We performed a retrospective trial to determine asymptomatic CMV reactivation and CMV disease in kidney allograft recipients with positive CMV serostatus.
MethodsPreemptive modified strategy under low dose thymoglobulin versus basiliximab induction was evaluated. Patients were monitored by CMV-polymerase chain reaction (PCR); if the viral load was >4000copies/μl, they received valganciclovir adjusted for their renal function.
Results132 recipients were included in the study, 84 and 48 receiving basiliximab and thymoglobulin induction respectively, and followed up for 12 months. Asymptomatic CMV reactivation was significantly higher for thymoglobulin (77.1% vs. 16.7%, p<0.001). Treatment groups had similar rates of CMV disease (3.6% vs. 2.1%, p 0.538). The significant difference in asymptomatic CMV reactivation between two treatment groups did not have any impact on 1 year graft function (71±26ml/min vs. 74±19ml/min; p=0.475) and no histological differences in protocol biopsies were observed among patients with asymptomatic CMV reactivation vs those without CMV reactivation.
ConclusionsDue to the high asymptomatic CMV reactivation incidence in patients who received thymoglobulin induction, our results suggest that valganciclovir prophylaxis may be advantageous in CMV seropositive renal transplant recipients after low dose thymoglobulin induction. A preemptive strategy appeared to significantly reduce the likelihood of CMV disease in both groups. Rejection risk and negative impact in renal function associated with asymptomatic CMV reactivation was not found in our series.
Llevamos a cabo un estudio retrospectivo para determinar la reactivación y enfermedad por CMV en receptores de trasplante renal CMV seropositivos bajo diferentes esquemas de inducción.
MétodosUna estrategia preventiva modificada bajo inducción con basiliximab y timoglobulina en dosis bajas fue evaluada. Se llevó a cabo un seguimiento de la carga viral-reacción de cadena de la polimerasa-CMV; los valores mayores de 4000 copias/μl recibieron valganciclovir ajustado a la función renal.
ResultadosUn total de 132 receptores de trasplante renal fueron incluidos; 84 y 48 recibieron inducción con basiliximab y timoglobulina respectivamente. Seguimiento hasta el mes 12 postrasplante. La reactivación asintomática de CMV fue significativamente mayor para timoglobulina (77,1% vs. 16,7%, p<0,001). La tasa de enfermedad por CMV fue similar en ambos grupos de tratamiento (3,6% vs. 2,1%, p=0,538). Ningún impacto en la función renal un año postrasplante fue encontrado entre los grupos a pesar de la diferencia significativa en reactivación asintomática de CMV (71±26ml/min vs. 74±19ml/min; p=0,475); igualmente, no encontramos diferencias en los hallazgos histológicos en biopsias por protocolo entre receptores con reactivación asintomática por CMV y aquellos sin reactivación.
ConclusionesLa alta incidencia de reactivación asintomática por CMV en receptores seropositivos a pesar del uso de bajas dosis de timoglobulina sugiere que la profilaxis con valganciclovir es una estrategia apropiada en este grupo; sin embargo, una estrategia preventiva reduce significativamente la probabilidad de enfermedad por CMV en ambos grupos de tratamiento. El riesgo de rechazo y el impacto negativo en la función renal asociado a la reactivación asintomática por CMV no fue encontrado en nuestra experiencia.
Asymptomatic CMV reactivation has been associated with notorious effects on morbidity and mortality in renal transplantation recipients.1–3 CMV risk is determined according to the overall immunosuppression status as a result of host conditions such as age, co-morbidity, and genetic factors. However, the two most relevant risk factors depend on the CMV serological status, which has the highest incidence among high-risk (donor (D)+/recipient (R)−) and intermediate-risk groups (R+/D+, R+/D−),4 as well as on the selected immunosuppression protocol.5 Clinical studies have demonstrated important differences between anti-thymocyte globulin (thymoglobulin) and basiliximab with regard to efficiency, particularly for outcomes such as biopsy-proven acute rejection, delayed graft function, graft loss, and death.6–10 Induction with thymoglobulin causes profound and long term CD4+ T cell depletion increasing the risk of CMV11,12; however, the impact of basiliximab is less clear.13,14 Clinical trials comparing the two drugs in different populations to assess the risk of CMV infection or disease onset have generally provided sparse evidence of significant differences; however, these findings have been difficult to interpret due to inclusion of multiples CMV risk groups.6,9,15–17
Since the introduction of CMV prophylaxis, disease incidence was reduced from greater than 60% to less than 30% in the first three months post-transplant.2,18,19 Two strategies for preventing CMV disease have been adopted: (1) universal prophylaxis with administration of an antiviral agent to all transplant recipients, or (2) a preemptive strategy that administers an antiviral agent only when a laboratory-confirmed infection is found during a scheduled screening. Preemptive therapy was found to be effective compared to placebo; however, its effectiveness relative to universal prophylaxis is unclear due to significant heterogeneity of the published studies.20
International consensus guidelines on CMV management in solid organ transplantation recommend the use of universal prophylaxis over preemptive therapy for high-CMV-risk patients21; however, recommendations for seropositive recipients vary, particularly in the context of varying induction therapies. Few studies have evaluated the efficiency of preemptive therapy in this population. Our study's aim was to evaluate the effectiveness of preemptive therapy in two renal transplantation cohorts, induced with low dose thymoglobulin or basiliximab at two transplantation centers in Colombia.
Outcomes: CMV infection, CMV disease, mortality, acute rejection, renal function and histological findings.
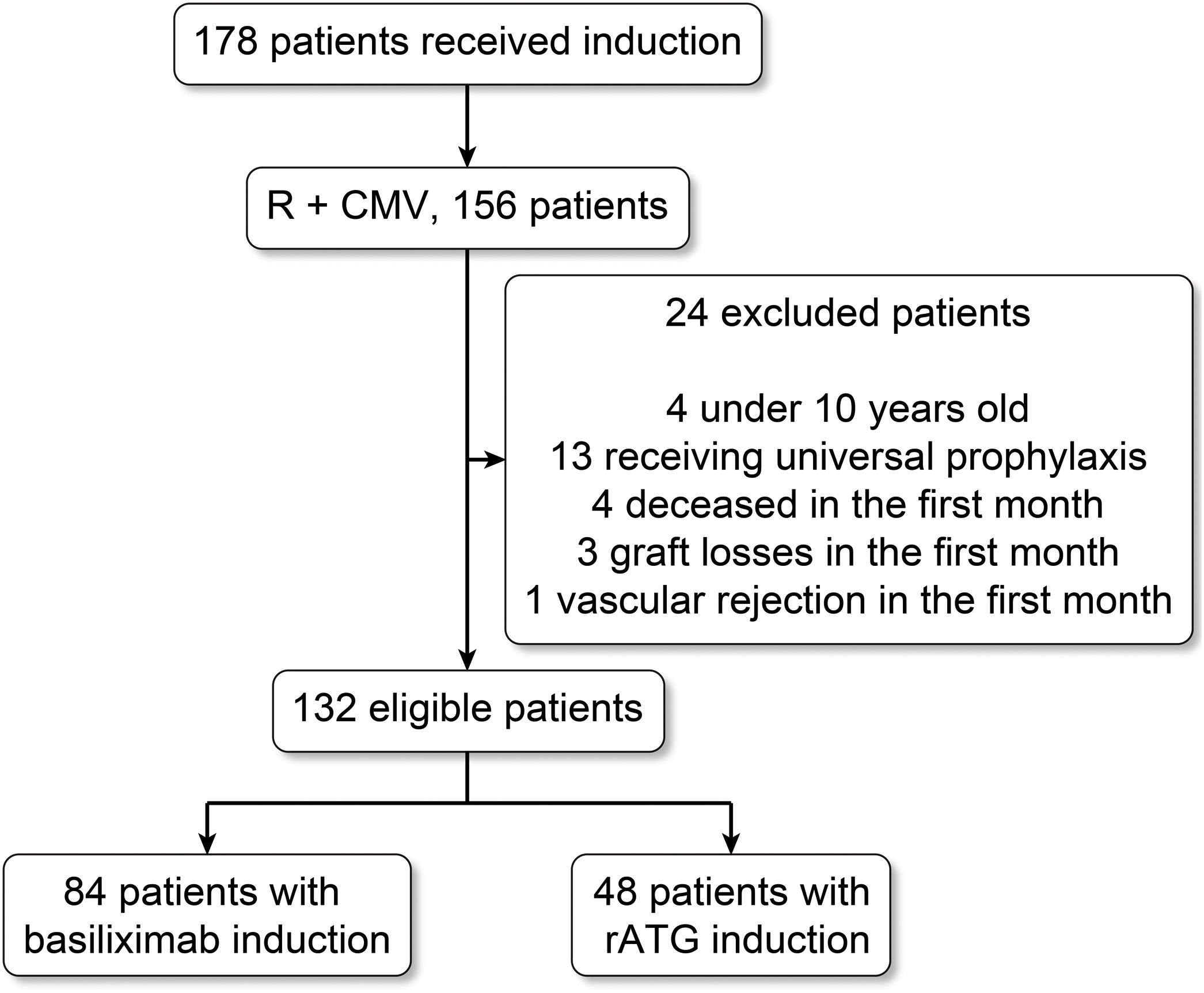
Materials and methodsStudy design and patientsThis retrospective cohort study was conducted in two renal transplantation centers in Colombia. The inclusion criteria were as follows: renal transplantation patients with related living or deceased donors performed between February 2009 and September 2012; patient age ≥10 years; CMV seropositivity (D+/R+ or D−/R+) and induction therapy with basiliximab or thymoglobulin. The exclusion criteria were patients who received universal CMV prophylaxis and patients who presented with graft loss, death, or vascular rejection requiring thymoglobulin during the first month post-transplant. The patients were treated according to the local site's standard of care, and the group received approval from the institutional review board.
Induction and immunosuppressant therapyBetween February 2009 and March 2011, from either a deceased or living donor received induction therapy with basiliximab and maintenance therapy comprising extended-release tacrolimus (Prograf XL; 5–10ng/ml), mycophenolate mofetil (2g/day) or mycophenolate sodium (1440mg/day) and prednisone. In the third month, a protocol biopsy and a switch to mTOR inhibitors were performed unless contraindicated because acute rejection, proteinuria, glomerular disease or severe interstitial fibrosis. The outcome analysis of the first two years yielded a subclinical rejection rate of 17.7% among transplant recipients, according to an analysis of the protocol biopsies. As a result, the clinical practice guidelines of our centers were modified, and induction therapy with thymoglobulin was formalized, in the same way; a protocol biopsy and a switch to mTOR inhibitors after third month were performed unless contraindicated. The initial transplant patient cohort (between February 2009 and March 2011) received induction with basiliximab, given in a 20-mg dose within two hours prior to graft revascularization and in a second dose on the fourth day post-transplant. The patients in the second cohort were transplanted between April 2011 and September 2012 and received thymoglobulin in two 1.5-mg/kg/day doses; thymoglobulin was reconstituted and administered via central-line infusion over a 6–8-h period, according to institution protocol. The treatment was initiated before graft reperfusion. All patients received 500mg of intravenous methylprednisolone before graft revascularization and on the first and second days post-transplant. Twenty minutes prior to surgery, all patients received surgical prophylaxis with cefazoline (1g). A nystatin was administered as an anti-mycotic prophylaxis and trimethoprim/sulfamethoxazole as a prophylaxis against Pneumocystis jiroveci during the first three months.
Laboratory methodsCMV viral load (real-time PCR): To detect and quantify CMV DNA, total blood samples were collected in BD Vacutainer® K2 EDTA 7.2-mg tubes (BD Biosciences, Franklin Lakes, NJ, USA), and viral DNA was extracted using MagNA Pure Compact equipment (Roche/Hoffman-La Roche AG, Basel, Switzerland) and the MagNA Pure Compact Nucleic Acid Isolation Kit I Version 1.1 kit (Roche #3039990). The extracted DNA from each sample was amplified with the CMV Light Cycler Set V2 kit (TIB MOLBIOL LLC, Adelphia, NJ, USA) according to the manufacturer's processing protocol on the real-time PCR LightCycler V2.0 apparatus (Roche). Additionally, a negative control (reaction mixture from the kit plus Ultra Clean® PCR Water; MO-BIO Laboratories, INC., Carlsbad, CA, USA) and a quantified positive control (AmpliRun® Cytomegalovirus DNA Control, Vircell Microbiologists, Granada, Spain) were included as general assay controls. The DNA detection limit of the assay used in this study was 10copies/μl.
Preemptive strategyCMV loads were assessed via real-time PCR of blood samples beginning at week four post-transplant and subsequently every four weeks in the basiliximab cohort and every two weeks in the thymoglobulin cohort until week 16. All patients with viral loads >4000copies/ml received an oral valganciclovir bid and dose was adjusted based on renal function until a negative CMV load was obtained. Follow-up viral load evaluations were conducted every 2 weeks.
Clinical definitionsClinical definitions were determined according to the international recommendations (21–23) considering the following categories:
Asymptomatic CMV reactivation: The presence of CMV replication
CMV disease onset: CMV reactivation accompanied by clinical signs and symptoms. Includes the following categories:
- -
CMV syndrome: characterized by a fever >38.8°C for at least two days in association with general malaise, leukopenia, thrombocytopenia, and aminotransferase elevation.
- -
Invasive CMV disease: characterized by organic involvement such as colitis or pneumonitis, hepatitis, nephritis, myocarditis, pancreatitis, or retinitis, among others.22
All graft biopsies were performed via interventional echograph-guided radiology and evaluated by a group of nephropathologists according to the Banff’07 classification.
Virological parameter definitionsViremia-free duration: Interval between transplantation and the detection of CMV DNA in total blood.
CMV viremia: detection of CMV DNA above the detection limit (>500copies/ml) in blood.
Viremia duration: total number of days between the first positive and first negative viral loads.
Replication post-treatment or repeated viremia incident: detectable presence of CMV DNA in total blood after the clearance of a previous incident with a documented negative viral load.
Viremia without treatment: duration of viremia without receiving treatment.
Statistical analysisThe associations between the type of induction and the qualitative clinical variables of effectiveness (incidence of CMV infection, CMV disease onset, late CMV disease onset, acute rejection, acute subclinical rejection, graft loss, renal function, graft survival and patient survival) were evaluated using Pearson's chi-squared test or Fisher's exact test (2×2 tables) and exact likelihood ratios (expected values <5). Quantitative variables were evaluated using Student's T-test for two independent groups (heterogeneous or homogeneous variances); variance homogeneity was previously evaluated with Levene's test and normality with the Shapiro–Wilk test. For cases in which these assumptions were not met, the Mann–Whitney asymptotic non-parametric test was used. To evaluate the associations and association strength, the relative risk (RR) and a 95% asymptotic or exact Katz confidence interval (CI) were used. These same tests were used to compare the two cohorts. Stratification for confounding variables was controlled by the Mantel Haenszel formula and a regression logistic unconditional model; OR adjusted and a 95% confidence interval were used. Statistical tests were evaluated at a significance level of 5% (p<0.05).
ResultsPatient characteristicsThe initial cohort comprised 178 patients treated via induction, 157 of whom were R+; of these patients, four younger than 10 years, 13 treated with universal prophylaxis, four who died during the first month, and three patients who suffered graft loss (focal segmental sclerosis, two patients, and vascular thrombosis, one patient) were excluded (Fig. 1).
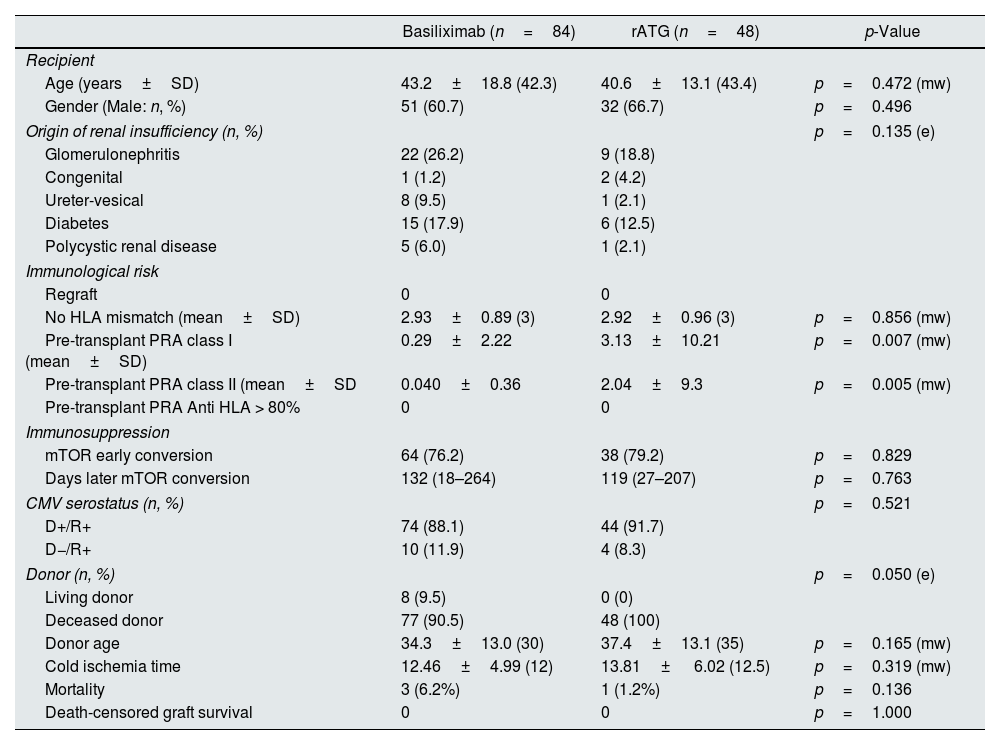
The cohorts comprised 132 patients who met the study selection criteria and were induced with basiliximab (n=84) and thymoglobulin (n=48). There were no statistically significant differences between the two cohorts with respect to demographic characteristics, age, gender, clinical variables, type of treatment received, etiology of renal insufficiency, and donor characteristics; however, there were significant differences in the class I and II pre-transplant panel-reactive antibody screening results (Table 1).
Demographic and clinical characteristics according to induction type.
| Basiliximab (n=84) | rATG (n=48) | p-Value | |
|---|---|---|---|
| Recipient | |||
| Age (years±SD) | 43.2±18.8 (42.3) | 40.6±13.1 (43.4) | p=0.472 (mw) |
| Gender (Male: n, %) | 51 (60.7) | 32 (66.7) | p=0.496 |
| Origin of renal insufficiency (n, %) | p=0.135 (e) | ||
| Glomerulonephritis | 22 (26.2) | 9 (18.8) | |
| Congenital | 1 (1.2) | 2 (4.2) | |
| Ureter-vesical | 8 (9.5) | 1 (2.1) | |
| Diabetes | 15 (17.9) | 6 (12.5) | |
| Polycystic renal disease | 5 (6.0) | 1 (2.1) | |
| Immunological risk | |||
| Regraft | 0 | 0 | |
| No HLA mismatch (mean±SD) | 2.93±0.89 (3) | 2.92±0.96 (3) | p=0.856 (mw) |
| Pre-transplant PRA class I (mean±SD) | 0.29±2.22 | 3.13±10.21 | p=0.007 (mw) |
| Pre-transplant PRA class II (mean±SD | 0.040±0.36 | 2.04±9.3 | p=0.005 (mw) |
| Pre-transplant PRA Anti HLA > 80% | 0 | 0 | |
| Immunosuppression | |||
| mTOR early conversion | 64 (76.2) | 38 (79.2) | p=0.829 |
| Days later mTOR conversion | 132 (18–264) | 119 (27–207) | p=0.763 |
| CMV serostatus (n, %) | p=0.521 | ||
| D+/R+ | 74 (88.1) | 44 (91.7) | |
| D−/R+ | 10 (11.9) | 4 (8.3) | |
| Donor (n, %) | p=0.050 (e) | ||
| Living donor | 8 (9.5) | 0 (0) | |
| Deceased donor | 77 (90.5) | 48 (100) | |
| Donor age | 34.3±13.0 (30) | 37.4±13.1 (35) | p=0.165 (mw) |
| Cold ischemia time | 12.46±4.99 (12) | 13.81± 6.02 (12.5) | p=0.319 (mw) |
| Mortality | 3 (6.2%) | 1 (1.2%) | p=0.136 |
| Death-censored graft survival | 0 | 0 | p=1.000 |
rATG: rabbit anti-thymocyte globulin; e: Exact Fisher's test; mw: non-parametric Mann–Whitney U test.
Four patients developed CMV disease. Three (3.6%) were in the basiliximab group (one patient with pancreatitis and two with colitis), and one (2.1%) was in the thymoglobulin group (pancreatitis); the latter died as a consequence of the disease (RR=1.71; 95% CI: 0.18–16.03; p=0.538, Fisher's exact test). All of these cases developed within 90 days post-transplantation, and no significant difference was observed between the groups. CMV syndrome or late infection was not identified beyond 90 days post-transplantation.
ViremiaThe incidence was significantly higher in the thymoglobulin cohort (85.4%) relative to the basiliximab cohort (27.4%; RR=3.120; 95% CI: 2.160–4.504; OR=15.534, IC 95%: 6.104, 39.532, p<0.001, Pearson's chi-squared test).
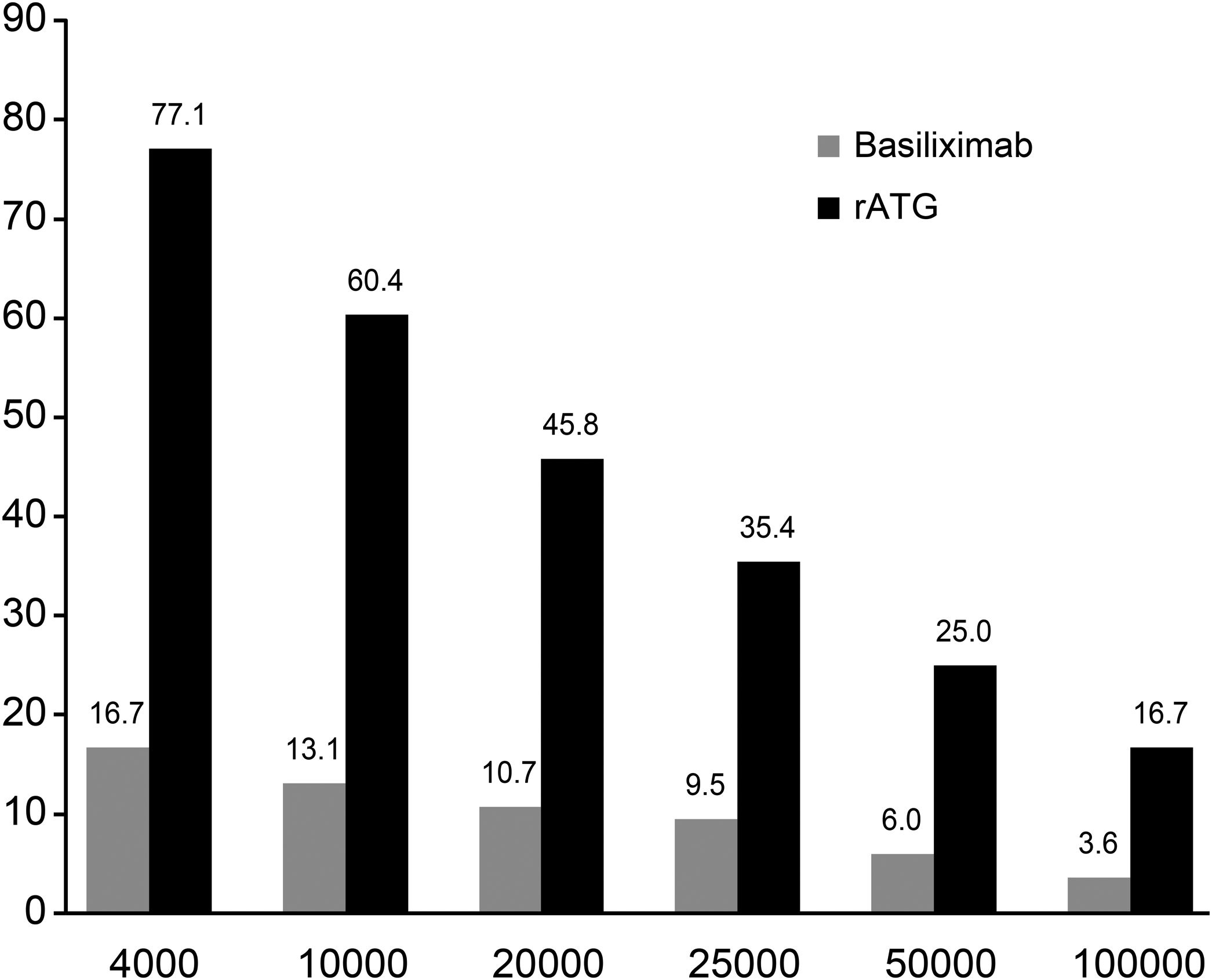
The incidence of CMV infection (viremia >4000copies) was higher in the thymoglobulin group than the basiliximab group (77.1% vs. 16.7%; p<0.001).
At different cut-off points, significant differences were observed between the cohorts; specifically, the incidence rates were significantly higher in the thymoglobulin cohort than in the basiliximab cohort (4000, p<0.001; 10,000, p<0.001; 20,000, p<0.001; 25,000, p<0.001; 50,000, p=0.002 and 100,000, p=0.018, Fisher's exact test; Fig. 2).
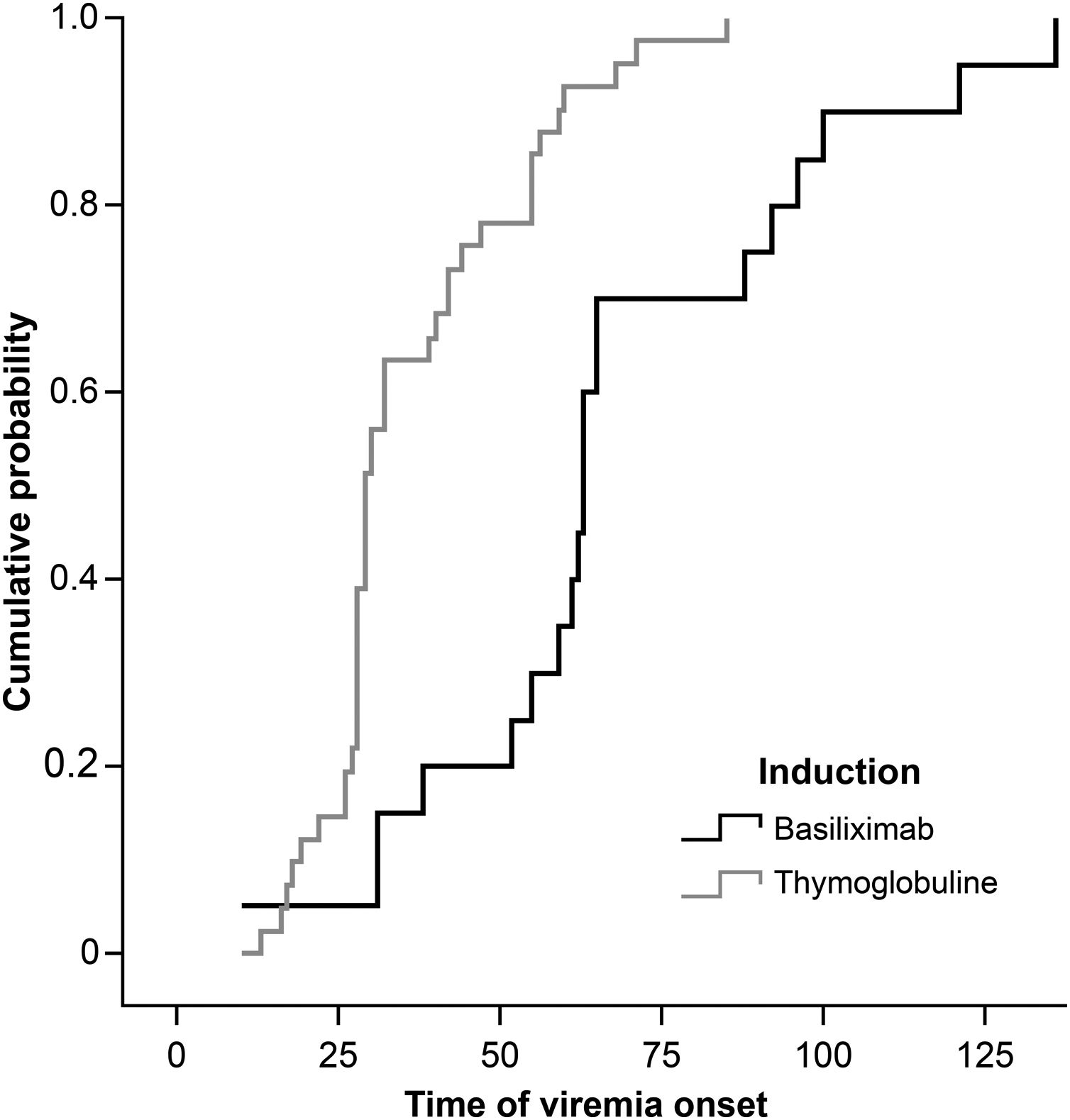
The viremia-free intervals were 51.45± 26.65 days (median, 60 days) in the basiliximab group and 36.56±16.36 days (median, 29 days) in the thymoglobulin group (Fig. 3). The treatment times were similar in both groups (basiliximab, 28.30±13.48 days (median, 26 days) and thymoglobulin, 27.57±17.90 days (median, 26 days)).
The median viremia clearance time, calculated as the number of days between the initial viremia detection (viremia >4000copies) and the first negative viremia test, did not differ between the groups (basiliximab, 32.0 days; 95% CI: 25.3–38.7 and thymoglobulin, 35.0 days; 95% CI: 22.8–47.2).
The analysis found no association between viremia and clinical rejection and did not show a confusion effect between induction therapy and positive viremia (OR adjusted 15.139, IC 95%: 5.936,38,607).
The thymoglobulin dose was higher in patients with viremia than in the patients without viremia (3.6±1.09mg versus 3.08±0.46mg; p=0.0115, Student's T for heterogeneous variances). 17/48 (35.4%) and 2/48 (4.2%) received three and four doses respectively due mainly to long ischemic times.
Positive viremia was higher in the IgG+Donor population but showed no confusion effect between induction therapy and positive viremia (OR adjusted 15.914, IC 95%: 6.154,41.155), although in the IgG+donor population, the association was significant (p<0.001).
Protocol adherenceThe number of days between the initial positive viremia test (viremia >4000copies) and the treatment initiation date was similar in the two groups (basiliximab, 7.40±6.47 days (median, 7 days) and thymoglobulin, 5.67±4.18 days (median, 5.0 days)).
The proportions of PCR samples not processed according to the protocol were 22.7% in the basiliximab group and 31.9% in the thymoglobulin group (p=0.276, Pearson's chi-squared test).
Renal functionThere was no significant difference between the cohorts in terms of renal function at 12 months post-transplant (basiliximab, 70.84±25.62ml/min vs. thymoglobulin, 73.96±19.17ml/min; p=0.475, Student's T-test). Additionally, no significant difference was observed between patients with and without viremia (71.92±22.41ml/min vs. 71.95±24.66ml/min; p=0.994).
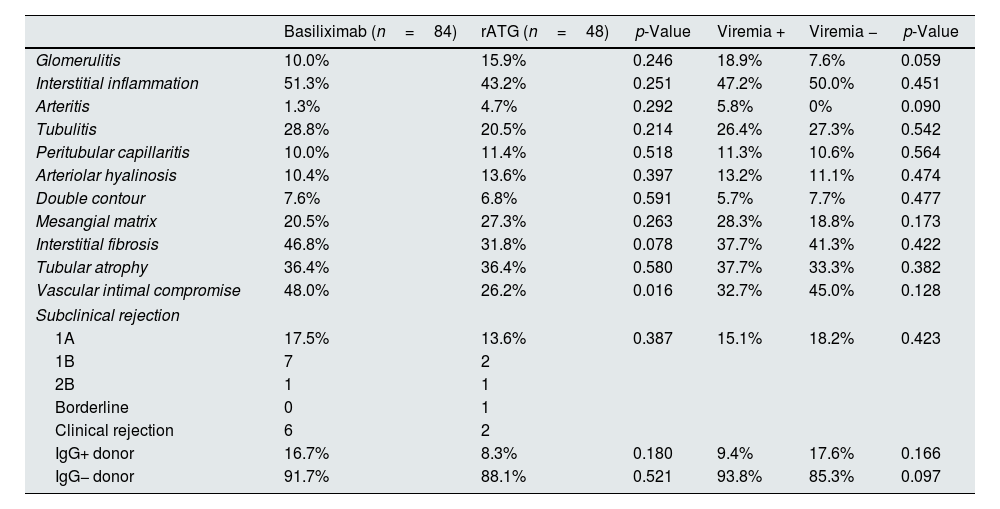
Histological outcomesA protocol biopsy was performed in 95% of the patients; the remaining 5% included four patients under 15 years of age for whom there was no biopsy protocol and two patients who died within the first three months post-transplantation. There were no indications of differences with respect to the subclinical rejection incidence according to the type of induction or the presence of viremia. There were no significant differences in the renal biopsy histological findings, except for compromised vascular intima and a similar but greater difference with respect to interstitial fibrosis among basiliximab patients. No histological differences were observed corresponding to the presence or absence of viremia, although the incidence rates of glomerulitis and arteritis were nearly significantly higher among patients with viremia. No differences were observed in the CADI index with respect to the induction therapy (basiliximab, 2.65±2.42 (median, 2) vs. thymoglobulin, 2.02±1.91 (median, 1.5); p=0.110) or the presence or absence of viremia (2.29±2.07 (median, 2) vs. 2.48±2.41 (median, 2); p=0.298) (Table 2).
Histological and clinical markers according to the induction type and presence or absence of viremia.
| Basiliximab (n=84) | rATG (n=48) | p-Value | Viremia + | Viremia − | p-Value | |
|---|---|---|---|---|---|---|
| Glomerulitis | 10.0% | 15.9% | 0.246 | 18.9% | 7.6% | 0.059 |
| Interstitial inflammation | 51.3% | 43.2% | 0.251 | 47.2% | 50.0% | 0.451 |
| Arteritis | 1.3% | 4.7% | 0.292 | 5.8% | 0% | 0.090 |
| Tubulitis | 28.8% | 20.5% | 0.214 | 26.4% | 27.3% | 0.542 |
| Peritubular capillaritis | 10.0% | 11.4% | 0.518 | 11.3% | 10.6% | 0.564 |
| Arteriolar hyalinosis | 10.4% | 13.6% | 0.397 | 13.2% | 11.1% | 0.474 |
| Double contour | 7.6% | 6.8% | 0.591 | 5.7% | 7.7% | 0.477 |
| Mesangial matrix | 20.5% | 27.3% | 0.263 | 28.3% | 18.8% | 0.173 |
| Interstitial fibrosis | 46.8% | 31.8% | 0.078 | 37.7% | 41.3% | 0.422 |
| Tubular atrophy | 36.4% | 36.4% | 0.580 | 37.7% | 33.3% | 0.382 |
| Vascular intimal compromise | 48.0% | 26.2% | 0.016 | 32.7% | 45.0% | 0.128 |
| Subclinical rejection | ||||||
| 1A | 17.5% | 13.6% | 0.387 | 15.1% | 18.2% | 0.423 |
| 1B | 7 | 2 | ||||
| 2B | 1 | 1 | ||||
| Borderline | 0 | 1 | ||||
| Clinical rejection | 6 | 2 | ||||
| IgG+ donor | 16.7% | 8.3% | 0.180 | 9.4% | 17.6% | 0.166 |
| IgG− donor | 91.7% | 88.1% | 0.521 | 93.8% | 85.3% | 0.097 |
rATG: rabbit anti-thymocyte globulin.
Fourteen of 36 patients (38, 9%) in the thymoglobulin group who were treated with valganciclovir until achieving negative viral load results presented with replication at a later time point and therefore required another round of treatment.
Renal graft and patient survivalThere was no significant difference in mortality. Three patients in the thymoglobulin group died because of pulmonary embolism, sudden death, and CMV-related pancreatitis, in which early replication at day 16 post-transplant and replication at 30 days after receiving negative viral load results had been documented. In the basiliximab group, one patient died because of pulmonary aspergillosis. There were no cases of death-censored graft losses.
DiscussionOur study provides the first preemptive strategy-based analysis of seropositive recipients with a comparison of two different induction strategies.
This retrospective analysis found that preemptive therapy via two different induction strategies appeared to have a significant impact in the prevention of CMV disease in CMV R+ renal transplantation recipients (3.6% in the basiliximab group and 2.1% in the thymoglobulin group). These results were similar to the recent report of a study of 1239 patients from a multicenter prospective cohort in Switzerland; a 4.4% incidence of disease onset was observed after implementing a preemptive strategy in CMV seropositive transplant recipients; however, the Swiss study did not compare the outcomes associated with different induction modalities.22
Some guidelines recommend that all renal transplantation recipients, except in cases of CMV D−/R−, receive antiviral prophylaxis treatment.23–25 There is evidence regarding the efficiency of antiviral prophylaxis among CMV R+ recipients compared to placebo/no treatment26; however, only recently has the efficiency of a preemptive strategy been compared with the efficiency of antiviral prophylaxis in CMV R+.27–29 In a retrospective study, Couzi and collaborators highlighted that preemptive therapy and universal prophylaxis were equally effective at preventing CMV disease onset in CMV R+ transplant recipients who received thymoglobulin induction.27 In contrast; Witzke, in a prospective randomized clinical trial, compared the efficiencies of preemptive therapy and universal prophylaxis in seropositive recipients. They found that CMV disease was significantly higher in recipients receiving the preemptive strategy (12.7% vs. 4.1%; p=0.01). This was a higher incidence compared to other studies. Interestingly, only 3% of the patients received induction with thymoglobulin.28 Weclawiak conducted a retrospective study to evaluate the efficiency of preemptive ganciclovir therapy vs valganciclovir prophylaxis in CMV R+ patients who received different induction treatments (52% basiliximab and 25% thymoglobulin; preemptive group) and found that prophylactic treatment significantly reduced the risk of CMV disease (9.8% vs. 2.68%; p<0.021).29 The increased incidence of CMV disease in patients receiving preemptive therapy in these previous studies has been attributed to the two- to four-week follow-up intervals during the critical period from the first to fourth month; in contrast, our study showed a low incidence of CMV disease onset with the same frequency of follow-up. This may be explained by the difference in protocol involving early conversion to mTOR inhibitors, guided by the implemented protocol biopsy in our center; affecting 77.2% of patients; 64/84 (76.3%) and 38/48 (79.2%) basiliximab and thymoglobulin respectively. Recently, Andrassy et al. performed a meta-analysis and systematic review and concluded that mTOR inhibitor treatment significantly reduced the incidence of CMV after organ transplantation30 and that such treatment might similarly reduce the infection severity in renal transplantation recipients.31
Our study did not find statistically significant differences in the incidence of disease onset between the basiliximab and thymoglobulin cohorts, despite previous reports claiming that the use of thymoglobulin was associated with up to a five-fold increase in the CMV disease onset1,32 compared to lower disease onset in patients receiving basiliximab induction.19,33 However, Khoury, in a single-center study, demonstrated that both preemptive and prophylactic strategies may effectively reduce symptomatic CMV reactivation in patients receiving thymoglobulin as the induction therapy.34 It is possible that the lower incidence of CMV disease onset in the thymoglobulin group may have resulted from use of low doses thymoglobulin, in our experience. The higher incidence of acute rejection episodes and increased use of immunosuppression in the basiliximab group may have been associated.
Regarding the risk of acute renal graft rejection, certain authors have suggested that the post-transplant viremia observed in patients subjected to a preemptive strategy is associated with a higher alloreactive immune response that might be responsible for the higher risk of acute renal graft rejection, chronic rejection, graft loss and mortality.35–44 These data are controversial and have not been verified in systematic comparisons of antiviral prophylactic and preemptive strategies.41 Our findings are supported by a higher level of validity; 95.5% of the patients were subjected to protocol biopsies, which revealed reduced subclinical rejection rates among patients induced basiliximab vs thymoglobulin (18% vs 14% p 0.387) and viremic patients vs non-viremic patients (15% vs 18% p 0.423). Similarly, Atabani identified equal numbers of viremic and non-viremic patients with biopsy-proven rejection (21/158 vs. 19/210) within three months post-transplant; although in most cases, the rejection was detected prior to viremia onset (17/21, 81%).45 In our experience, 92% of the biopsies were performed after viremia onset. An observational study performed by Sagedal and collaborators reported an association between asymptomatic viremia and CMV disease onset along with a higher risk of acute renal graft rejection, although only 38% of the rejections were biopsy-confirmed.35 In contrast to the aforementioned study, no clear association was found between viremia and acute rejection in our cohort, even when utilizing thymoglobulin induction therapy; viremia was early onset and was more intense than was observed in the basiliximab cohort.
In line with recent publications,19,46 we did not find a negative impact on mortality and graft survival within 12 months post-transplant when patients with or without viremia were compared. Reischig, performed a long-term comparison of antiviral prophylaxis and preemptive strategies in a single-center, randomized, open clinical trial and found that the cumulative incidence of viremia at 36 months was significantly higher in the preemptive strategy group than in the antiviral prophylaxis group (92% vs. 59%; p<0.001); despite these results, the patient survival rates were similar in both groups. The four-year graft survival rate was significantly higher in the preemptive strategy group (92% vs. 74%; p=0.049), suggesting a possible negative impact of late viral replication in patients who received antiviral prophylaxis.47 However, Kliem reported other findings from a randomized clinical trial that compared antiviral prophylactic and preemptive strategies found that the graft survival rate was significantly lower in the preemptive strategy group (9% vs. 25% in the prophylactic group; p=0.00117).42 For our cohort, we plan on continuing the long-term follow-up to determine the existence of a negative impact in the patients who presented with asymptomatic viremia.
In contrast to reports by Reischig and collaborators, describing an increased incidence of interstitial fibrosis and tubular atrophy in protocol biopsies collected three months post-transplant in patients receiving a preemptive strategy vs. patients receiving antiviral prophylaxis,48 our study did not find differences in the chronicity indexes. There was a tendency toward a higher incidence of glomerulitis in the group presenting with viremia compared to the group without viremia. Although this difference did not reach statistical significance, it warrants long-term historically follow up to determine if a difference exits.
Preemptive therapy has been associated with lower drug costs and fewer side effects; however, there are also different limitations such as difficulties in technique standardization, consequent absence of a validated cut-off point that signals therapy initiation due to variations in test performance, trial design, and study population diversity,48 and the implementation of a strict protocol. First; in our cohort the cut-off point was 4000copies, although four patients with CMV disease onset presented viral loads exceeding 50,000copies, suggesting the possibility of introducing higher cut-off points for therapy initiation. In this case, a kinetic evaluation of replication according to the different immunosuppression protocols is important. Second, the study conducted at our transplant center underscored the relevance of establishing strict protocols, follow-up systems, surveillance and adherence strategies that aim to ensure compliance. By monitoring the viral load, initiating early therapy once infection has been detected, we have provided evidence that approximately 77% and 68% of patients in the basiliximab and thymoglobulin cohorts complied with the viral load schedules. The average time intervals between viremia detection and treatment initiation were 7 days in the basiliximab group and 6 days in the thymoglobulin group; these delays represented logistical issues with respect to patient compliance and were related to the geographical locations of their homes, socioeconomic situations, or barriers in local administrative processes with respect to medication dispensation. Consequently the preemptive strategy was effective for the prevention of CMV infection and disease onset, it continues to require strict and coordinated processes between the transplant centers and other institutions, this requirement has been confirmed by others.49
The short follow-up duration did not allow assessment of the impact of viral replication on graft survival, patient survival and histological changes; however, our study suggest that preemptive therapy can effectively prevent CMV disease onset during the first year post-transplant without affecting subclinical or acute rejection under basiliximab induction. The high incidence rates of CMV infection and early onset and the high post-treatment replication rate in the thymoglobulin group, despite the use of low doses, suggests that universal prophylaxis might be a better strategy in this population; in contrast, the preemptive strategy might be more cost-effective among patients receiving basiliximab.
The results obtained using different induction treatments (basiliximab and thymoglobulin) in CMV R+ recipients exhibited differences with respect to the CMV infection incidence and time to viremia onset. Even at low doses of thymoglobulin (thymoglobulin dose was higher in patients with viremia than in the patients without viremia), this result explains why the influences of different induction schemes on solid-organ transplants should be evaluated much more stringently to identify preventive behaviors based on different immunosuppression regimens.
Finally, anecdotal observations have indicated that transplant centers use different protocols for handling post-transplant CMV that are generally not sufficiently rigorous; Le Page recently performed an international multi-center study to evaluate the CMV handling strategies in accordance with the published guidelines and provided evidence that 26% of the respondents reported no implementation of preventive measures50; which should change.
Different limitations are found, between these a higher frequency of samples in basiliximab group expecting less risk CMV reactivation which could lead to greater number of cases in thymoglobulin group and follow up until week 16 due to limited resources.
FundingFunding for this research was provided in part by Roche. Dr. Montero and Dr. Torres have received research support from Roche, Bristol-Myers Squibb, Pfizer, Novartis, and Janssen and participated in Speaker's Bureaus for Wyeth, Novartis, and Roche.
Conflict of interestsThe authors declare that they have no conflict of interest.
Qadri Irfan, Alquadan Kawther and Montero Paola.