Acute kidney injury (AKI) is frequent in hospitalized patients and contributes to adverse short- and long-term outcomes. We aimed to evaluate the association of AKI and long-term adverse renal events and mortality in a cohort of patients hospitalized with COVID-19.
Material and methodsSingle-center and retrospective study of hospitalized patients admitted to a Dedicated Unit for COVID-19 at Centro Hospitalar Universitário Lisboa Norte, Portugal, between March 2020 and October 2020. AKI was defined and classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) classification, using SCr criteria. The analyzed outcomes were development of major adverse kidney events (MAKE), major adverse renal cardiovascular events (MARCE), and mortality over a two-year follow-up period.
ResultsFrom the included 409 patients, AKI occurred in 60.4% (n=247). Within two years after discharge, 31.8% (n=130) of patients had an eGFR<60mL/min/1.73m2 and/or a 25% decrease on eGFR and 1.7% (n=7) of patients required RRT, 6.1% (n=25) of patients had CV events and 27.9% (n=114) of patients died. The incidence of MAKE was 60.9% (n=249), and MARCE was 62.6% (n=256). On a multivariate analysis, older age (adjusted HR 1.02 (95% CI: 1.01–1.04), p=0.008), cardiovascular disease (adjusted HR 2.22 (95% CI: 1.24–3.95), p=0.007), chronic kidney disease (adjusted HR 5.15 (95% CI: 2.22–11.93), p<0.001), and AKI (adjusted HR 1.76 (95% CI: 1.12–2.78), p=0.015) were independent predictors of MAKE. Older age (adjusted HR 1.06 (95% CI: 1.04–1.08), p<0.001) and neoplasia (adjusted HR 4.88 (95% CI: 2.37–10.04), p<0.001) were independent predictors of mortality.
ConclusionsIn this cohort of hospitalized patients with COVID-19, AKI was independently associated with the risk of long-term need for dialysis and/or renal function decline and/or mortality after hospital discharge.
La insuficiencia renal aguda (IRA) es frecuente en los pacientes hospitalizados, y contribuye a resultados adversos a corto y a largo plazo. Nuestro objetivo fue evaluar la asociación entre la IRA y los episodios renales adversos a largo plazo y la mortalidad en una cohorte de pacientes hospitalizados con COVID-19.
Material y métodosEstudio unicéntrico y retrospectivo de pacientes hospitalizados ingresados en una unidad especializada en COVID-19 en el Centro Hospitalar Universitário Lisboa Norte, Portugal, entre marzo y octubre de 2020. La IRA se definió y clasificó de acuerdo con la escala Kidney Disease: Improving Global Outcomes (KDIGO) utilizando los criterios SCr. Los resultados analizados fueron el desarrollo de episodios Major Adverse Kidney Events (MAKE), Major Adverse Renal Cardiovascular Events (MARCE) y la mortalidad a lo largo de un periodo de seguimiento de dos años.
ResultadosEn los 409 pacientes incluidos, se produjo IRA en el 60,4% (n=247) de ellos. En un periodo de dos años desde la recepción del alta, el 31,8% (n=130) de los pacientes tuvo un índice eGFR <60ml/min/1,73m2 y/o una reducción de dicho eGFR del 25%, requiriendo TRR el 1,7% (n=7) de los pacientes. El 6,1% (n=25) de los pacientes tuvieron episodios CV, falleciendo el 27,9% (n=114) de los mismos. La incidencia de MAKE fue del 60,9% (n=249), y la de MARCE, del 62,6% (n=256). En un análisis multivariante, la edad avanzada (HR ajustado 1,02 [IC95%: 1,01-1,04], p=0,008), la enfermedad cardiovascular (HR ajustado 2,22 [IC95%: 1,24-3,95], p=0,007), la enfermedad renal crónica (HR ajustado 5,15 [IC95%: 2,22-11,93], p<0,001) y la IRA (HR ajustado 1,76 [IC95%: 1,12-2,78], p=0,015) fueron factores predictivos independientes de MAKE. La edad avanzada (HR ajustado 1,06 [IC95%: 1,04-1,08], p<0,001) y la neoplasia (HR ajustado 4,88 [IC95%: 2,37-10,04], p<0,001) fueron factores predictivos independientes de la mortalidad.
ConclusionesEn esta cohorte de pacientes hospitalizados con COVID-19, la IRA estuvo asociada de manera independiente al riesgo de necesidad de diálisis a largo plazo y/o a la reducción de la función renal y/o mortalidad tras el alta hospitalaria.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was responsible for a global pandemic affecting 769 million people worldwide, as of August 2023.1 Acute kidney injury (AKI) was reported in 30–50% of hospitalized patients with COVID-19, and nearly 20% required dialysis.2 COVID-19 patients with AKI had more severe disease, greater requirement of dialysis, in-hospital mortality, and less kidney recovery at discharge.3,4
AKI in patients with COVID-19 has a complex and multifactorial pathogenesis including endothelial dysfunction, complement activation, coagulopathy, and systemic inflammation. AKI is known to contribute to adverse short- and long-term outcomes, namely mortality, cardiovascular events and incident or progressive chronic kidney disease (CKD).5 Additionally, 10–20% of people with COVID-19 experience post-COVID-19 conditions, defined as a syndrome of persistent symptoms after infection. Post-COVID-19 can cause multi-organ damages with a wide spectrum of manifestations. Indeed, COVID-19-associated AKI has been linked with worse renal outcomes within the first months after discharge.6
At 3 months of follow-up, 16% of 313 AKI critically ill patients with COVID-19 developed CKD, and in survivors who had no renal recovery at discharge, the incidence of CKD was 44%.7
Lu et al. reported a higher incidence of dialysis dependence, death or eGFR decline ≥25% and cardiovascular events at 90 days of follow-up (p<0.05) in a cohort of 3296 COVID-19 patients with AKI.8 Salgueira et al. in 703 Spanish patients with COVID-19 described a more severe pattern of late onset AKI with greater need for renal replacement therapy (RRT).9
Also, in a cohort of 1612 patients, AKI associated with COVID-19 was associated with a greater decline of estimated glomerular filtration rate compared with AKI due to other causes, independent of underlying comorbidities or AKI severity (−16.7 (95% CI −43.4 to 10.0) vs −2.7mL/min/1.73m2/year (95% CI −26.8 to 21.4), p=0.01) on the first 6 months after hospital discharge.10
Whether this is associated with greater severity of AKI, or if SARS CoV2 infection has a direct effect on the kidney function decline is unknown and the long-term impact of COVID-19 in the kidney remains to be determined.
The purpose of this study was to evaluate the association of AKI and adverse renal events and mortality on a long-term follow-up in hospitalized patients with COVID-19.
MethodsWe performed a single-center and retrospective study of hospitalized patients admitted to a Dedicated Unit for COVID-19 patients (UICIVE) at the Department of Medicine of the Centro Hospitalar Universitário Lisboa Norte (CHULN), in Lisbon, Portugal, between March 2020 and October 2020. Data collection was performed in March 2023.
The Ethical Committee approved of this study, in agreement with institutional guidelines and informed consent was waived, given its retrospective and non-interventional nature.
PatientsWe selected as eligible all adult patients (≥18 years of age) with hospital discharge after being admitted to the UICIVE with a positive polymerase chain reaction (PCR) test of a nasopharyngeal sample for COVID-19 from March 2020 to October 2020.
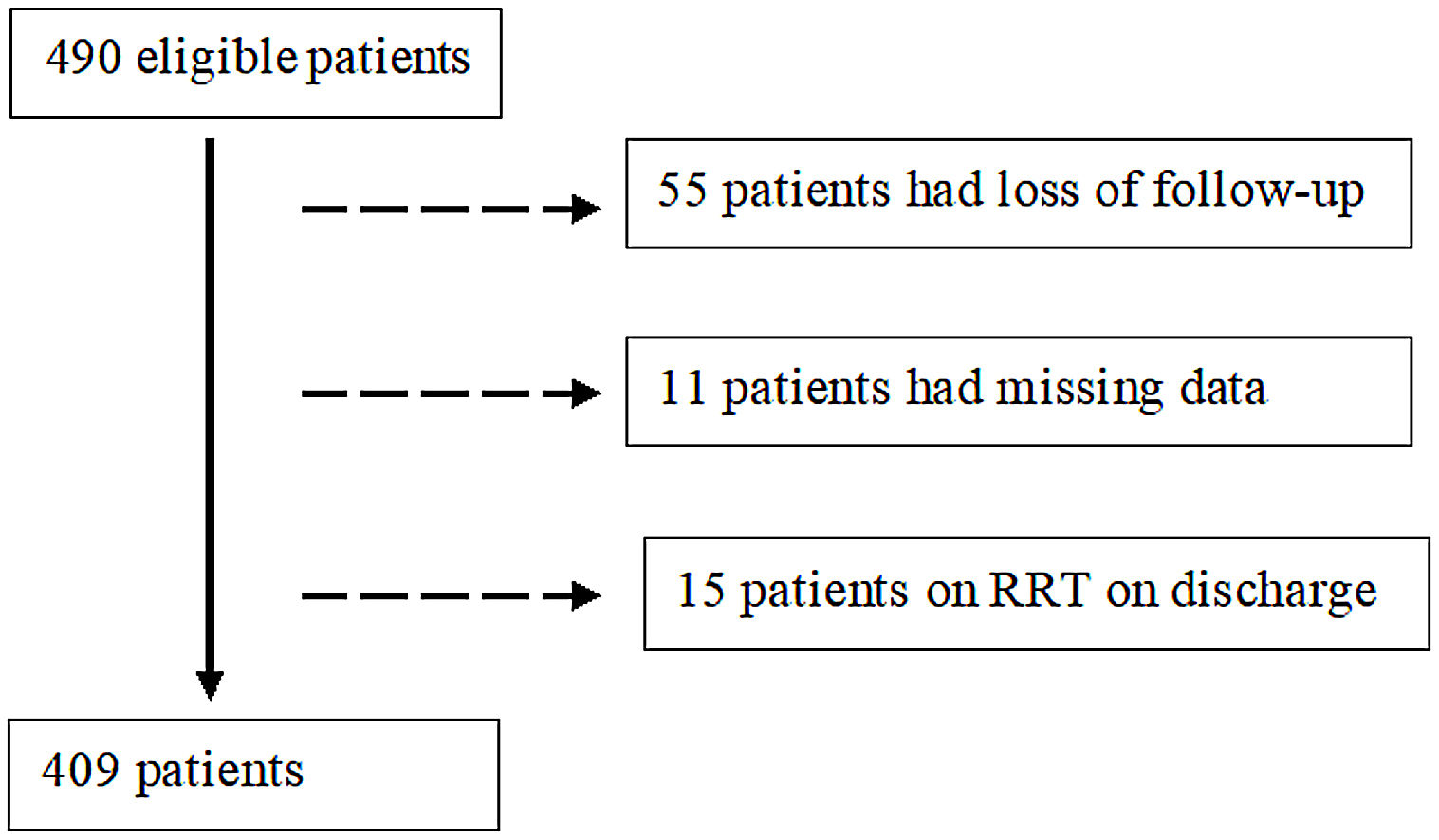
Exclusion criteria comprised (a) loss to follow-up, (b) missing data and (c) RRT on discharge.
VariablesData was collected from individual electronic clinical records. The analyzed variables included patient demographic characteristics (age, gender and ethnicity); comorbidities [hypertension, diabetes mellitus, cardiovascular disease (CVD), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), cirrhosis, and/or active malignancy]; baseline serum creatinine (SCr); laboratory values at admission [SCr, hemoglobin, serum albumin and C reactive protein (CRP)]; need for intensive care unit (UCI) admission; need for mechanical ventilation; vasopressor use; renal replacement therapy (RRT) requirement during hospitalization; SCr and RRT requirement on discharge; laboratory values at the last follow-up [SCr and estimated glomerular filtration rate (eGFR)] and RRT requirement at the last follow-up.
AKI was defined as indicated on the Kidney Disease: Improving Global Outcomes (KDIGO) classification, using the SCr criteria. Therefore, AKI was defined as an increase in SCr by ≥0.3mg/dl within 48h or an increase in SCr≥1.5 times the baseline value, which is known or presumed to have occurred within the prior 7 days. This classification also categorizes AKI according to its severity: Stage 1: increase in SCr by 0.3mg/dl within 48h or 1.5–1.9 times increase in SCr from baseline within 7 days; stage 2: 2.0–2.9 times increase in SCr from baseline within 7 days; stage 3: 3.0 times increase in SCr from baseline within 7 days or increase in SCr to ≥4.0mg/dl or initiation of RRT.11 Patients were stratified according to the highest AKI stage attained during their hospital stay.
Pre-admission SCr was assessed through centralized medical record and considered as baseline value.
Presence of CKD was estimated according to the baseline SCr as an estimated GFR lower than 60mL/min/1.73m2, present for >3 months.12
CVD was considered whenever a history of cerebrovascular disease, chronic heart failure, cardiac ischemic disease and/or peripheral arterial disease was documented. Diagnosis of hypertension was based on the European Society of Cardiology and European Society of Hypertension (ESC/ESH) Guidelines.13Diabetes mellitus was diagnosed according to the American Diabetes Association criteria.14COPD comprised emphysema and chronic bronchitis.15
OutcomesThe primary outcomes were development of major adverse kidney event (MAKE) and mortality during the follow-up period. MAKE was defined as a composite of death from any cause, RRT dependence or worsened kidney function (decrease in eGFR to <25% of baseline and/or eGFR<60mL/min/1.73m2).16,17 It can be used to assess clinically significant events after an AKI.
We also analyzed the development of cardiovascular events (cerebrovascular disease, heart failure, acute myocardial infarction, arrhythmia and/or pulmonary embolism) and major adverse renal cardiovascular events (MARCE) during the follow-up period. MARCE consists of major adverse kidney event (death, RRT dependence or worsened renal function) and/or major adverse cardiovascular event (myocardial infarction, stroke, and heart failure).18
Statistical analysisCategorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean±standard deviation. Normally distributed continuous variables were compared with the Student's t-test, non-normally distributed continuous variables were compared with the Mann–Whitney U test and categorical variables were compared with the chi-square test. To determine risk factors for MAKE and mortality, univariate and multivariate Cox logistic regression analysis was employed. In the multivariate analysis (enter model), only variables with statistical significance in the univariate analysis were included. Data were expressed as hazard ratios (HR) with 95% confidence intervals. To determine cumulative survival curves, the Kaplan–Meier method was used, using the log-rank test to compare groups. Patients were censored at the last follow-up date if alive. Statistical significance was defined as a p value <0.05. Statistical analysis was performed with the statistical software package SPSS for Windows (version 21.0; SPSS, Chicago, IL).
ResultsParticipantsFour hundred and ninety patients survived the initial hospitalization for COVID-19 and were potentially eligible. Of these, 81 patients were excluded: 55 were lost to follow-up, 11 patients had missing data and 15 patients were on RRT on discharge. Thereby, we focused on a final cohort of 409 patients as shown in Fig. 1.
Our study is a subanalysis of a previously published article about AKI in hospitalized patients with COVID-19, where the patients’ baseline characteristics have been described.19
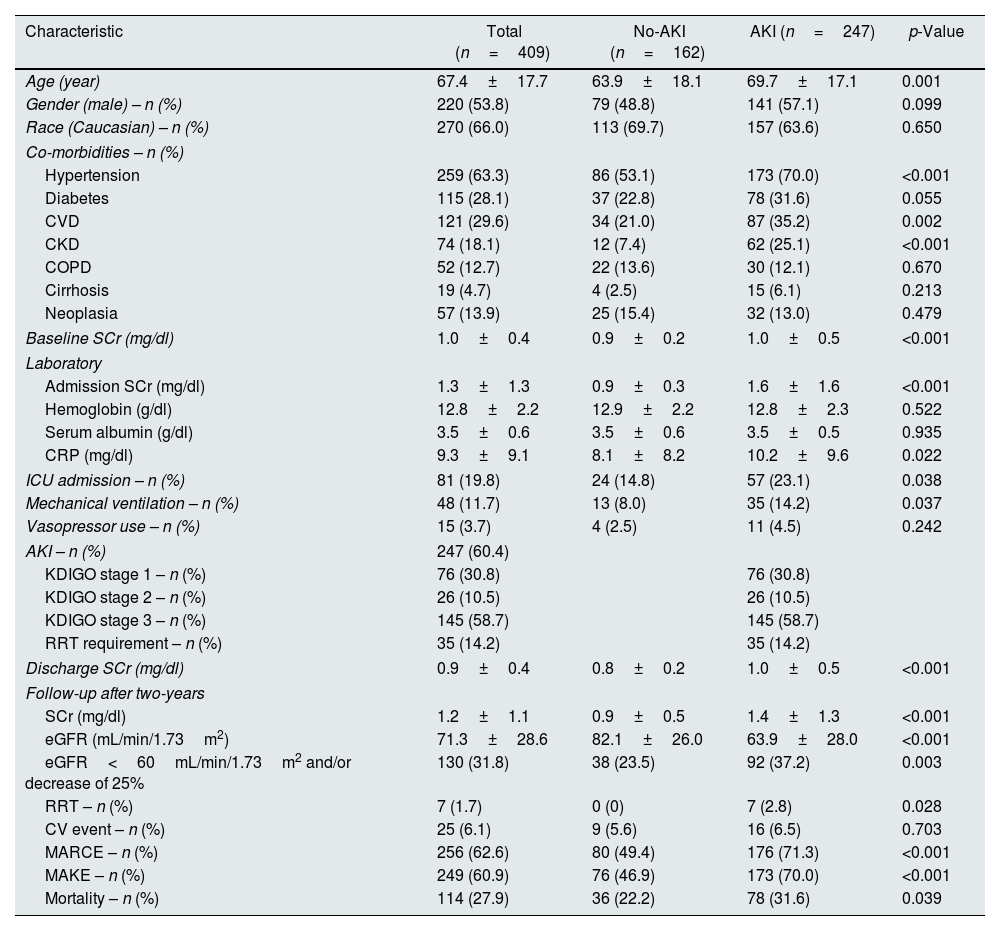
Patients’ baseline characteristics and comparison between the AKI and non-AKI groups are shown in Table 1. Most patients were Caucasian (n=270, 66%), male (n=220, 53.8%) and had a mean age of 67.4±17.7 years. There was a high prevalence of hypertension (n=259, 63.3%), CVD (n=121, 29.6%), diabetes (n=115, 28.1%) and CKD (n=74, 18.1%). Mean baseline SCr was 1.0±0.4mg/dl.
Patients’ baseline characteristics and according to acute kidney injury.
| Characteristic | Total (n=409) | No-AKI (n=162) | AKI (n=247) | p-Value |
|---|---|---|---|---|
| Age (year) | 67.4±17.7 | 63.9±18.1 | 69.7±17.1 | 0.001 |
| Gender (male) – n (%) | 220 (53.8) | 79 (48.8) | 141 (57.1) | 0.099 |
| Race (Caucasian) – n (%) | 270 (66.0) | 113 (69.7) | 157 (63.6) | 0.650 |
| Co-morbidities – n (%) | ||||
| Hypertension | 259 (63.3) | 86 (53.1) | 173 (70.0) | <0.001 |
| Diabetes | 115 (28.1) | 37 (22.8) | 78 (31.6) | 0.055 |
| CVD | 121 (29.6) | 34 (21.0) | 87 (35.2) | 0.002 |
| CKD | 74 (18.1) | 12 (7.4) | 62 (25.1) | <0.001 |
| COPD | 52 (12.7) | 22 (13.6) | 30 (12.1) | 0.670 |
| Cirrhosis | 19 (4.7) | 4 (2.5) | 15 (6.1) | 0.213 |
| Neoplasia | 57 (13.9) | 25 (15.4) | 32 (13.0) | 0.479 |
| Baseline SCr (mg/dl) | 1.0±0.4 | 0.9±0.2 | 1.0±0.5 | <0.001 |
| Laboratory | ||||
| Admission SCr (mg/dl) | 1.3±1.3 | 0.9±0.3 | 1.6±1.6 | <0.001 |
| Hemoglobin (g/dl) | 12.8±2.2 | 12.9±2.2 | 12.8±2.3 | 0.522 |
| Serum albumin (g/dl) | 3.5±0.6 | 3.5±0.6 | 3.5±0.5 | 0.935 |
| CRP (mg/dl) | 9.3±9.1 | 8.1±8.2 | 10.2±9.6 | 0.022 |
| ICU admission – n (%) | 81 (19.8) | 24 (14.8) | 57 (23.1) | 0.038 |
| Mechanical ventilation – n (%) | 48 (11.7) | 13 (8.0) | 35 (14.2) | 0.037 |
| Vasopressor use – n (%) | 15 (3.7) | 4 (2.5) | 11 (4.5) | 0.242 |
| AKI – n (%) | 247 (60.4) | |||
| KDIGO stage 1 – n (%) | 76 (30.8) | 76 (30.8) | ||
| KDIGO stage 2 – n (%) | 26 (10.5) | 26 (10.5) | ||
| KDIGO stage 3 – n (%) | 145 (58.7) | 145 (58.7) | ||
| RRT requirement – n (%) | 35 (14.2) | 35 (14.2) | ||
| Discharge SCr (mg/dl) | 0.9±0.4 | 0.8±0.2 | 1.0±0.5 | <0.001 |
| Follow-up after two-years | ||||
| SCr (mg/dl) | 1.2±1.1 | 0.9±0.5 | 1.4±1.3 | <0.001 |
| eGFR (mL/min/1.73m2) | 71.3±28.6 | 82.1±26.0 | 63.9±28.0 | <0.001 |
| eGFR<60mL/min/1.73m2 and/or decrease of 25% | 130 (31.8) | 38 (23.5) | 92 (37.2) | 0.003 |
| RRT – n (%) | 7 (1.7) | 0 (0) | 7 (2.8) | 0.028 |
| CV event – n (%) | 25 (6.1) | 9 (5.6) | 16 (6.5) | 0.703 |
| MARCE – n (%) | 256 (62.6) | 80 (49.4) | 176 (71.3) | <0.001 |
| MAKE – n (%) | 249 (60.9) | 76 (46.9) | 173 (70.0) | <0.001 |
| Mortality – n (%) | 114 (27.9) | 36 (22.2) | 78 (31.6) | 0.039 |
AKI, acute kidney injury; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; CV, cardiovascular; CVD, cardiovascular disease; GFR, glomerular filtration rate; ICU, intensive care unit; KDIGO, Kidney Disease Improving Global Outcomes; MAKE, major adverse kidney events; MARCE, major adverse renal cardiovascular event; RRT, renal replacement therapy; SCr, serum creatinine.
At admission, SCr was 1.3±1.3mg/dl, hemoglobin was 12.8±2.2g/dl, serum albumin was 3.5±0.6g/dl and CRP was 9.3±9.1mg/dl. Twenty percent (n=81) of patients were admitted to the ICU, 11.7% (n=48) required mechanical ventilation and 3.7% (n=15) received vasopressors.
AKI occurred in 60.4% (n=247) of patients who survived the initial hospitalization. Regarding AKI severity, most patients were KDIGO stage 3 (n=145, 58.7%), followed by KDIGO stage 1 (n=76, 30.8%) and KDIGO stage 2 (n=26, 10.5%). Fourteen percent (n=35) of these patients required RRT. At discharge, mean SCr was 0.9±0.4mg/dl.
Mean follow-up was 19.7±10.5 months (minimum 1 month, maximum 36 months). At the last follow-up, mean SCr was 1.2±1.1mg/dl, eGFR was 71.3±28.6mL/min/1.73m2, 31.8% (n=130) of patients had an eGFR<60mL/min/1.73m2 and/or a 25% decrease on eGFR and 1.7% (n=7) of patients required RRT. The incidence of MAKE was 60.9% (n=249), 6.1% (n=25) of patients had CV events, 62.6% (n=256) experienced MARCE and 27.9% (n=114) of patients died.
AKIPatients with AKI were older (69.7±17.1 vs. 63.9±18.1, p=0.001), more likely to have pre-existing hypertension (70% vs. 53.1%, p<0.001), CVD (35.2% vs. 21%, p=0.002) and CKD (25.1% vs. 7.4%, p<0.001) and had higher SCr (1.6±1.6 vs. 0.9±0.3, p<0.001) and CRP (10.2±9.6 vs. 8.1±8.2, p=0.022) at admission. These patients required more ICU admission (23.1% vs 14.8%, p=0.038) and mechanical ventilation (14.2% vs 8%, p=0.037). At discharge, AKI patients had higher SCr (1.0±0.5 vs. 0.8±0.2, p<0.001).
At the last follow-up, they also had higher SCr (1.4±1.3 vs. 0.9±0.5, p<0.001) and were more likely to be on RRT (2.8% vs. 0%, p=0.028). They experienced more MARCE (71.3% vs. 49.4%, p<0.001) and MAKE (70% vs. 46.9%, p<0.001), and had higher mortality (31.6% vs. 22.2%, p=0.039).
MAKEThe characteristics of the 249 patients with MAKE are described in Table 2.
Characteristics of patients according to MAKE.
| Characteristic | No-MAKE (n=160) | MAKE (n=249) | p-Value |
|---|---|---|---|
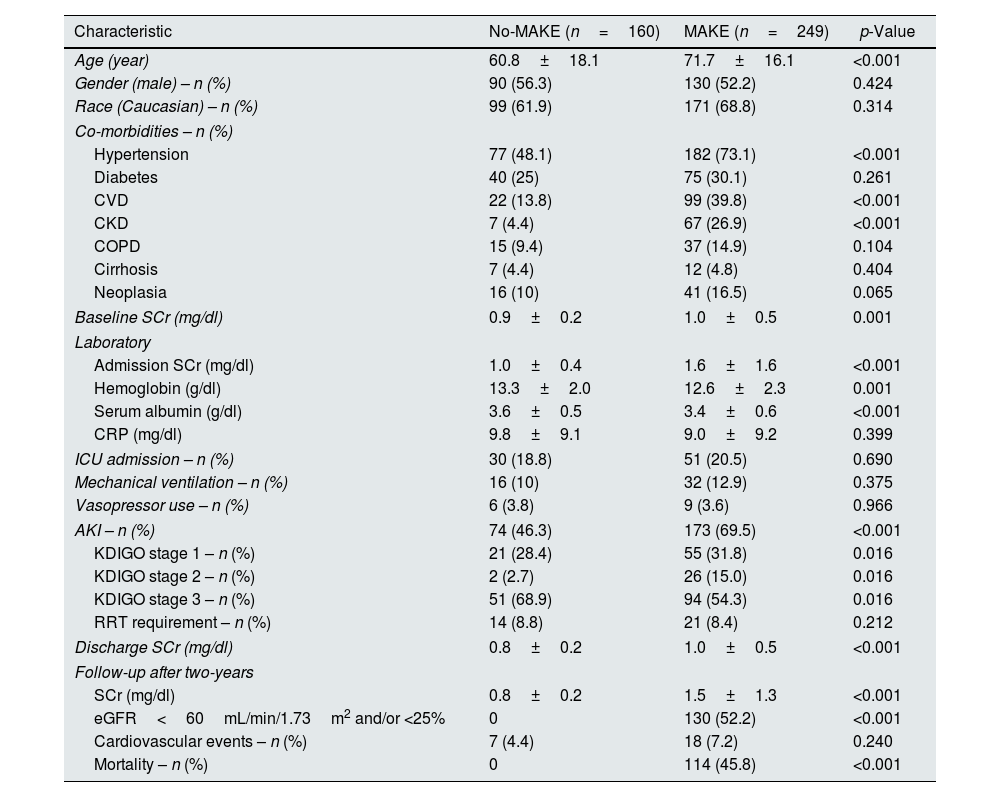
| Age (year) | 60.8±18.1 | 71.7±16.1 | <0.001 |
| Gender (male) – n (%) | 90 (56.3) | 130 (52.2) | 0.424 |
| Race (Caucasian) – n (%) | 99 (61.9) | 171 (68.8) | 0.314 |
| Co-morbidities – n (%) | |||
| Hypertension | 77 (48.1) | 182 (73.1) | <0.001 |
| Diabetes | 40 (25) | 75 (30.1) | 0.261 |
| CVD | 22 (13.8) | 99 (39.8) | <0.001 |
| CKD | 7 (4.4) | 67 (26.9) | <0.001 |
| COPD | 15 (9.4) | 37 (14.9) | 0.104 |
| Cirrhosis | 7 (4.4) | 12 (4.8) | 0.404 |
| Neoplasia | 16 (10) | 41 (16.5) | 0.065 |
| Baseline SCr (mg/dl) | 0.9±0.2 | 1.0±0.5 | 0.001 |
| Laboratory | |||
| Admission SCr (mg/dl) | 1.0±0.4 | 1.6±1.6 | <0.001 |
| Hemoglobin (g/dl) | 13.3±2.0 | 12.6±2.3 | 0.001 |
| Serum albumin (g/dl) | 3.6±0.5 | 3.4±0.6 | <0.001 |
| CRP (mg/dl) | 9.8±9.1 | 9.0±9.2 | 0.399 |
| ICU admission – n (%) | 30 (18.8) | 51 (20.5) | 0.690 |
| Mechanical ventilation – n (%) | 16 (10) | 32 (12.9) | 0.375 |
| Vasopressor use – n (%) | 6 (3.8) | 9 (3.6) | 0.966 |
| AKI – n (%) | 74 (46.3) | 173 (69.5) | <0.001 |
| KDIGO stage 1 – n (%) | 21 (28.4) | 55 (31.8) | 0.016 |
| KDIGO stage 2 – n (%) | 2 (2.7) | 26 (15.0) | 0.016 |
| KDIGO stage 3 – n (%) | 51 (68.9) | 94 (54.3) | 0.016 |
| RRT requirement – n (%) | 14 (8.8) | 21 (8.4) | 0.212 |
| Discharge SCr (mg/dl) | 0.8±0.2 | 1.0±0.5 | <0.001 |
| Follow-up after two-years | |||
| SCr (mg/dl) | 0.8±0.2 | 1.5±1.3 | <0.001 |
| eGFR<60mL/min/1.73m2 and/or <25% | 0 | 130 (52.2) | <0.001 |
| Cardiovascular events – n (%) | 7 (4.4) | 18 (7.2) | 0.240 |
| Mortality – n (%) | 0 | 114 (45.8) | <0.001 |
AKI, acute kidney injury; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; CVD, cardiovascular disease; GFR, glomerular filtration rate; ICU, intensive care unit; MAKE, Major Adverse Kidney Events; MARCE, major adverse renal cardiovascular event; RRT, renal replacement therapy; SCr, serum creatinine.
Patients with MAKE were older (71.7±16.1 vs. 60.8±18.1, p<0.001) and more likely to have pre-existing hypertension (72.5% vs. 48.7%, p<0.001), CVD (39.4% vs. 13.9%, p<0.001) and CKD (26.7% vs 4.4%, p<0.001).
At admission, patients with MAKE had higher SCr (1.6±1.6 vs. 1.0±0.4, p<0.001), lower hemoglobin (12.6±2.3 vs. 13.3±2.0, p=0.001) and lower serum albumin (3.4±0.6 vs. 3.6±0.5, p<0.001).
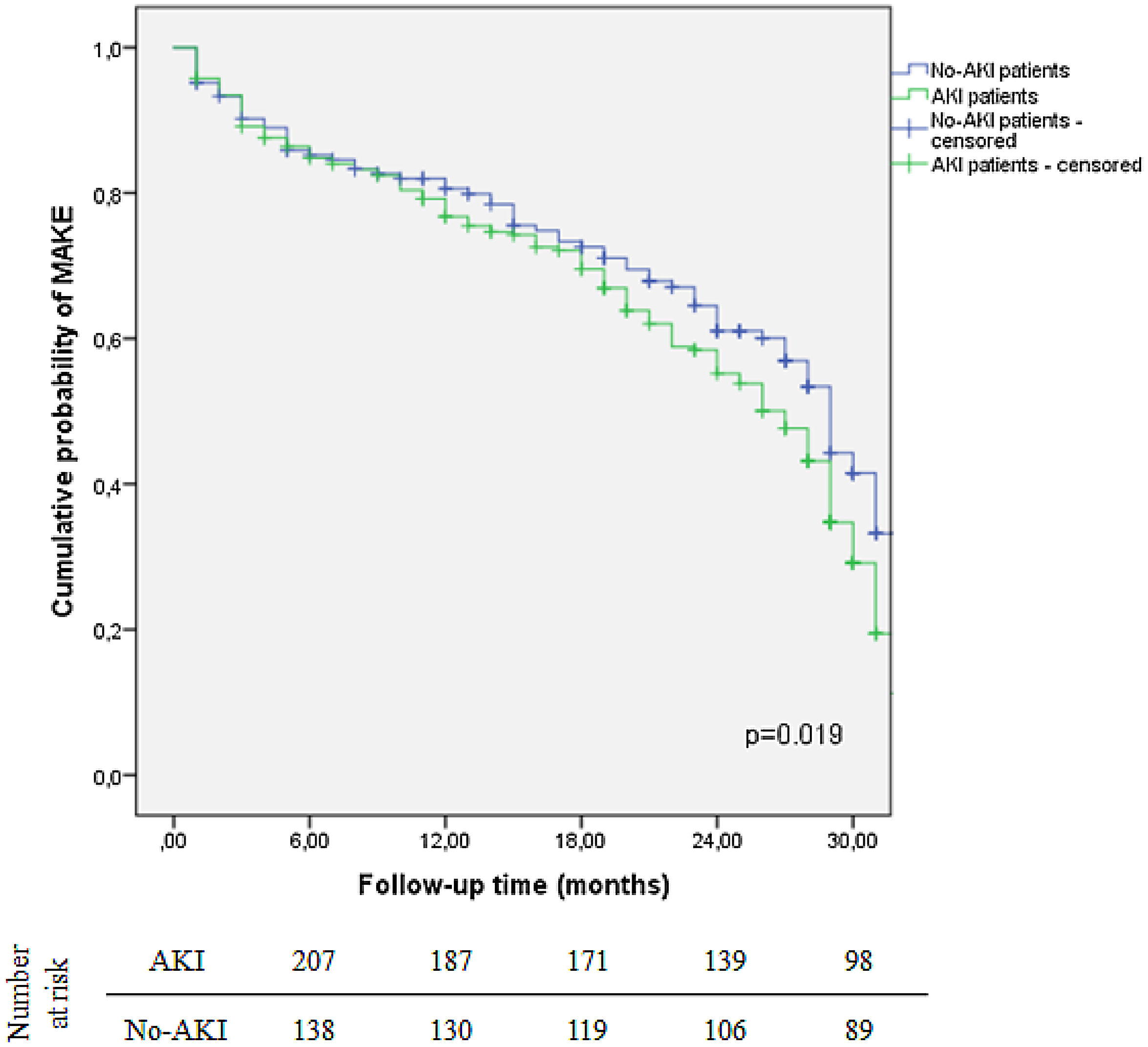
MAKE was more frequent in patients who experienced AKI during hospitalization (68.9% vs. 46.9%, p<0.001). The two-year probability of MAKE was 44.8% for AKI patients, while it was 39% for patients with no AKI (log-rank test, p=0.019) (Fig. 2). Concerning AKI severity, patients who developed MAKE were more likely KDIGO stage 3 (p=0.016). Patients with higher SCr at discharge also had higher risk of MAKE (1.0±0.5 vs. 0.8±0.2, p<0.001).
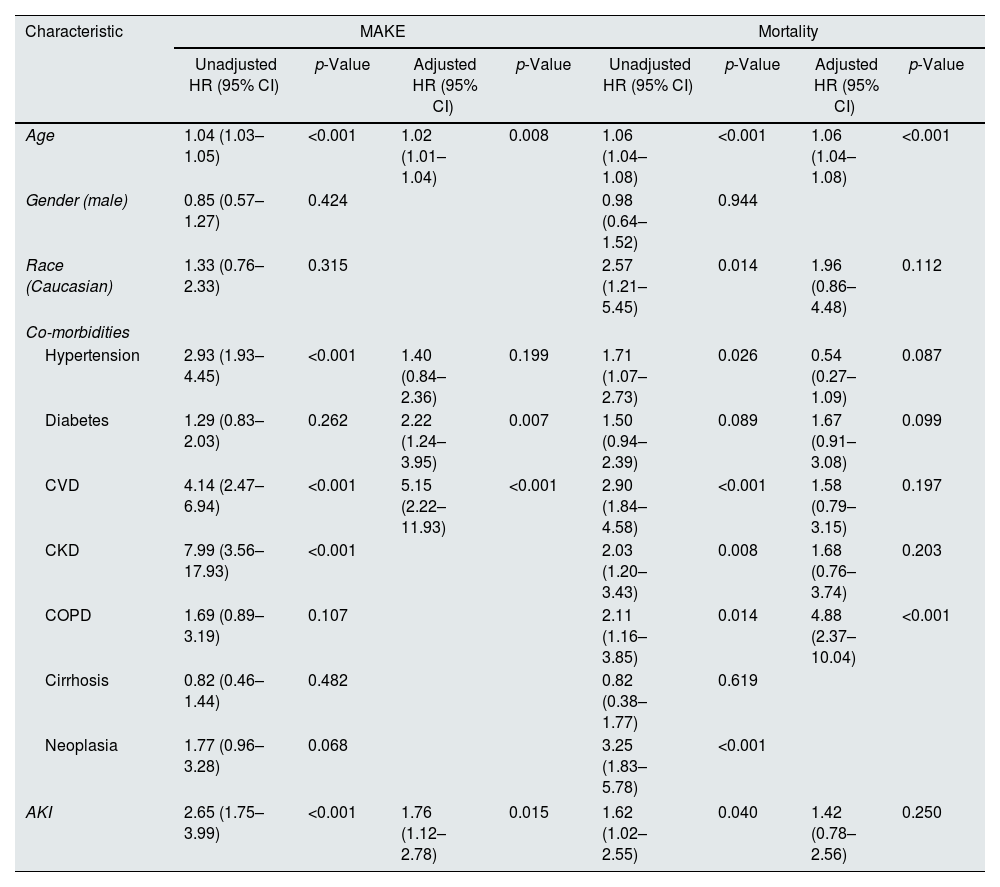
On a multivariate analysis, older age (adjusted HR 1.02 (95% CI: 1.01–1.04), p=0.008), CVD (adjusted HR 2.22 (95% CI: 1.24–3.95), p=0.007), CKD (adjusted HR 5.15 (95% CI: 2.22–11.93), p<0.001), and AKI (adjusted HR 1.76 (95% CI: 1.12–2.78), p=0.015) were independent predictors of MAKE (Table 3).
Univariate and multivariate analysis of factors predictive of MAKE and Mortality in COVID-19 patients.
| Characteristic | MAKE | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
| Age | 1.04 (1.03–1.05) | <0.001 | 1.02 (1.01–1.04) | 0.008 | 1.06 (1.04–1.08) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| Gender (male) | 0.85 (0.57–1.27) | 0.424 | 0.98 (0.64–1.52) | 0.944 | ||||
| Race (Caucasian) | 1.33 (0.76–2.33) | 0.315 | 2.57 (1.21–5.45) | 0.014 | 1.96 (0.86–4.48) | 0.112 | ||
| Co-morbidities | ||||||||
| Hypertension | 2.93 (1.93–4.45) | <0.001 | 1.40 (0.84–2.36) | 0.199 | 1.71 (1.07–2.73) | 0.026 | 0.54 (0.27–1.09) | 0.087 |
| Diabetes | 1.29 (0.83–2.03) | 0.262 | 2.22 (1.24–3.95) | 0.007 | 1.50 (0.94–2.39) | 0.089 | 1.67 (0.91–3.08) | 0.099 |
| CVD | 4.14 (2.47–6.94) | <0.001 | 5.15 (2.22–11.93) | <0.001 | 2.90 (1.84–4.58) | <0.001 | 1.58 (0.79–3.15) | 0.197 |
| CKD | 7.99 (3.56–17.93) | <0.001 | 2.03 (1.20–3.43) | 0.008 | 1.68 (0.76–3.74) | 0.203 | ||
| COPD | 1.69 (0.89–3.19) | 0.107 | 2.11 (1.16–3.85) | 0.014 | 4.88 (2.37–10.04) | <0.001 | ||
| Cirrhosis | 0.82 (0.46–1.44) | 0.482 | 0.82 (0.38–1.77) | 0.619 | ||||
| Neoplasia | 1.77 (0.96–3.28) | 0.068 | 3.25 (1.83–5.78) | <0.001 | ||||
| AKI | 2.65 (1.75–3.99) | <0.001 | 1.76 (1.12–2.78) | 0.015 | 1.62 (1.02–2.55) | 0.040 | 1.42 (0.78–2.56) | 0.250 |
AKI, acute kidney injury; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; MAKE, Major Adverse Kidney Events; SCr, serum creatinine.
On a multivariate analysis, older age (adjusted HR 1.06 (95% CI: 1.04–1.08), p<0.001) and neoplasia (adjusted HR 4.88 (95% CI: 2.37–10.04), p<0.001) were independent predictors of mortality (Table 3).
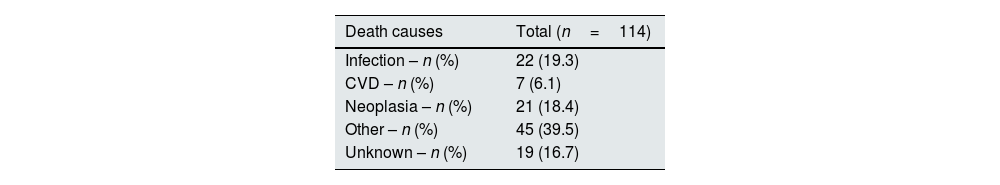
Table 4 shows mortality causes: 19.3% (n=22) of them died due to infection, while 18.4% (n=21) died due to neoplasia and 6.1% (n=7) died due to CVD. Forty percent (n=45) of patients died of other causes. The remaining 16.7% (n=19) died of unknown causes.
DiscussionIn this single-center and retrospective cohort of 409 hospitalized patients with COVID-19, we demonstrated that AKI was independently associated with the risk of long-term need for renal replacement therapy and/or renal function decline and/or mortality after hospital discharge.
In our study, AKI occurred in 60.4% of patients who survived the initial hospitalization and after two-years of follow-up 33.5% had a decline in kidney function or required kidney replacement therapy. This is in line with previously published literature although this cohort has the longest follow-up. Bowe et al. demonstrated that a group of 89,216 30-day survivors of COVID-19 had approximately 60% higher risk of MAKE than a non-infected control group of 1,637,437 patients.20 MAKE was significantly more frequent in patients who experienced AKI as well (70% vs. 46.9%, p<0.001). This is consistent with our cohort's results, as we reported 60.9% MAKE incidence. In fact, the two-year probability of MAKE in our cohort was 44.8% for AKI patients, while it was 39% for patients without AKI. On the other hand, Lu et al. when assessing 90-day MAKE incidence in COVID-19 survivors with AKI was only 22.6%. However, in the sub-group of patients with prolonged recovery of AKI MAKE incidence was significantly higher (22.6% vs 44.3%, p<0.001).21 In a cohort of 2212 patients, Atiquzzaman et al. also have shown the negative impact of COVID-19 infection in kidney function with a 3.4% decrease in eGFR after 1-year follow-up. This decrease is greater than expect for age adjusted decrease.22 Similarly, in our cohort, other than AKI, older age, CVD and CKD were also independent predictors of MAKE.
Regarding AKI severity, most patients who experienced MAKE were KDIGO stage 3, followed by KDIGO stages 1 and 2. These findings are compatible with previously published data which demonstrates the impact of AKI severity on long-term outcomes. Gu et al. reported a higher decrease on eGFR and reduced renal function in those with higher AKI stage.23 In a different study, Wan et al. demonstrated that adverse outcomes were strongly associated with increased severity of AKI.24
It is well known that AKI is a common complication of COVID-19 and has a complex and diverse etiology, being a result of both direct cytopathic effects and indirect mechanisms, such as ischemic injury from hypotension and hypoxemia, exposure to nephrotoxins, rhabdomyolysis, cytokine storm, activation and dysregulation of the angiotensin II pathway and complement system, endothelial dysfunction, abnormal platelet activation, hypercoagulation and microangiopathy.19,25,26
Some of the COVID-19 survivors may experience post-acute manifestations, with multiple system involvement. Studies on post-COVID-19 kidney events are limited in number and in follow-up duration.27,28 Our study adds to the literature by providing outcomes up to 2-years after hospital discharge, helping us understand the post-acute sequelae of COVID-19.
At the same time, it is vital to be aware of the risk of progression from AKI to CKD. Indeed, in our study, patients with AKI were older, had more CVD and CKD, which are some of the risk factors associated with poorer outcomes post-AKI, as well as AKI severity and duration. Another interesting finding of our study is that discharge serum creatinine was not associated with higher risk of kidney events after AKI. This has also been reported in previous studies suggesting that creatinine is an inaccurate marker to assess AKI recovery, reflecting fluid overload and muscle mass loss.29 Data from the NARA-AKI study demonstrated that creatinine and eGFR at a 3-month follow-up were stable or even increased after AKI most likely due to prolonged inflammatory and catabolic state.30 Haredasht et al. also reported that assessing eGFR using Cystatin C could detect more CKD events than creatinine in the follow-up of 101 AKI patients.31 As such, it is fundamental to identify high-risk patients and more precise biomarkers for CKD progression after AKI.
Simultaneously, AKI has been associated with an increased risk of cardiovascular events and short- and long-term mortality as demonstrated in this cohort.5 In a retrospective multicenter observational cohort of 12.891 hospitalized patients with a diagnosis of SARS-CoV-2 infection, Tan et al. reported a 50.5% incidence of AKI, as well as higher 1-year mortality in these patients (32.5% vs. 10.4%).32 In another study, Hadadi et al. demonstrated a 29.3% incidence of AKI. Furthermore, unresolved kidney injury at the time of discharge and AKI stages 2 and 3 were associated with an increased risk of mortality in the 10-month follow-up period.33 In a cohort of 2425 hospitalized patients with influenza and COVID-19, Strobenhn et al. showed a significant higher incidence of AKI and mortality in COVID-19 patients.34 Sullivan et al. in the ISARIC WHO CCP-UK cohort, demonstrated a 31.5% incidence of AKI and also reported greater 28-day mortality rates in these group of patients.35 Our study performed a long-term analysis with a mean of almost 20 months. Whether AKI contributes directly to the risk of mortality or is more frequent in patients with more comorbidities and thus higher mortality risk remains to be determined.
In the present study, some limitations must be acknowledged. First, the single-center and retrospective nature of the study may limit, in part, its generalizability. Second, the relatively small cohort of patients may have compromised, in part, the results.
Despite these limitations, our study has numerous strengths. Firstly, to our knowledge this study has the longest follow-up after COVID-19 investigating kidney outcomes. Secondly, even though we had a small cohort of patients, we were able to identify an association between AKI and long-term adverse renal events and mortality. Thirdly, we defined AKI according to the KDIGO classification using SCr criteria. Additionally, the current study addresses cause-specific mortality.
To conclude, AKI was independently associated with long-term kidney function decline and/or kidney replacement therapy requirement and/or mortality. This highlights the need for adequate follow-up care in these high-risk patients.
Ethical approvalThe Ethical Committee of ULS Santa Maria approved of this study, in agreement with institutional guidelines. The work was conducted in line with the Declaration of Helsinki.
FundingThere was no funding for this study.
Authors’ contributionsThe authors participated as follows: BMS and JG drafted the article. JLT participated in the collection of data. CC, CB, JO, JB, FB, JAF and JAL critically revised the manuscript.
Informed consentInformed consent was waived given retrospective and non-interventional nature of the study.
Conflict of interestsThere is no conflict of interest. The results presented in this paper have not been published previously in whole or part.
Data availability statementThe data underlying this article will be shared on reasonable request to the corresponding author.
The authors have no acknowledgements.