Diabetic nephropathy (DN) is a life-threatening complication of diabetes mellitus (DM) and the leading cause of end-stage renal disease. Ferroptosis, a novel iron-dependent mode of cell death, has been identified to participate in the pathogenesis of DN. Isorhynchophylline (IRN) is a tetracyclic indole oxide alkaloid present in Uncaria rhynchophylla (Rubiaceae), which shows protective effects against diabetic encephalopathy and acute kidney injury. Our study intends to determine whether IRN ameliorates DN progression through inhibiting ferroptosis.
MethodsThe db/db diabetic mice and high glucose (HG)-stimulated human kidney tubular epithelial HK-2 cells were used to explore the potential therapeutic value of IRN in vivo and in vitro. Blood glucose levels, body weight, kidney weight, serum creatinine (SCr), blood urea nitrogen (BUN), and albumin-to-creatinine ratio (UACR) were detected to assess diabetic symptoms and renal functions in db/db mice. Hematoxylin–eosin (H&E) and periodic acid-Schiff staining (PAS) staining were performed to observe renal pathohistological changes in diabetic mice. Iron contents as well as malondialdehyde (MDA) and glutathione (GSH) in mouse tissue homogenates and HK-2 cell supernatants were examined to assess iron accumulation and oxidative stress. The levels of ferroptosis-related proteins and Nrf2/HO-1 signaling-related proteins as well as Nrf2 nuclear translocation in mouse renal tissues and HK-2 cells were detected by western blotting and immunofluorescence staining.
ResultsIRN administration alleviated diabetic symptoms and improved renal functions in diabetic mice. IRN mitigated renal histologic damage, including glomerular hypertrophy, mesangial matrix accumulation, capillary basement membrane thickening, and thylakoid stroma expansion in diabetic mice. IRN treatment inhibited ferroptosis in both diabetic mice and HG-induced HK-2 cells by reducing iron content and MDA levels, elevating GSH levels, upregulating the protein levels of FTH-1, GPX4, and SLC7A11, and downregulating the protein levels ofTFR-1 and NCOA4. Mechanistically, IRN treatment enhanced Nrf2 and HO-1 protein levels and Nrf2 nuclear translocation in renal tissues of diabetic mice and HG-exposed HK-2 cells.
ConclusionIRN plays a renoprotective role in DN by suppressing ferroptosis, which might be ascribed to the Nrf2/HO-1 pathway activation, highlighting the potential therapeutic application of IRN for DN treatment.
La nefropatía diabética (ND) es una complicación potencialmente letal de la diabetes mellitus (DM), siendo la causa principal de enfermedad renal en etapa terminal. La ferroptosis, un modo nuevo de muerte celular dependiente del hierro, ha sido identificada para participar en la patogenia de la ND. Isorincofilina (IRN) es un alcaloide oxindólico tetracíclico presente en Uncaria rhynchophylla (rubiácea), que muestra efectos protectores contra la encefalopatía diabética y la lesión renal aguda. El objetivo de nuestro estudio es determinar si IRN mejora la progresión de la ND mediante la inhibición de la ferroptosis.
MétodosSe utilizaron ratones diabéticos db/db y células HK-2 epiteliales de túbulo renal humano estimuladas con alta glucosa (HG) para explorar el valor terapéutico potencial de IRN in vivo e in vitro. Se detectaron los niveles glucémicos, peso corporal, peso renal, creatinina sérica (SCr), nitrógeno ureico en sangre (BUN) y ratio albúmina-creatinina (UACR) para evaluar los síntomas diabéticos y las funciones renales en los ratones db/db. Se realizaron tinciones hematoxilina–eosina (H&E) y de ácido peryódico de Schiff (PAS) para observar los cambios patohistiológicos renales en los ratones diabéticos. Se examinaron los contenidos férricos y de malondialdehído (MDA) y glutatión (GSH) en homogeneizados de tejido de ratones y sobrenadantes de células HK-2, para evaluar la acumulación de hierro y el estrés oxidativo. Se detectaron los niveles de proteínas relacionadas con la ferroptosis y las proteínas relacionadas con la señalización de Nrf2/HO-1, así como la traslocación nuclear de Nrf2 en tejidos renales de los ratones y las células HK-2 mediante inmunotransferencia y tinción por inmunofluorescencia.
ResultadosLa administración de IRN alivió los síntomas diabéticos y mejoró las funciones renales en los ratones diabéticos. IRN mitigó el daño histológico renal, incluyendo la hipertrofia glomerular, la acumulación en la matriz mesangial, el engrosamiento de la membrana basal capilar y la expansión del estroma tilacoide en los ratones diabéticos. El tratamiento de IRN inhibió la ferroptosis tanto en los ratones diabéticos como en las células HK-2 inducidas por HG, reduciendo el contenido férrico y los niveles de MDA, elevando los niveles de GSH, aumentando los niveles proteicos de FTH-1, GPX4 y SLC7A11, y reduciendo los niveles proteicos de TFR-1 y NCOA4. Mecánicamente, el tratamiento de IRN mejoró los niveles proteicos de Nrf2 y HO-1 y la traslocación nuclear de Nrf2 en los tejidos renales de los ratones diabéticos y las células HK-2 expuestas a HG.
ConclusiónIRN juega un papel nefroprotector en la ND, suprimiendo la ferroptosis, lo cual podría atribuirse a la activación de la vía Nrf2/HO-1, destacando la aplicación terapéutica potencial de IRN para el tratamiento de la ND.
Due to unhealthy lifestyles and the growing prevalence of obesity, the global prevalence of diabetes mellitus (DM) is projected to gradually rise, which will bring medical and economic burdens.1 Diabetic nephropathy (DN), as a significant microvascular complication of DM, can cause irreversible damage to the kidneys.2 DN is the leading cause that contributes to the occurrence and development of end-stage renal disease (ESRD), which is responsible for about half of ESRD cases.3 The golden standard for the diagnosis of DN is renal biopsy, and the pathological characteristics include glomerular basement membrane thickening, glomerular hypertrophy, mesangial expansion, and glomerular sclerosis.4 Currently, blocking the renin–angiotensin–aldosterone system (RAAS) is the first recommended treatment option for patients with DN in addition to controlling blood pressure and blood glucose.5 Nevertheless, the application of specific inhibitors of the RAAS in the clinic is not satisfying since they fail to reverse or completely prevent the progression of DN to ESRD.6 Hence, developing novel renoprotective drugs to prevent or delay the onset of DN is extremely critical, which can help to address a major public health problem.
The pathogenesis of DN is complicated. Recently, a new form of regulated cell death, known as ferroptosis, has come into the field of medical research.7 Ferroptosis is driven by iron-dependent lipid peroxidation and is distinct from other forms of cell death in morphology and biochemistry.8 The essence of ferroptosis is that the depletion of glutathione (GSH) and the decrease in glutathione peroxidase 4 (GPX4) activity results in the oxidation of lipids by divalent iron ions to produce reactive oxygen species (ROS), which triggers the onset of ferroptosis.9 The process of ferroptosis is accompanied by the accumulation of large amounts of iron ions and ROS. Accumulating evidence has suggested that abnormal ferroptosis is closely related to the development of diabetic complications including DN.10 The persistent hyperglycemic environment leads to iron overload, which induces ROS overproduction, oxidative stress, and eventually ferroptosis.11 Multiple studies have demonstrated that inhibiting ferroptosis slows DN progression in various cellular and animal models.12 Nrf2 is a key transcriptional factor responsible for modulating the redox and antioxidant genes and thereby playing a pivotal role in the anti-oxidative stress defense system.13 In addition, Nrf2 also prevents lipid peroxidation and inhibits ferroptosis by regulating GSH and GPX4 expression.14 Nrf2 depletion has been identified to cause excessive production and deposition of iron contents in tissues and organs.15 Accordingly, upregulating Nrf2 is a promising strategy to repress ferroptosis and oxidative stress and delay DN progression.16
Over the past decades, Chinese medicine therapies have exhibited unique advantages in preventing and treating clinical-stage DN.17 Either the use of Chinese herbal medicines alone or their combined use with chemical drugs helps to reduce proteinuria and improve renal function in DN patients.18 More importantly, increasing herbal plant-derived natural compounds have been discovered to prevent and treat DN through modulating ferroptosis.19 Isorhynchophylline (IRN) is a tetracyclic indole oxide alkaloid extracted from Uncaria rhynchophylla (Rubiaceae).20 Modern pharmacological researches suggest that IRN possesses various biological activities, such as neuroprotection, anti-inflammation, anti-oxidative, anti-proliferation, and anti-hypertension effects.21,22 IRN has been used in the treatment of cardiovascular and neurological diseases such as hypertension, numbness, convulsions, lightheadedness, arrhythmia, amnesia brachycardia, amnesia, and vascular dementia.23 Additionally, IRN was reported to improve insulin homeostasis and alleviate cognitive impairment in diabetic encephalopathy, a DM-induced central diabetic neuropathy, through stimulating sXBP1 nuclear translocation.24 IRN upregulates Tollip to attenuate oxidative stress and mitochondrial damage, thereby ameliorating paraquat-induced acute kidney injury.25 However, whether IRN plays a protective role in DN remains unknown.
Our research intends to determine the beneficial effects of IRN on DN progression and delineate the underlying mechanism. Given that Nrf2-mediated ferroptosis plays a central role in DN development, we hypothesized that IRN may exert its renoprotective effects on DN by inhibiting ferroptosis through upregulation of Nrf2. Our results may provide a theoretical basis for the potential of IRN as a therapeutic agent for DN.
Materials and methodsAnimal experimentsMale C57BLKs/J db/m and db/db mice (8-week-old) were provided by Nanjing Junke Bioengineering (Jiangsu, China). All animal procedures were authorized by the Animal Ethics Committee of The First Affiliated Hospital of Soochow University. After 2 weeks of adaptive rearing under a 12:12h light–dark cycle (humidity, 45–55%; temperature, 22–24°C), the mice were arbitrarily assigned into the db/m, db/m+IRN, db/db, db/db+IRN, and db/db+Fer-1 group (n=6/group). IRN (purity ≥98%; #SI8310; Solarbio, Beijing, China) was suspended in 0.5% sodium carboxymethyl cellulose (CMC-Na; #HY-Y0703; MedChemExpress, Shanghai, China), and then given to the mice in db/m+IRN and db/db+IRN groups at a dose of 40mg/kg through oral gavage for 12 weeks (once daily for 5 consecutive days 1 week), while the same volume of 0.5% CMC-Na was given to the mice in db/m and db/db groups for the same time. The ferroptosis inhibitor ferrostatin-1 (Fer-1) (purity ≥98%; #CSGC10380; Chemstan, Wuhan, China) was dissolved in 1% dimethyl sulfoxide (DMSO; #abs9185; Absin, Shanghai, China) and then injected intraperitoneally into the mice in the db/db+Fer-1 group at a dose of 5mg/kg for 12 weeks (once daily for 5 consecutive days 1 week). The dosages of IRN24 and Fer-126,27 were selected according to the previous studies. After 12 weeks of administration, the blood glucose and body weight of mice were measured, and the mice were separately placed in metabolic cages to obtain 24h urine. Afterwards, the mice were humanely euthanized with isoflurane, and blood samples from the left ventricle were collected for biochemical examination. Besides, the kidneys were rapidly dissected, one fixed in formalin for histological evaluation, whereas the other preserved at −80°C for the homogenization process.
Biochemical analysisHemoCue B-Glucose kit (HemoCue AB, Angelholm, Sweden) was used to measure the fasting blood glucose concentration of mice. An automatic biochemical analyzer (Hitachi7060, Tokyo, Japan) was employed to detect the concentrations of serum creatinine (SCr), blood urea nitrogen (BUN), urinary albumin, and urinary creatinine. Urinary albumin-to-creatinine ratio (UACR) was calculated to indicate urine albumin excretion.
Histopathological stainingThe kidney tissues were fixed with 4% paraformaldehyde overnight, followed by dehydration with gradient alcohol, washing with phosphate-buffered saline (PBS), paraffin-embedding, and cutting into 5μm thick sections. After dewaxing and rehydration, the sections were separately processed for hematoxylin–eosin (H&E; #mlsw-1249; mlBio, Shanghai, China) and periodic acid-Schiff staining (PAS; #C0142M; Beyotime, Shanghai, China) staining to observe histopathological changes. For H&E staining, the sections were stained with hematoxylin for 10min and eosin for 5min. For PAS staining, the sections were incubated with 0.1% periodic acid for 10min and Schiff's reagent for 17min. After washing in tap water, the samples were counterstained with hematoxylin for 2min. Lastly, the stained samples were cleared in xylene, sealed with neutral balsam, and observed under a microscope (Zeiss, Germany). Slices stained with H&E revealed the glomerular size and the degree of glomerular hypertrophy, and PAS staining revealed the expansion of the mesangial matrix and the damage degree of the renal tubule.
Cell culture and treatmentHuman kidney tubular epithelial cells (HK-2) were acquired from Procell (#CL-0109; Wuhan, China) and cultured at 37°C and 5% CO2 in DMEM/F12 medium (#PM150310B; Procell) containing 1% antibiotics (penicillin/streptomycin) and 10% fetal bovine serum (FBS). To construct the cellular model of DN, HK-2 cells were incubated in a high-glucose medium (HG; 30mM glucose) for 48h, with a normal-glucose medium (NG; 30mM glucose) and a high-mannitol medium (HM; 5.6mM glucose combined with 24.4mM mannitol) as the controls. For treatment groups, HK-2 cells were incubated in a high-glucose medium containing IRN at different concentrations (5, 10, 25, or 50μM) or Fer-1 (1μM) for 48h.
CCK-8 assayHK-2 cells were seeded (3,000cells/well) in 96-well plates and treated as described above. Forty-eight hours later, CCK-8 solution (10μL; #C4018; Warbio, Nanjing, China) was placed into each well using a pipette tip, followed by another 2h incubation. Lastly, cell viability was determined by detecting the absorbance at 450nm with a microplate reader (Bio-Rad, USA).
Measurement of iron contents and MDA and GSH levelsTen milligrams of mouse renal tissues were homogenized by a tissue homogenizer (TissueRuptor, Qiagen, Hilden, Germany). HK-2 cells were homogenized by an ultrasonic cell pulverizer (Beyotime) and centrifuged for 10min to obtain the supernatant. Iron, MDA, and GSH levels in tissue homogenates and cell supernatants were respectively examined using the Iron Assay Kit (#KA0814; AmyJet, Wuhan, China), the Lipid Peroxidation MDA Assay Kit (#K084; FineTest, Wuhan, China), and Micro Reduced GSH Assay Kit (#BC1175; Reanta, Beijing, China) as directed by the manufacturer. The results were finally analyzed by a microplate reader at the corresponding wavelength (593nm for iron, 532nm for MDA, and 412nm for GSH).
Western blottingMouse renal tissues and HK-2 cells were homogenized in RIPA lysis buffer (#LM-609; LAMI Bio, Shanghai, China) and then centrifuged at 12,000g for 5min at 4°C to harvest the supernatant. After determining the protein concentration using the bicinchoninic acid assay kit (#AR1189; Boster, Wuhan, China), 40μg protein samples were resolved by electrophoresis on 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The membranes were subjected to 60min blockage with 5% non-fat milk, followed by overnight incubation on a shaker at 4°C with primary antibodies against SLC7A11 (#AF7992; Beyotime), GPX4 (#AF7020; Beyotime), TFR-1 (#AF8136; Beyotime), FTH-1 (#FNab10519; FineTest), NCOA4 (#A04368-3; Boster), Nrf2 (#AF7623; Beyotime), HO-1 (#FNab03937; FineTest), and β-actin (#AF5003; Beyotime) diluted to 1:1000 and 1h incubation at 25°C with HRP-labeled IgG secondary antibody (#A0208; Beyotime) diluted to 1:1000. After that, the membranes were developed with an enhanced chemiluminescence reagent (#P1010; Applygen, Beijing, China). Image-Pro Plus 6.0 software was utilized to quantify the relative gray intensity of each band, with β-actin as an internal reference.
Immunofluorescence stainingHK-2 cells were incubated on coverslips in 35mm dishes. After washing with PBS and fixation with 4% paraformaldehyde for 20min, cells were permeabilized with PBS containing 0.5% Triton X-100 for 10min and sealed with 3% bovine serum albumin for 60min. Thereafter, cells were probed with the Nrf2 primary antibody (#abs130481; 1:100; Absin) at 4°C overnight and fluorescently labeled IgG secondary antibody (Alexa Fluor 647; #A0468; 1:500; Beyotime) for 60min at indoor temperature protected from light. The nucleus was stained with DAPI (#HXSJ-021134; JISSKANG, Qingdao, China) for 10min. The fluorescence signals were finally viewed under a confocal laser scanning microscope (Nikon, Japan) and quantified with Image-Pro Plus 6.0 software.
Statistical analysisAll statistical analyses were carried out using SPSS (version 20, IBM Corp, Armonk, NY, USA). Data are presented as mean±standard deviation of at least three experimental repeats conducted independently. Comparisons between two or more groups were analyzed through Student's t-test or one-way ANOVA followed by the Bonferroni post hoc test. p-Value <0.05 were regarded statistically significant in all analyses.
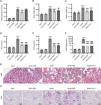
ResultsIRN ameliorates renal injury in diabetic miceThe influence of IRN on DN progression in type 2 diabetes db/db mice was investigated. Diabetic db/db mice had markedly increased blood glucose levels, body weight, and kidney weight compared with control db/m mice, which however, were notably reduced after IRN treatment. Similarly, Fer-1 treatment remarkably attenuated body weight and kidney weight but did not significantly affect the blood glucose level of diabetic mice (Fig. 1A–C). Next, biochemical analysis was performed to estimate the levels of renal function indexes. As expected, SCr, BUN, and UACR levels were prominently higher in db/db mice than in db/m mice. Intriguingly, both IRN and Fer-1 treatment notably reduced SCr, BUN, and UACR levels in diabetic mice (Fig. 1D–F). To be mentioned, IRN administration exerted no significant effects on body and kidney weight as well as biochemical parameters in db/m mice (Fig. 1A–F). In addition, the renal tissues of mice were subjected to H&E and PAS pathological staining. It was observed that db/db mice exhibited obvious histologic signs of renal damage, such as glomerular hypertrophy, mesangial matrix accumulation, capillary basement membrane thickening, and thylakoid stroma expansion. Nevertheless, both IRN and Fer-1 treatment partly relieved the above renal pathological changes (Fig. 1G and H). Collectively, these findings demonstrated that IRN dramatically mitigated diabetes-related renal injury and dysfunction in db/db mice.
IRN ameliorates renal injury in db/db mice. (A–F) Measurement of blood glucose levels, body weight, renal weight, serum creatinine (SCr) levels, blood urea nitrogen (BUN) levels, and urinary albumin creatinine ratio (UACR) in db/m and db/db mice treated with vehicle, IRN, or Fer-1. (G and H) Representative H&E and PAS staining images showing pathological changes in mouse renal tissues. In H&E staining, black arrows indicate glomerular hypertrophy, red arrows indicate renal tubule atrophy, and blue arrows indicate inflammatory cell infiltration. In PAS staining, red arrows indicate glomerular basement membrane thickening, blue arrows indicate thickening of the basement membrane of the renal tubules, and black arrows indicate thickening of the basement membrane of the renal capsule. Scale bar: 50μm. Results are presented as the mean±SD of 6 mice. ***p<0.001 versus db/m; ##p<0.01, ###p<0.001 versus db/db.
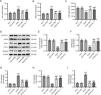
Next, whether IRN affects ferroptosis in diabetic mice was assessed. The iron content of kidney tissues was noticeably elevated in db/db mice versus db/m mice, which however, was reduced after both IRN and Fer-1 treatment (Fig. 2A). The levels of lipid peroxidation product MDA and anti-oxidant GSH in mouse renal tissues were detected to evaluate oxidative stress. As depicted in Fig. 2B and C, MDA levels were considerably higher while GSH levels were lower in the kidney tissues of db/db mice than in those of db/m mice. Nevertheless, both IRN and Fer-1 treatment reversed the increment in MDA levels and decrement in GSH levels in db/db mice. Moreover, the levels of ferroptosis-related proteins in mouse kidney tissues were examined through western blotting, which revealed that SLC7A11, GPX4, and FTH-1 protein levels were weakened and TFR-1 and NCOA4 protein levels were enhanced in db/db mice versus db/m mice. Both IRN and Fer-1 treatment overturned the changes in the levels of these proteins in db/db mice (Fig. 2D–I). Overall, IRN alleviated ferroptosis and oxidative stress in the kidneys of db/db mice.
IRN inhibits ferroptosis in db/db mice. (A–C) Examination of iron content, MDA levels, and GSH levels in mouse renal tissues. (D–I) Evaluation of SLC7A11, GPX4, TFR-1, FTH-1, and NCOA4 protein expression in mouse kidney tissues through western blotting and densitometric analysis of the bands. Results are presented as the mean±SD of 6 mice. ***p<0.001 versus db/m; ###p<0.001 versus db/db.
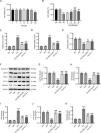
Based on the CCK-8 assay, we discovered that IRN had significant cytotoxicity against HK-2 cells at 100μM, while IRN at concentrations lower than 50μM exhibited no marked effect on HK-2 cell viability (Fig. 3A). HG-stimulated HK-2 cells were then treated with IRN (5, 10, 25, or 50μM), and we found that IRN rescued HG-induced decline in HK-2 cell viability, with 25μM showing the best improvements (Fig. 3B). Besides, HG caused a substantial elevation in iron content and MDA levels and a decline in GSH levels in HK-2 cells, which however, were counteracted after receiving IRN or Fer-1 treatment (Fig. 3C–E). What's more, as corroborated by western blotting, both IRN and Fer-1 treatment abrogated HG-induced decrement in SLC7A11, GPX4, and FTH-1 protein levels and increment in TFR-1 and NCOA4 protein levels in HK-2 cells (Fig. 3F–K). To sum up, IRN repressed HG-induced ferroptosis and oxidative stress in HK-2 cells.
IRN curbs HG-induced HK-2 cell injury and ferroptosis. (A) Detection of the cytotoxicity of IRN against HK-2 cells through CCK-8 assay. *p<0.05. (B) Assessment of HK-2 cell viability after treatment with normal glucose, normal glucose+mannitol, high glucose, or high glucose+IRN (5, 10, 25, or 50μM) by CCK-8 assay. (C–E) Measurement of iron content, MDA levels, and GSH levels in HK-2 cells. (F–K) Estimation of SLC7A11, GPX4, TFR-1, FTH-1, and NCOA4 protein levels in HK-2 cells via western blotting and densitometric analysis of the bands. Results are presented as the mean±SD of three independent experiments. ***p<0.001 versus NG; #p<0.05, ##p<0.01, ###p<0.001 versus HG.
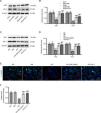
Finally, we explored the mechanism by which IRN protects against ferroptosis in DN. As revealed in Fig. 4A and B, Nrf2 and HO-1 protein levels were prominently lower in the kidney tissues of db/db mice than in those of db/m mice. Conversely, both IRN and Fer-1 administration enhanced Nrf2 and HO-1 protein levels in db/db mice. Consistent with the in vivo results, HG-induced reduction in Nrf2 and HO-1 protein levels in HK-2 cells was offset by both IRN and Fer-1 treatment (Fig. 4C and D). The immunofluorescence results further proved that both IRN and Fer-1 treatment promoted Nrf2 expression and nuclear translocation in HK-2 cells (Fig. 4E and F). Thus, IRN stimulated the Nrf2/HO-1 signaling activation in cellular and animal models of DN.
IRN activates the Nrf2/HO-1 pathway in db/db mice and HG-stimulated HK-2 cells. (A and B) Determination of Nrf2 and HO-1 protein levels in db/m and db/db mice administrated with vehicle, IRN, or Fer-1 through western blotting and densitometric analysis of the bands. Results are presented as the mean±SD of 6 mice. ***p<0.001 versus db/m; ###p<0.001 versus db/db. (C and D) Evaluation of Nrf2 and HO-1 protein levels in HK-2 cells following treatment with normal glucose, normal glucose+mannitol, high glucose, or high glucose+IRN (25μM) by western blotting and densitometric analysis of the bands. (E and F) Representative immunofluorescence staining images showing Nrf2 expression and nuclear translocation in HK-2 cells and quantitative analysis of Nrf2 fluorescence intensity. Scale bar: 50μm. Results are presented as the mean±SD of three independent experiments. ***p<0.001 versus NG; ###p<0.001 versus HG.
As a primary cause of ESRD, DN has high morbidity and mortality, which can bring severe health damage and pose a huge economic burden on human society. The efficacy of the existing treatment therapies, mainly oral administration of hypoglycaemic and ACEI or ARB antihypertensive drugs and subcutaneous insulin injection, is unsatisfactory.28 Increasing natural herbs and their bioactive ingredients have been revealed to be beneficial in the treatment and management of DN.29 IRN, as the major active component of U. rhynchophylla, exhibits significant protective effects against a wide range of diseases.30,31 The present study evaluated the therapeutic effects of IRN against DN in both cellular and animal models. We discovered that IRN improved kidney functions by attenuating blood glucose levels, body and kidney weight, and SCr, BUN, and UACR levels in db/db mice. Histological examination corroborated the beneficial effects of IRN. IRN treatment ameliorated ferroptosis and oxidative damage in db/db mice and HG-stimulated HK-2 cells, which might be attributed to the Nrf2/HO-1 pathway activation.
Long-term hyperglycemia prompts the body to produce a large number of oxygen free radicals, inducing a large accumulation of ROS, which causes oxidative damage to the body's intracellular DNA. Abundant evidence has confirmed that ferroptosis, a type of regulatory cell death caused by iron-catalyzed and ROS-induced lipid peroxidation, is implicated in the progression of DN.32 GPX4 is an antioxidant enzyme that can neutralize lipid peroxidation and protect cell membrane fluidity.33 Upregulating GPX4 curbs lipid peroxidation and inhibits ferroptosis by reducing cytotoxic lipid hydroperoxides (L-OOH) to their corresponding alcohols (L-OH).34 Downregulation of SLC7A11 can indirectly attenuate GPX4 activity through restraining the cysteine metabolic pathway.35 TFR1, also known as TFRC, is an essential membrane protein that modulates intracellular iron transport.36 The main physiological function of TFR1 is to bind to transferrin and mediate iron uptake through endocytosis, providing an increased flux for the overaccumulation of intracellular Fe2+.37 Decreased or aberrant expression of TFR1 leads to iron deficiency in cells, while excess iron may catalyze ROS and damage biomolecules.38 Yasumura et al. reported that renal fibrosis was reduced in heterozygous TFR-1-deficient (TFR-1+/−) diabetic mice compared with wild-type mice.39 FTH-1, as ferritin heavy chain, is degraded in the autophagic lysosome, followed by the rapid release of Fe2+, which results in cellular iron overload and thereby promotes ferroptosis.40 The process of ferritin degradation is mediated by NCOA4, a selective cargo receptor of ferritin. NCOA4-dependent autophagy is defined as ferritinophagy and leads to increased intracellular iron levels and Fenton reaction, which subsequently causes excessive lipid peroxidation to induce cell death.41 Previously, multiple herbs and their derived natural compounds and extracts have been shown to possess anti-ferroptosis properties and participate in delaying the progression of DN.42,43 Importantly, IRN was revealed to protect neuronal cells from ferroptosis after intracerebral hemorrhage through regulating the miR-122-5p/TP53/SLC7A11 pathway.44 Herein, our results revealed that IRN treatment reduced iron deposition and MDA levels and enhanced GSH levels in db/db mice and HG-induced HK-2 cells. Additionally, IRN upregulated SLC7A11, GPX4, and FTH-1 protein levels and downregulated TFR-1 and NCOA4 protein levels, suggesting the inhibition of IRN on ferroptosis under hyperglycemia conditions.
Nrf2 is a pivotal transcription factor involved in modulating multiple biological processes, including antioxidant response, immunity, inflammation, unfolded protein response, autophagy, heme and iron metabolism, lipid metabolism, amino acid metabolism, and drug detoxification.45 Several studies have found that patients with DN had markedly lower levels of circulating Nrf2 than healthy controls.46 More serious diabetic symptoms, enhanced mesangiolysis, and intensified interstitial renal fibrosis and renal inflammation can be observed in Nrf2-knockout Akita diabetic mice compared with mutant control mice.47 In contrast, activation of Nrf2 delays the progression of DN through upregulating the levels of HO-1 and NQO-1, the Nrf2 downstream antioxidant enzymes.48 Besides, a large body of studies indicate that Nrf2 is a crucial regulator of ferroptosis.49 Many target genes of Nrf2 have been confirmed to participate in preventing lipid peroxidation and modulating cellular iron homeostasis.50 New evidence suggests that enhancement in Nrf2 expression contributes to suppressing ferroptosis and in turn mitigating renal damage in diabetic mice, while Nrf2 knockdown enhances the sensitivity of HK-2 cells to ferroptosis under hyperglycaemic conditions in vitro.51 Till now, several natural compounds have been identified to hinder DN progression by restraining Nrf2-mediated ferroptosis. For example, Feng et al. disclosed that the flavonoid quercetin exhibited nephroprotective effects in diabetic db/db mice and HG-stimulated HK-2 cells by suppressing ferroptosis through activating the Nrf2/HO-1 signaling.26 Jin and Chen clarified that umbelliferone repressed oxidative stress and ferroptosis in db/db mice and HG-treated HK-2 cells by upregulating Nrf2 and HO-1 expression.52 Yu et al. discovered that leonurine inhibited ferroptosis in a high-fat diet and streptozotocin-induced DN mice and HG-induced human umbilical vein endothelial cells through increasing Nrf2-mediated GPX4 expression.53 Previously, IRN was reported to prevent cardiac hypertrophy in mice by stimulating the Nrf2 nuclear translocation.54 IRN treatment improves the paraquat-triggered oxidative damage in the renal cortex of acute kidney injury rat models by increasing Nrf-2, NQO-1, and HO-1 levels.25 Similarly, current research indicated that IRN treatment upregulated Nrf2 and HO-1 protein levels and promoted Nrf2 nuclear translocation in DN cellular and animal models.
Collectively, our study demonstrated that IRN had renoprotective effect against DN by inhibiting ferroptosis and oxidative stress, which might be linked with the Nrf2/HO-1 pathway activation. Our study might provide a vital experimental basis for the development of IRN as a therapeutic agent for DN treatment in the near future. However, the main weakness of this study lies in the lack of further experiments to validate whether inhibition of the Nrf2/HO-1 signaling pathway will abrogate the protective effects of IRN against renal damage and ferroptosis in DN. In future investigations, we can verify that the renoprotective and anti-ferroptotic effects of IRN in DN depend on the Nrf2/HO-1 pathway activation through the use of Nrf2 inhibitor ML385 or genetic knockdown of Nrf2.
CRediT authorship contribution statementTing Yu conceived and designed the experiments. Ting Yu, Fengling Chen, Bing You, Xiaopan Zhang, Yilin Wang, Ying Huang, Xiang Shao and Bo Sun carried out the experiments. Ting Yu, Fengling Chen, Bing You, Xiaopan Zhang, Yilin Wang, Ying Huang, Xiang Shao and Bo Sun analyzed the data. Ting Yu and Bo Sun drafted the manuscript. All authors agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Ethics and consent to participate declarationAll animal procedures were authorized by the Animal Ethics Committee of The First Affiliated Hospital of Soochow University.
Registry, trial registration number, and data of registrationNot applicable.
Consent to publishNot applicable.
Funding- 1.
Pre-research Project of Suzhou Jiulong Hospital in 2022 (SZJL202203).
- 2.
Suzhou Medical and Health Science and Technology Innovation in 2023 – applied basic research (SKY2023113).
None declared.
Data availabilityThe datasets used or analyzed during the current study are available from the corresponding author on reasonable request.