups were compared in terms of MCT. The patient group was evaluated in terms of a peritonitis history, and the correlations with MC were analyzed.
ResultsPatient group with CKD consisted of 16 females and 24 males with a mean age of 32.4 years; healthy individuals in the control group consisted of 17 women and 22 men with a mean age of 33.3 years. There was not a significant difference in terms of mean MC time in patients with CKD when compared with the individuals in the control group. The comparison between the mean MCT in the patients who had a history of peritonitis and patients without peritonitis was statistically significant (p<0.05).
ConclusionsUnique for being conducted with patients in continuous ambulatory peritoneal dialysis, the current study shows that although the MC of CKD patients and healthy individuals is similar, patients with low rates of MC appear to present an increased incidence of peritoneal infection. Considering the small sample investigated, an invitation to future confirmatory studies would be appropriate.
El aclaramiento mucociliar (AM) es un mecanismo de defensa fundamental para la protección del sistema respiratorio. La colonización nasal de algunos patógenos y las infecciones nasales crónicas son factores de riesgo importantes de peritonitis. Cualquier alteración en el AM provoca estasis de secreciones e infecciones secundarias.
ObjetivoEl objetivo de este estudio fue evaluar el AM nasal de los pacientes con nefropatía crónica (NC) que recibían diálisis peritoneal ambulatoria continua (DPAC). Más concretamente, el objetivo fue estudiar la posible relación entre el AM nasal y la peritonitis.
MétodosCuarenta pacientes en DPAC y 39 voluntarios sanos participaron en el estudio. El AM nasal se evaluó con la prueba de sacarina, en la que se colocó cuidadosamente una partícula de sacarina, de 1 mm de diámetro, en la superficie anteromedial del cornete nasal inferior. El tiempo transcurrido desde el momento en que se colocó la partícula hasta que los pacientes percibieron el sabor dulce se consideró el tiempo de aclaramiento mucociliar (TAM), parámetro que se empleó para hacer la comparación entre los grupos. Se evaluaron los antecedentes de peritonitis en el grupo de pacientes y se analizaron las correlaciones con el AM.
ResultadosEl grupo de pacientes con NC constó de 16 mujeres y 24 hombres con una media de edad de 32,4 años. Los pacientes sanos en el grupo control fueron 17 mujeres y 22 hombres con una media de edad de 33,3 años. No se observó una diferencia significativa en el tiempo medio de AM en pacientes con NC respecto a los pacientes del grupo control. La comparación entre el TAM medio en los pacientes con antecedentes de peritonitis y en pacientes sin peritonitis fue estadísticamente significativa (p < 0,05).
ConclusionesÚnico por llevarse a cabo en pacientes con diálisis peritoneal ambulatoria continua, el estudio actual muestra que, aunque el AM de pacientes con NC y pacientes sanos es similar, los pacientes con tasas bajas de AM parecen presentar un aumento de la incidencia de infección peritoneal. Teniendo en cuenta la pequeña muestra estudiada, consideramos conveniente realizar nuevos estudios de confirmación.
Mucociliary clearance (MC) is a critical defense mechanism for the protection of the entire respiratory system. Nasal or lower airways can also be cleaned with a sneeze or cough reflex, however, the paranasal sinuses solely depend on MC for cleaning.1 Effective MC requires appropriate mucus/periciliary fluid production and coordinated ciliary activity.2 Several tests have been used to investigate MC, and the saccharin transit test (STT) is the easiest, cheapest and most widely used one.3
Chronic kidney disease (CKD) refers to the progressive loss in renal functions over a period of months or years with important extrarenal systemic consequences such as metabolic, cardiovascular and respiratory system problems.4 For the patients with CKD, peritonitis is currently one of the leading complications of continuous ambulatory peritoneal dialysis (CAPD).5 Nasal colonization of some pathogens and chronical nasal infections are important risk factors for peritonitis. Recent studies have reported deteriorated nasal mucociliary activity in patients with CKD.6,7 Any disturbance in the MC causes stasis of secretions and secondary infections. In other words, disordered MC leads to impaired health of respiratory tract and can manifest as chronical infections of the nose, sinuses and the whole airway which can be risky in CKD patients, especially ones receiving CAPD.8 Our aim of this study was to evaluate the patients with CKD receiving CAPD in terms of nasal MC and to investigate whether there is a relation between the MC and risk of infection for this particular patient group.
MethodsPatients and control groupThis single-center study was performed in patients undergoing CAPD at the Nephrology Department of a University Hospital for a period of 38 months between May 2014 and July 2017. The study was approved by the University Ethics Committee and Local Hospital Review Committee. All participants provided written informed consent.
In the center, the majority (∼70%) of dialysis patients are treated with CAPD. One hundred and twenty-five CAPD patients are currently being followed. The patients between 18 and 70 years of age who have CAPD as the first renal replacement therapy were included in the study. CAPD peritonitis was diagnosed when the peritoneal fluid white cell count was 100/mm3 or greater and when 50% or more of these cells were polymorphonuclear leukocytes. After considering this inclusion criterion, 61 patients were found to be eligible. Six patients refused to participate in the study and 15 were excluded because of having at least one of the following conditions; rhinosinusitis, nasal polyps, allergic rhinitis, septal problems such as deviations and perforations, a history of previous nasal surgery, obstructive adenoid vegetation, cystic fibrosis, immunodeficiency, ciliary dyskinesia or other diseases/medications affecting nasal mucosa and its mucociliary transport mechanism and smoking. Finally, a total of 40 CAPD patients with or without peritonitis history were enrolled in this study.
Evaluation of mucociliary transportAll participants underwent a fiberoptic rigid endoscopic nasal examination without any topical medications before the evaluation of MC. Before the MC measurement, nasal swabs for Staphylococcus aureus carriage were taken from each patient.
Nasal MC was measured with STT, which is first described by Andersen et al.,9 and modified by Rutland and Cole3 by the same clinician in all participants.
Patients were asked not to consume coffee or alcohol for 6h before their test. A particle of 1-mm-diameter sodium saccharin was placed on the antero-medial surface of the inferior nasal turbinate. The patients were instructed to sit quietly in a neutral position without/avoiding eating, drinking, coughing, sneezing, sniffing, deep breathing and report whenever they feel a sweet taste on their tongue. The time between placement of saccharin and sweet taste was first sensed was measured in minutes and seconds with a stopwatch. If no response was reported at 30th min, the test was concluded after confirming the normal perception of sweet taste by placing saccharin particle on the tongue directly.
Statistical analysisAll tests or examinations were performed by the same investigator. The collected data were analysed via SPSS 22.0 (IBM Corp., Armonk, New York). The normal distribution of numerical variants were analyzed with Shapiro–Wilk test of normality and Q-Q graphics. The intergroup comparisons were performed with two sample t test in normally distributed variables. The relationships between numerical variants were analyzed with Pearson and Spearman correlation analysis. The relations between categorical variants were analyzed with exact method of Fisher Chi Square test on rxc tables. The saccarin clearance time was evaluated with ROC (Receiver Operating Characteristic) curve. p<0.05 value is named statistically significant.
Additionally, univariate and multiple binary logistic regression analysis were applied to identify the risk factors of peritonitis. Significant variables at p<0.25 were included into multiple model, and backward elimination was performed using Wald statistic to identify the independent risk factors of peritonitis. The goodness-of-fit of the model was assessed using Hosmer–Lemeshow test, Omnibus test and Nagelkerke R2 statistic. Analyses were conducted using TURCOSA (Turcosa Analytics Ltd Co, Turkey, www.turcosa.com.tr) statistical software.
ResultsA total of 40 patients with CKD, 16 females/24 males with a mean age of 32.4 years (range 22–52 years); and 39 healthy individuals in the control group, 17 females/22 males with a mean age of 33.3 years (range 20–56 years) completed the study. There were no significant differences between the CKD and control groups in terms of age (p>0.05). Table 1 shows the clinical and demographical features of patients. Also the etiological spectrum of peritonitis in CAPD patients are listed in Table 2.
Clinical and demographical features of the patients.
| Parameters | Patients (n=40) |
|---|---|
| Age (year) | 32 (22–52) |
| Gender male (%) | 24 (60) |
| Cause of chronic renal failure | |
| Diabetes mellitus | 18 |
| Hypertension | 13 |
| Polycystic kidney disease | 2 |
| Pyelonephritis | 2 |
| Glomeruloephritis | 0 |
| Amyloidosis | 1 |
| Unknown | 4 |
| Duration of CAPD (month) | 33.5±22.0 |
| Body mass index (kg/m2) | 28.5±4.2 |
| WBC (mm3) | 8030±3092 |
| Hemoglobin (g/dl) | 11.1±1.6 |
| Albumin (g/dl) | 3.32±0.4 |
| Residual urine volume (ml/day) | 950±520 |
| Kt/V | 2.35±0.66 |
| D/P creatinine | 0.73±0.07 |
CAPD: continuous ambulatory peritoneal dialysis, WBC: white blood cells, D/P: dialysate to plasma ratios.
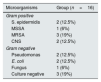
Causative organisms isolated from peritoneal effluent fluid.
| Microorganisms | Group (n=16) |
|---|---|
| Gram positive | |
| S. epidermidis | 2 (12.5%) |
| MSSA | 1 (6%) |
| MRSA | 3 (19%) |
| CNS | 2 (12.5%) |
| Gram negative | |
| Pseudomonas | 2 (12.5%) |
| E. coli | 2 (12.5%) |
| Fungus | 1 (6%) |
| Culture negative | 3 (19%) |
MSSA: methicillin sensitive Staphylococcus aureus, MRSA: methicillin resistant Staphylococcus aureus, CNS: coagulase negative staphylococcus.
The nasal swab results were positive for S. aureus in only 4 patients who had a history of peritonitis. As the number was low, these results were not included in analysis.
Mean MC times in the CKD and control groups were 382.55s (range 90–941s) and 373.33s (range 115–616s) respectively. There was not a significant difference in terms of mean MC time in patients with CKD when compared with the individuals in the control group (p>0.05).
There were no correlations between the MC time and age, gender, body mass index and the duration of CAPD. The correlation analysis between the MC time and blood glucose was significant (p=0.004).
In the patient group, there were 16 patients who had had at least one peritonitis episode. The mean MC time in the patients who had a history of peritonitis was 588.8s while it was 256.5s in the 24 patients without peritonitis which revealed a statistically significant difference (p<0.05).
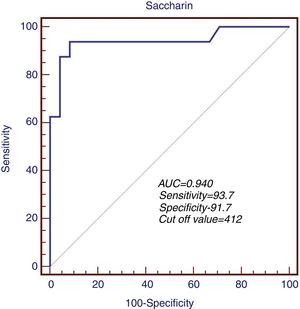
The predictive role of MC time in determination of peritonitis risk was first measured in ROC analysis. The sensitivity and specificity of MC time were 93.7% and 91.7% when the cut-off level was accepted as 412s during the evaluation of the patient group in terms of a previous history of peritonitis. ROC area was measured as 0.940 (95% CI: 0.697–0.990, p<0.001) (Fig. 1).
The Omnibus test (χ2=38.089, p=0.008) revealed that the built multiple model is significantly better than the baseline logistic regression model. Nagelkerke R2 statistic was calculated as 0.830. The WBC and MC time variables in the multiple model predicted 83% of the variability of peritonitis. Not surprisingly, the WBC is related to the inflammatory process and is a predictor. The Hosmer–Lemeshow test resulted as χ2=8.172, p=0.417. These results revealed the appropriateness of the built multiple binary logistic regression model in order to predict the peritonitis (Table 3). In the multivariate analysis, the OR (95% CI) of WBC and MC were 1.74(1.05–2.88) (p=0.032) and 0.98(0.97–0.99) (p=0.008) respectively.
Univariate binary logistic regression analysis results in identifying the risk factors of peritonitis. OR: odds ratio, CI: confidence interval. Omnibus test: χ2=38.089, p=0.008; Hosmer and Lemeshow test: χ2=8.172, p=0.417; Nagelkerke R2: 0.830. WBC: white blood cell, iPTH: intact parathormone.
| Variables | Univariate | |
|---|---|---|
| OR (95%CI) | p | |
| Age (years) | 0.98 (0.92–1.04) | 0.502 |
| Gender (female/male) | 1.19 (0.33–4.36) | 0.792 |
| Diabetes mellitus (present/absent) | 0.19 (0.05–0.74) | 0.017 |
| Peritonitis number | 0.69 (0.37–1.30) | 0.691 |
| Body mass index (kg/m2) | 1.12 (0.95–1.32) | 0.189 |
| Duration of CAPD (months) | 1.00 (0.97–1.03) | 0.926 |
| Serum glucose (mg/dl) | 1.00 (0.97–1.03) | 0.812 |
| Serum albumin (g/dl) | 0.75 (0.22–2.53) | 0.641 |
| Hemoglobin (g/dl) | 1.18 (0.85–1.63) | 0.328 |
| WBC (μ3/l) | 1.36 (1.04–1.77) | 0.026 |
| iPTH (pg/ml) | 0.99 (0.98–1.01) | 0.128 |
| Urine volume (ml) | 0.99 (0.98–1.01) | 0.074 |
| Mucociliary clearance time (sec) | 0.98 (0.97–0.99) | 0.001 |
This prospective study aimed to investigate the nasal MC in patients with CKD receiving CAPD. Although an alteration in the nasal functions and MC were reported in the literature, which might be an expected consequence of CKD, no significant difference was observed between the patients with CKD and healthy individuals in the control group in terms of nasal MC in this study. However, in the patient group, the MC of the subjects who had a history of peritonitis was found to be significantly altered. This interesting finding may prompt a conception that a relation between the peritonitis and MC exists and needs further investigation in this particular patient group.
Mucociliary clearance is an important mechanism for protecting upper and lower airways by eliminating foreign particles and pathogens and keeping the mucosa moist and fresh. Mucociliary clearance is dependent on the volume and composition of mucus and periciliary fluid, the ciliary structure and function, and the mucociliary interaction.10 The ciliary cells line upper and lower airways; so as any alteration in any mechanism MC depends on, such as cystic fibrosis or sinonasal manifestations of systemic diseases, leads to the stasis of secretions and recurrent middle ear, sinonasal, and lower respiratory tract infections.11
Many factors are known for affecting ciliary function and mucus production, thus the MC. Moisture, temperature, smoking, partial oxygen pressure and pH are modifiable environmental factors affecting MC; however, systemic diseases such as hypothyroidism and cystic fibrosis, obstructive nasal pathologies such as septal deviation and adenoid vegetations, infectious or allergic rhinitis also affect MC by altering ciliary function, production of mucus or obstructing the airway.12 Hence, the study was conducted in the same environmental condition; smokers and subjects with rhinosinusitis, nasal polyps, allergic rhinitis, septal deviations or perforations, a history of previous nasal surgery, obstructive adenoid vegetation, cystic fibrosis or other subjects with chronical pulmonary infections, immunodeficiency, ciliary dyskinesia were excluded from study.
Many techniques have been reported for measuring MC, but the easiest, cheapest and most widely used one is the STT. Originally described by Andersen et al.,9 then modified by Rutland and Cole,3 STT is conducted by placing a 1mm saccharin on inferior turbinate, 1cm behind the mucocutaneous junction, under direct visualization. Time between the placement of saccharin and first sensation of sweet taste on the tongue is measured with a timer.13 Broad range of times for normal MC have been reported in the literature from 4.4 to 20min.14–17 If there was no perception within 30min, another saccharin particle was placed directly on the tongue to determine if the patient tastes saccharin at all. Some authors dissolve saccharin with methylene blue and confirm the patient's taste perception by presence of blue dye in oropharynx.18
Other methods for measuring MC consist of a variety of in vivo and in vitro techniques. Technetium-99 scintigraphy 19 and microdialysis probes20 are other in vivo choices, more objective than STT, but also more expensive and invasive. In vitro techniques consist of human airway cell cultures,21 photodiodide, photomultiplier and digital high-speed video capturing as an indirect method for quantifying ciliary beat frequency.13,22 Among all these different techniques, the STT is the most serviceable, easiest and cheapest technique for measuring MC; therefore, it was used in this study.
Chronic renal disease is an irreversible pathophysiological renal process resulting in the inevitable destruction of renal structure and function with many extrarenal manifestations. Oxidative stress, hypoxia and endothelial dysfunctions are some of blamed mechanisms behind end-organ damage and the extrarenal manifestations. It has been reported in previous studies that patients with CKD have increased rate of nasal problems such as epistaxis, spontaneous perforations of nasal septum and deteriorated mucociliary clearance rate.6,7
Possible and unclear causes have been reported in previous studies for deteriorated MC in patients with CKD. Chronic renal disease causes progression of atherosclerosis and microvascular disease of small vessels, and these processes may affect MC by altering production of mucus or ciliary cell function on a cellular basis.4,6 Another study speculated that damage of dynein arms and microtubules by free oxygen radicals and uremic toxins and endothelial dysfunction, vasoconstriction and hypoxia in ciliary cells may have a role on decreased mucociliary function.7 Increased mucoid viscosity by liquid–electrolyte imbalance is also blamed for making MC ineffective.7 In another study, it has been shown that CKD patients have fibrosis, metaplasia and chronic inflammation in nasal biopsies, and all these pathophysiologies may have a role in this.23 In the current study, however, there was no significant difference between the MC of patient and control groups. Furthermore, the correlation analysis between the MC time and blood glucose was significant (p=0.004) in the current study, parallel to what has also been previously mentioned in the literature.24
Chronic kidney disease has a myriad of renal and extrarenal complications, but infectious complications are the second cause of mortality and main cause of morbidity in patients undergoing haemodialysis and CAPD.25,26 Increased infection rates in CKD can be explained by impaired leucocyte function and numbers, new pathways for microorganisms to enter, such as catheter exit site in patients undergoing CAPD, and ineffective defense mechanisms such as deteriorated MC.6 A vast number of prophylactic strategies have been used to reduce the occurrence of these infectious complications, including the use of oral, nasal and topical antibiotics and cautionary advice for the patients about infectious complications.6,26 Gram positive microorganisms, particularly S. aureus is an important causative microorganism in CAPD peritonitis. The microbiological evaluation of patients in the current study showed similar results in line with the current literature. Probably, these microorganisms may transfer from upper respiratory tract to peritoneal microvasculature via blood stream. It has been well established that poor glycemic control is related with increased risk of peritonitis in PD patients.27 The potential effect of nasal Staph. carriage on peritonitis rate has not been clarified yet. Moreover it has been recently demonstrated that Staph. carriage rates were similar in diabetic patients compared to general population.28 It might be speculated that diabetes may affect host-defense mechanisms in addition to lowering of MC clearance depending upon our study results. Thus further larger studies are needed to explain the relationship between MC time and peritonitis in diabetic population. Although it was stated that no data is present on the effectiveness of routine screening and eradication of S. aureus nasal carriage before catheter insertion; screening for nasal S. aureus carriage prior to PD catheter insertion, and if found, treating by topical nasal application of mupirocin is suggested, while further randomized controlled studies are needed to strengthen the evidence.29
To date, two studies evaluated MC in CKD patients. Study by Kucur et al.,6 has revealed significant difference in MC times when healthy controls and patients with CKD were compared (8.97±1.83min and 12.51±3.74min, respectively). The study by Uluyol et al.,7 has a different method; they compared pre-dialysis patients, dialysis patients and control groups for MC time. They have found that MC time was higher in patients with CKD than healthy controls, but paradoxically, higher MC times were observed in pre-dialysis patients than dialysis patients with a longer CKD duration. They suggested that haemodialysis reduces the effects of CKD on MC by filtering toxins, oxidants and balancing the electrolytes.
The patients with CAPD have a high risk of peritonitis especially when a nasal pathogenic colonization or an infection is present and ‘theoretically’, a deteriorated MC might cause such consequences. Kucur et al.,6 concluded in their study that MC time in CKD patients is prolonged and should be followed-up closely in terms of respiratory tract diseases and sinonasal and middle ear infections.
Univariate and multiple binary logistic regression analysis performed in the current study demonstrated the WBC and MC as independent risk factors for peritonitis. The WBC, in our opinion, instead is a consequence of this inflamatorry condition however the detoriated MC may be a risk factor. On the other hand, monitoring all patients with CKD in terms of MC might not be practical in daily clinical practice since the STT of those patients are similar to the ones of the healthy individuals according to the findings of the current study.
The current study, which is the first, investigating the patients receiving CAPD in terms of nasal MC however also has weaknesses. A correlation between the MC and nasal microbiological environment could not be performed although conducted during the study, which would obviously supplement the findings.
ConclusionsAiming to enrich the information of MC in CKD patients, the current study is the first with a large sample size and conducted with patients with CAPD shows that, there is no significant difference in terms of MC and CKD, however a relation between the nasal MC and peritonitis occurs. We think that this relation must be taken seriously and further studies should be conducted with this patient group especially correlating with S. aureus presence in order to shed light to a possible relation between the MC, nasal colonization and peritonitis.
Conflict of interestNone.
The authors declare that they have no competing interests. This study was conducted after the approval of Local Ethical Committee, and a written informed consent was taken from each of the participants. No funding was received for this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.