AKI is frequent in critically ill patients, in whom the leading cause of AKI is sepsis. The role of intrarenal and systemic inflammation appears to be significant in the pathophysiology of septic-AKI. The neutrophils to lymphocytes and platelets (N/LP) ratio is an indirect marker of inflammation. The aim of this study was to evaluate the prognostic ability of N/LP ratio at admission in septic-AKI patients admitted to an intensive care unit (ICU).
MethodsThis is a retrospective analysis of 399 septic-AKI patients admitted to the Division of Intensive Medicine of the Centro Hospitalar Universitário Lisboa Norte between January 2008 and December 2014. The Kidney Disease Improving Global Outcomes (KDIGO) classification was used to define AKI. N/LP ratio was calculated as: (Neutrophil count×100)/(Lymphocyte count×Platelet count).
ResultsFifty-two percent of patients were KDIGO stage 3, 25.8% KDIGO stage 2 and 22.3% KDIGO stage 1. A higher N/LP ratio was an independent predictor of increased risk of in-hospital mortality in septic-AKI patients regardless of KDIGO stage (31.59±126.8 vs 13.66±22.64, p=0.028; unadjusted OR 1.01 (95% CI 1.00–1.02), p=0.027; adjusted OR 1.01 (95% CI 1.00–1.02), p=0.015). The AUC for mortality prediction in septic-AKI was of 0.565 (95% CI (0.515–0.615), p=0.034).
ConclusionsThe N/LP ratio at ICU admission was independently associated with in-hospital mortality in septic-AKI patients.
La LRA es frecuente en pacientes críticos, en quienes la causa principal de LRA es la sepsis. El papel de la inflamación intrarrenal y sistémica parece ser significativo en la fisiopatología de la LRA por sepsis. La relación entre neutrófilos y linfocitos y plaquetas (N/LP) es un marcador indirecto de inflamación. El objetivo de este estudio fue evaluar la capacidad pronóstica de N/LP en el momento del ingreso en pacientes con LRA por sepsis ingresados en una unidad de cuidados intensivos.
MétodosSe trata de un análisis retrospectivo de 399 pacientes con LRA por sepsis ingresados en la Unidad de Medicina Intensiva del Centro Hospitalario Universitario Lisboa Norte, entre enero de 2008 y diciembre de 2014. Se usó la clasificación del Kidney Disease Improving Global Outcomes (KDIGO) para definir la LRA. La relación N/LP se calculó como: (recuento de neutrófilos×100)/(recuento de linfocitos×recuento de plaquetas).
ResultadosEl 52% de los pacientes presentaba KDIGO estadio 3, el 25,8% KDIGO estadio 2 y el 22,3% KDIGO estadio 1. Un valor más elevado de N/LP fue un factor pronóstico independiente de mayor riesgo de mortalidad intrahospitalaria en pacientes con LRA por sepsis, independientemente del estadio KDIGO (31,59±126,8 frente a 13,66±22,64, p=0,028); OR sin ajustar 1,01 (IC del 95%: 1,00-1,02, p=0,027), OR ajustada 1,01 (IC del 95%: 1,00-1,02, p=0,015). El AUC para la predicción de mortalidad en la LRA por sepsis fue de 0,565 (IC del 95%: 0,515-0,615, p=0,034).
ConclusionesLa relación N/LP en el momento del ingreso en la unidad de cuidados intensivos se asoció de forma independiente con la mortalidad intrahospitalaria en pacientes con LRA por sepsis.
Acute kidney injury (AKI) is characterized by a rapid decrease in renal function.1 AKI is frequent in hospitalized patients and its incidence is higher in critically ill patients, in whom the leading cause of AKI is sepsis, substantiated by multiple cohorts.2–4 Sepsis is the systemic inflammatory response to an infectious insult, which often results in multiple organ dysfunction.5,6
Septic-AKI patients have distinct characteristics than non-septic AKI, that is higher severity scores at admission, more non-renal organ failure and requirement of vasopressors and mechanical ventilation.3,7
AKI has been associated with longer hospital stays, in-hospital mortality, cardiovascular events, progression to chronic kidney disease and long-term mortality.8–10 Septic-AKI also has distinct prognostic implications than non-septic AKI, namely higher short-term mortality rate, prolonged length of hospital stays and higher probability of renal function recovery at hospital discharge.3,7 Therefore, it is vital to detect predictors of AKI and mortality to timely prevent, diagnose and treat this complication.
Recent research has led to improvement in the understanding of the pathophysiology of septic-AKI, which is a complex interaction of hemodynamic, microcirculatory, inflammatory, and immune mechanisms.11–14 Indeed, the role of intrarenal and systemic inflammation appears to be significant in the pathophysiology of septic-AKI and in the associated multi-organ dysfunction.15–17
The neutrophil to lymphocyte ratio (N/L ratio) and neutrophil to lymphocytes and platelets ratio (N/LP ratio) have been associated with AKI in the emergency setting, sepsis, contrast induced-AKI, cardiovascular surgery and abdominal surgery.18–23 These are easily calculated, effective and low-cost markers of systemic inflammation which might be promising in AKI patients.
The prognostic ability of the neutrophils to lymphocytes and platelets ratio (N/LP ratio) has not previously been evaluated in septic-AKI. The aim of this study was to evaluate the prognostic ability of N/LP ratio at admission in septic-AKI patients admitted to an intensive care unit (ICU). For this purpose, we cross-examined data from a retrospective study of critically ill patients admitted with sepsis to the ICU in which the initial goal was to compare the diagnostic sensitivity and prognostic ability of the standard classifications for AKI.24
Materials and methodsThis is a single center retrospective analysis of septic-AKI patients admitted to the Division of Intensive Medicine of the Centro Hospitalar Universitário Lisboa Norte (CHULN) between January 2008 and December 2014. CHULN is an academic and referral center located in Lisbon, Portugal. This study was approved by the Ethical Committee in agreement with institutional guidelines. Due to the retrospective and non-interventional nature of the study, informed consent was waived by the Ethical Committee.
ParticipantsEligible patients were selected as adult patients (=18 years of age) with a diagnosis of sepsis at admission to the Division of Intensive Medicine which developed AKI within the first week of ICU hospitalization.
Exclusion criteria comprised (a) CKD patients on renal replacement therapy, (b) patients who underwent renal replacement therapy one week prior to admission to the ICU and (c) patients who were discharged or died less than two days after ICU admission.
Variables and outcomesPatient variables were collected from individual clinical records. The protocol for all patients in this ICU includes daily determination of SCr and hourly UO.
The following variables were analyzed: patient demographic characteristics (age, gender, ethnicity, body weight and height); comorbidities [diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), cirrhosis and/or malignancy]; main diagnosis on admission (medical vs surgical nature); source of infection; laboratory values at admission (serum hemoglobin, neutrophil, lymphocyte count and platelet count, serum albumin, SCr, arterial blood gas and pH analysis); disease severity according to the Simplified Acute Physiologic Score (SAPS) II25 as determined by the worst variables documented throughout the first 24h of ICU admission; fluid balance during ICU admission; mechanical ventilation, vasopressor use and requirement for renal replacement therapy.
The outcomes measured were length of stay and in-hospital mortality.
DefinitionsThe Kidney Disease Improving Global Outcomes (KDIGO) classification according to both serum creatinine (SCr) and urine output (UO) criteria was used to define AKI (Table 1).26 Pre-admission SCr (SCr within the previous three months) was considered as baseline value. When unavailable, baseline SCr was estimated from the MDRD equation,26 accepting the lower limit of a normal baseline GFR of 75ml/min/1.73m2.
Kidney Disease Improving Global Outcomes (KDIGO) classification.
| Stage | SCr/GFR | UO |
|---|---|---|
| 1 | ? SCr=26.5µmol/l (=0.3mg/dl)or ? SCr=150–200% (1.5–1.9×) | <0.5ml/kg/h (>6h) |
| 2 | ? SCr>200–300% (>2–2.9×) | <0.5ml/kg/h (>12h) |
| 3 | ? SCr>300% (=3×)or ? SCr to =353.6µmol/l (=4mg/dl)or initiation of renal replacement therapy | <0.3ml/kg/h (24h)or anuria (12h) |
Sepsis was diagnosed according to the third international consensus definitions as an acute change in total Sequential Organ Failure Assessment (SOFA) score =2 points consequent to the infection.27 Diabetes mellitus was diagnosed according to the American Diabetes Association criteria28 and hypertension was diagnosed according to the seventh report of the Joint National Committee.29 COPD comprised emphysema and chronic bronchitis and CVD was considered as present whenever a history of cerebrovascular disease, chronic heart failure of any cause, cardiac ischemic disease and/or peripheral arterial disease was documented, also, a previous diagnosis on clinical records was considered sufficient for the confirmation of these diagnosis.
N/LP ratio at admission was calculated as: (Neutrophil count×100)/(Lymphocyte count×Platelet count).
Statistical methodsCategorical variables were described as the total number and percentage for each category, whereas continuous variables were described as the mean±standard deviation. Normally distributed continuous variables were compared with the Student's t-test, non-normally distributed continuous variables were compared with the Mann–Whitney U test and categorical variables were compared with the chi-square test.
We performed univariate analysis in all variables to determine statistically significant factors that may have contributed to in-hospital mortality. Only variables with a significant statistical difference were included in the multivariate analysis using the Cox logistic regression method.
The discriminatory ability for N/LP ratio to predict mortality in septic-AKI patients was determined using the receiver operating characteristic (ROC) curve. A cut-off value was defined as that with the highest validity.
Data were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as a p-value <0.05. Statistical analysis was performed with the statistical software package SPSS for windows (version 21.0).
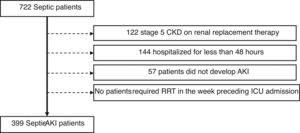
ResultsWe identified 722 septic patients. Of these, 323 were excluded as followed: 122 had stage 5 CKD on renal replacement therapy, 144 had been hospitalized for less than 48h and 57 patients did not develop AKI during ICU stay. No patients required renal replacement therapy in the week preceding ICU admission (Fig. 1).
A final cohort of 399 patients was studied. Demographic variables, clinical and laboratory characteristics are described in Table 2.
Patients’ baseline characteristics.
| Characteristic | Value |
|---|---|
| Age (year) | 64.1±15.9 |
| Gender (male) – n (%) | 229 (57.4) |
| Race (Caucasian) – n (%) | 379 (95) |
| Obese (IMC =30) – n (%) | 116 (29.1) |
| Co-morbidities – n (%) | |
| Hypertension | 182 (45.6) |
| Diabetes | 88 (22.1) |
| CVD | 31 (7.8) |
| COPD | 33 (8.3) |
| Cirrhosis | 17 (4.3) |
| Neoplasia | 96 (24.1) |
| Medical admission – n (%) | 222 (55.6) |
| Infection source – n (%) | |
| Abdominal | 168 (42.1) |
| Respiratory | 122 (30.6) |
| Kidney | 43 (10.8) |
| Skin | 14 (3.5) |
| Others | 20 (5.0) |
| Unknown | 14 (3.5) |
| SAPS II | 50.4±17.3 |
| Baseline SCr (mg/dl) | 1.27±0.6 |
| Admission SCr (mg/dl) | 2.44±1.6 |
| Hemoglobin (g/dl) | 10.5±2.0 |
| Serum albumin (g/dl) | 1.89±0.6 |
| Acidemia (pH <7.5) – n (%) | 143 (35.8) |
| NLP ratio | 20.1±78.4 |
| Mechanical ventilation – n (%) | 309 (77.4) |
| Vasopressors – n (%) | 290 (72.7) |
| Fluid balance (l) | 4.6±5.4 |
| RRT – n (%) | 108 (27.1) |
| LOS in hospital (days) | 36.7±38.6 |
| LOS in ICU (days) | 10.0±10.0 |
| KDIGO stage 1 – n (%) | 89 (22.3) |
| KDIGO stage 2 – n (%) | 103 (25.8) |
| KDIGO stage 3 – n (%) | 207 (51.9) |
| In-hospital mortality – n (%) | 143 (35.8) |
In this cohort of septic-AKI patients, mean age was 64.1±15.9 years, the majority were Caucasian (95%), males (57.4%) and the main diagnosis at ICU admission was medical in nature (55.6%). Forty-five percent of patients were hypertensive, 22.1% diabetic and 24.1% had malignancy. Baseline SCr was 1.27±0.6mg/dl. The infection source was abdominal in 42.1%, respiratory in 30.6% and urinary in 10.8% of patients. At ICU admission mean SAPS II value was 50.4±17.3, SCr was 2.44±1.6mg/dl, hemoglobin was 10.5±2.0, serum albumin was 1.89±0.6mg/dl and N/LP ratio was 20.1±78.4. Acidemia was present in 35.8% of patients at admission. The majority of patients were classified as KDIGO stage 3 (51.9%), 25.8% KDIGO stage 2 and 22.3% as KDIGO stage 1. The N/LP ratio was progressively higher with the KDIGO stages but the differences were not statistically significant (KDIGO stage 1 – 13.7±22.1, KDIGO stage 2 – 18.5±31.7, KDIGO stage 3 – 23.6±105.5, p=0.591). During ICU admission, 77.4% patients required mechanical ventilation, 72.7% required vasopressors, 27.1% required renal replacement therapy (RRT), and required mean fluid balance of 4.6±5.4l.
These patients had a length of stay in ICU of 10.0±10.0 days and in hospital of 36.7±38.6 days. The in-hospital mortality in this cohort of septic-AKI patients was 35.8% (KDIGO stage 1 – 13.3%, KDIGO stage 2 – 16.8%, KDIGO stage 3 – 69.9%, p<0.001). Overall, patient survival was 64.2%.
Septic-AKI patients who died within the hospital admission were older (66.6±15.9 vs 62.6±15.7, p=0.016), had a higher incidence of malignancy (32.2% vs 19.5%, p=0.005) and had fewer urinary tract infections (5.6% vs 13.7%, p=0.013). At ICU admission, higher SAPS II (58.1±17.7 vs 46.1±15.6, p<0.001), lower hemoglobin level (9.9±2.0 vs 10.8±1.9, p<0.001) and lower serum albumin (1.78±0.6 vs 1.94±0.56, p=0.010) correlated with mortality. The N/LP ratio at admission was associated with in-hospital mortality in septic-AKI patients (31.59±126.8 vs 13.66±22.64, p=0.028). Need for mechanical ventilation (88.8% vs 71.1%, p<0.001), vasopressors (81.8% vs 67.6%, p=0.002), higher fluid balance (6.87±5.99 vs 3.38±4.6, p<0.001) and RRT (45.5% vs 16.8%, p<0.001) also correlated with mortality (Table 3).
Demographic and clinical characteristics of septic AKI patients according to in-hospital mortality.
| Characteristics | Survivors (256) | In-hospital mortality (143) | p-value |
|---|---|---|---|
| Age (year) | 62.6±15.7 | 66.6±15.9 | 0.016 |
| Gender (male) – n (%) | 144 (56.3) | 85 (59.4) | 0.537 |
| Race (Caucasian) – n (%) | 245 (95.7) | 134 (93.7) | 0.381 |
| Obesity – n (%) | 77 (28.1) | 39 (26.1) | 0.554 |
| Co-morbidities – n (%) | |||
| Hypertension | 118 (46.1) | 64 (44.8) | 0.797 |
| Diabetes | 57 (22.3) | 31 (21.7) | 0.892 |
| CVD | 16 (6.3) | 15 (10.5) | 0.129 |
| COPD | 18 (7.0) | 15 (10.5) | 0.229 |
| Cirrhosis | 10 (3.9) | 7 (4.9) | 0.639 |
| Neoplasia | 50 (19.5) | 46 (32.2) | 0.005 |
| Medical admission – n (%) | 140 (54.7) | 82 (57.3) | 0.609 |
| Infection source – n (%) | |||
| Abdominal | 106 (41.4) | 62 (57.3) | 0.705 |
| Respiratory | 76 (29.7) | 46 (32.2) | 0.606 |
| Kidney | 35 (13.7) | 8 (5.6) | 0.013 |
| Skin | 20 (7.8) | 12 (8.4) | 0.838 |
| Others | 11 (4.3) | 9 (6.3) | 0.381 |
| Unknown | 8 (3.1) | 6 (4.2) | 0.577 |
| SAPS II | 46.1±15.6 | 58.1±17.7 | <0.001 |
| Baseline SCr (mg/dl) | 1.28±0.63 | 1.25±0.54 | 0.699 |
| Hemoglobin (g/dl) | 10.8±1.9 | 9.9±2.0 | <0.001 |
| Serum albumin (g/dl) | 1.94±0.56 | 1.78±0.6 | 0.010 |
| NLP ratio | 13.66±22.64 | 31.59±126.8 | 0.028 |
| Mechanical ventilation – n (%) | 182 (71.1) | 127 (88.8) | <0.001 |
| Vasopressors – n (%) | 173 (67.6) | 117 (81.8) | 0.002 |
| RRT – n (%) | 43 (16.8) | 65 (45.5) | <0.001 |
| Fluid balance (l) | 3.38±4.6 | 6.87±5.99 | <0.001 |
| LOS in hospital (days) | 37.7±36.1 | 35.1±12.8 | 0.521 |
| LOS in ICU (days) | 9.8±9.8 | 10.4±10.3 | 0.609 |
| KDIGO stage 1 – n (%) | 70 (27.3) | 19 (13.3) | 0.001 |
| KDIGO stage 2 – n (%) | 79 (30.9) | 24 (16.8) | 0.002 |
| KDIGO stage 3 – n (%) | 107 (41.8) | 100 (69.9) | <0.001 |
An adjusted multivariate analysis to demographic, clinical, ICU admission variables and during ICU stay factors was conducted, in which a higher N/LP ratio remained as an independent predictor of increased risk of in-hospital mortality in septic-AKI patients (31.59±126.8 vs 13.66±22.64, p=0.028; unadjusted OR 1.01 (95% CI 1.00–1.02), p=0.027; adjusted OR 1.01 (95% CI 1.00–1.02), p=0.015). After a sensitive analysis, a higher NL/P ratio was still associated with in-hospital mortality regardless of KDIGO stage (adjusted OR 1.01 (95% CI 1.00–1.02), p=0.030) (Table 4).
Univariate and multivariate analysis of factors predictive of mortality in septic-AKI patients.
| Mortality | ||||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Demographics | ||||
| Age | 1.02 (1.00–1.03) | 0.016 | 1.02 (1.00–1.04) | 0.004 |
| Male | 1.14 (0.75–1.73) | 0.537 | ||
| Caucasian | 0.67 (0.27–1.65) | 0.383 | ||
| Obesity | 0.87 (0.55–1.37) | 0.554 | ||
| Co-morbidities | ||||
| Hypertension | 0.95 (0.63–1.43) | 0.797 | ||
| Diabetes | 0.97 (0.59–1.89) | 0.892 | ||
| CVD | 1.76 (0.84–3.67) | 0.133 | ||
| COPD | 1.55 (0.76–3.18) | 0.232 | ||
| Cirrhosis | 1.27 (0.47–3.40) | 0.640 | ||
| CKD | 1.73 (0.91–3.29) | 0.095 | ||
| Neoplasia | 1.40 (1.11–1.78) | 0.005 | 1.11 (0.84–1.47) | 0.446 |
| Medical admission | 1.11 (0.74–1.69) | 0.609 | ||
| Infection source | ||||
| Abdominal | 1.08 (0.72–1.64) | 0.705 | ||
| Respiratory | 1.12 (0.72–1.75) | 0.606 | ||
| Kidney | 0.37 (0.17–0.83) | 0.016 | 0.27 (0.11–0.69) | 0.006 |
| Skin | 1.08 (0.51–2.28) | 0.838 | ||
| Others | 1.50 (0.61–3.70) | 0.383 | ||
| At UCI admission | ||||
| SAPS II | 1.05 (1.03–1.06) | 0.000 | 1.03 (1.01–1.05) | 0.001 |
| Baseline SCr | 0.93 (0.66–1.32) | 0.698 | ||
| Hemoglobin | 0.79 (0.70–0.88) | 0.000 | 0.79 (0.70–0.90) | 0.001 |
| Serum albumin | 0.61 (0.42–0.89) | 0.011 | 0.86 (0.56–1.33) | 0.507 |
| pH <7.35 | 1.73 (1.14–2.64) | 0.011 | 1.14 (0.69–1.88) | 0.614 |
| NLP ratio | 1.01 (1.00–1.02) | 0.027 | 1.01 (1.00–1.02) | 0.015 |
| NLP ratio >14 | 1.79 (1.13–2.83) | 0.013 | 2.14 (1.23–3.71) | 0.007 |
| During ICU admission | ||||
| Mechanical ventilation | 3.23 (1.80–5.80) | 0.000 | 1.28 (0.65–2.53) | 0.477 |
| Vasopressors | 2.16 (1.31–3.56) | 0.003 | 0.96 (0.53–1.75) | 0.903 |
| Fluid balance | 1.00 (1.00–1.00) | 0.000 | 1.00 (1.00–1.00) | <0.001 |
| RRT | 4.13 (2.59–6.57) | 0.000 | 3.13 (1.76–5.57) | <0.001 |
Older age (p=0.004), higher SAPS II (p=0.001), lower hemoglobin (p=0.001), higher fluid balance (p<0.001) and need for RRT (p<0.001) were also associated with mortality in the multivariate analysis (Table 4).
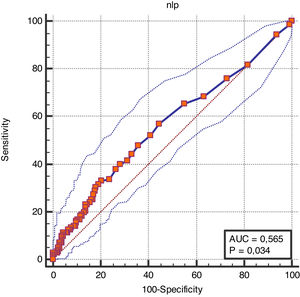
To assess the discriminative ability of N/LP ratio for mortality, a ROC curve was produced. The AUC for mortality prediction in septic-AKI was of 0.565 (95% CI (0.515–0.615), p=0.034) (Fig. 2). The optimal cut-off was assessed to be >14, which has a sensitivity of 33.1% and specificity of 79.7% with a positive predictive value of 1.63 and negative predictive value of 0.84, meaning that almost 80% of patients with a NL/P ratio lower than 14 will survive.
DiscussionIn this retrospective cohort, we demonstrated that a higher N/LP ratio at ICU admission was independently associated with in-hospital mortality in septic-AKI patients after adjustment to demographic and clinical variables.
The association of this marker with mortality in this setting highlights the role of inflammation in the pathophysiology of septic-AKI.30 The exact mechanisms involved in septic-AKI are unknown, still, this complex process appears to result from the interaction of hemodynamic changes, microcirculatory dysfunction, tubular cell injury, inflammation, coagulation disturbances and adaptive responses to injury.12,31
AKI develops despite an increased renal blood flow in the initial hyperdynamic state of sepsis.31 It is theorized that the ultrafiltration of toxins and release of inflammatory cytokines prompts tubular cell injury, endothelial dysfunction, reduced microcirculatory flow and interstitial inflammatory cell infiltration.13,15
The role of platelet and leukocyte interactions as a critical step in leukocyte recruitment, activation and migration in inflammation has emerged in recent years.32,33 Both innate and adaptive immune responses are modulated by the interaction of neutrophils, monocytes, lymphocytes and platelets.32,33 There is an intrinsic interaction between inflammation and coagulation pathways in sepsis.34 In the early phases of sepsis platelet/neutrophils complexes are raised, and reduced in severe and complicated sepsis due to peripheral sequestration or sepsis-associated thrombocytopenia.35–37
The systemic inflammation associated with septic-AKI is central in the development of multi-organ failure and long-term outcomes.38 Therefore, the N/LP ratio stands as an indirect and sensitive predictor of inflammation and outcomes in this setting.
The N/L ratio has been described in recent literature as a significant marker for inflammation and a useful predictor of the development of AKI.18–21 In patients with septic shock, Riche et al. reported that the N/L ratio correlated with short and long-term mortality.39 Also, Huang et al. demonstrated that the N/L ratio at admission was associated with mortality in patients with severe sepsis and septic shock.40 In a study by Bu et al., N/L ratio was associated with AKI in patients with sepsis, though there was no association with mortality.41
The N/LP ratio was developed afterwards by Koo et al. who described its association with AKI and short and long-term mortality after cardiovascular surgery.22 In a recent study, we have also reported the significant correlation between the N/LP ratio and AKI in major abdominal surgery, although the ratio did not correlate significantly with in-hospital mortality.23
The NL ratio was not predictive of mortality in this cohort; thus, platelet count was incorporated in the ratio to increase its sensitivity in predicting patient outcomes. This is the first study to report the association between the N/LP ratio and mortality in septic-AKI. In the sepsis setting, thrombocytopenia is a marker of critically ill patients and is a recognized risk factor for mortality, therefore, the incorporation of platelets in the ratio increases its sensitivity in predicting patient outcomes.33
Importantly, the N/LP ratio at admission was an independent predictor of mortality in patients who develop septic-AKI within the first week of admission. More importantly, a NL/P ratio lower than 14 is predictive of patient survival, which further reflects that lower inflammation is associated with lower mortality in critically ill septic patients. Thus, highlighting the role of the N/LP ratio as a significant marker of systemic inflammatory response at ICU admission.
We address some of the important limitations of this study. Firstly, the results may be compromised by the single-center and retrospective nature of the study with a small cohort of patients. Secondly, we could not assess factors which could influence UO, of which we had hourly measurements. Thirdly, the pre-admission SCr level was unknown in nearly 60% of patients, compelling us to calculate an estimated baseline function using the MDRD equation as recommended, which could overestimate AKI incidence. Fourthly, the power of the ROC curve is light and therefore further studies are necessary before generalization. Finally, we did not address cause-specific mortality.
Despite these limitations, our study has several notable strengths. To the best of our knowledge, this is the first study evaluating the association between the N/LP ratio and in-hospital mortality in septic-AKI patients in an ICU. Also, the high specificity of the NL/P ratio reflects that a low ratio is predictive of patient survival. Additionally, AKI was defined and categorized using both creatinine and UO. Finally, despite the retrospective nature of the study, most of the studied variables were registered as part of routine clinical practice on a daily basis and made accessible for analysis.
We highlight the need for further understanding of septic-AKI in order to identify at-risk populations, employ preventive strategies, guaranty an early diagnosis and prompt and adequate treatment, ultimately, improving patient outcomes.
ConclusionsIn conclusion, we confirmed that the N/LP ratio at ICU admission was independently associated with in-hospital mortality in septic-AKI patients. The assessment of this ratio is straightforward from a routine blood analysis in ICU patients and can prove useful in identifying patients at risk for mortality.
Ethics approvalEthics approval and consent to participate.
This study was approved by the Ethical Committee in agreement with institutional guidelines. Due to the retrospective and non-interventional nature of the study, informed consent was waived by the Ethical Committee.
FundingThere was no funding for this study.
Authors’ contributionsThe authors participated as follows: JG and JAF drafted the article, SJ and JG revised the article, JAL revised the article and approved the final version to be submitted for publication.
Conflict of interestThere is no conflict of interest. The results presented in this paper have not been published previously in whole or part.
Consent for publicationThe authors give their consent for publication.
Availability of data and materialAll datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
The authors acknowledge the remaining doctors in the investigation team at the Division of Nephrology and Renal Transplantation of CHULN for the collection of data.