In Fabry disease (FD), primary factors such as glycosphingolipid deposition that initiate kidney damage and secondary factors that advance kidney damage to fibrosis are different. Periostin is a molecule of proven importance in renal inflammation and fibrosis. It was previously shown that periostin plays an essential role in the process leading to renal fibrosis and its expression is increased in many kidney diseases. In the present study, we aimed to reveal the relationship between periostin and Fabry nephropathy.
Material-methodThis cross-sectional study included 18 FD patients (10 males, 8 females) with enzyme replacement therapy (ERT) indications and 22 healthy control patients of similar age and gender. At the time of diagnosis, plasma alpha-galactosidase A (α-gal-A) and globotriaosylsphingosine (lyso-Gb3), proteinuria, and kidney function tests of all FD patients before ERT were scanned from the hospital system. Periostin was studied from serum samples collected and stored before ERT. Parameters related to serum periostin levels in Fabry disease were investigated.
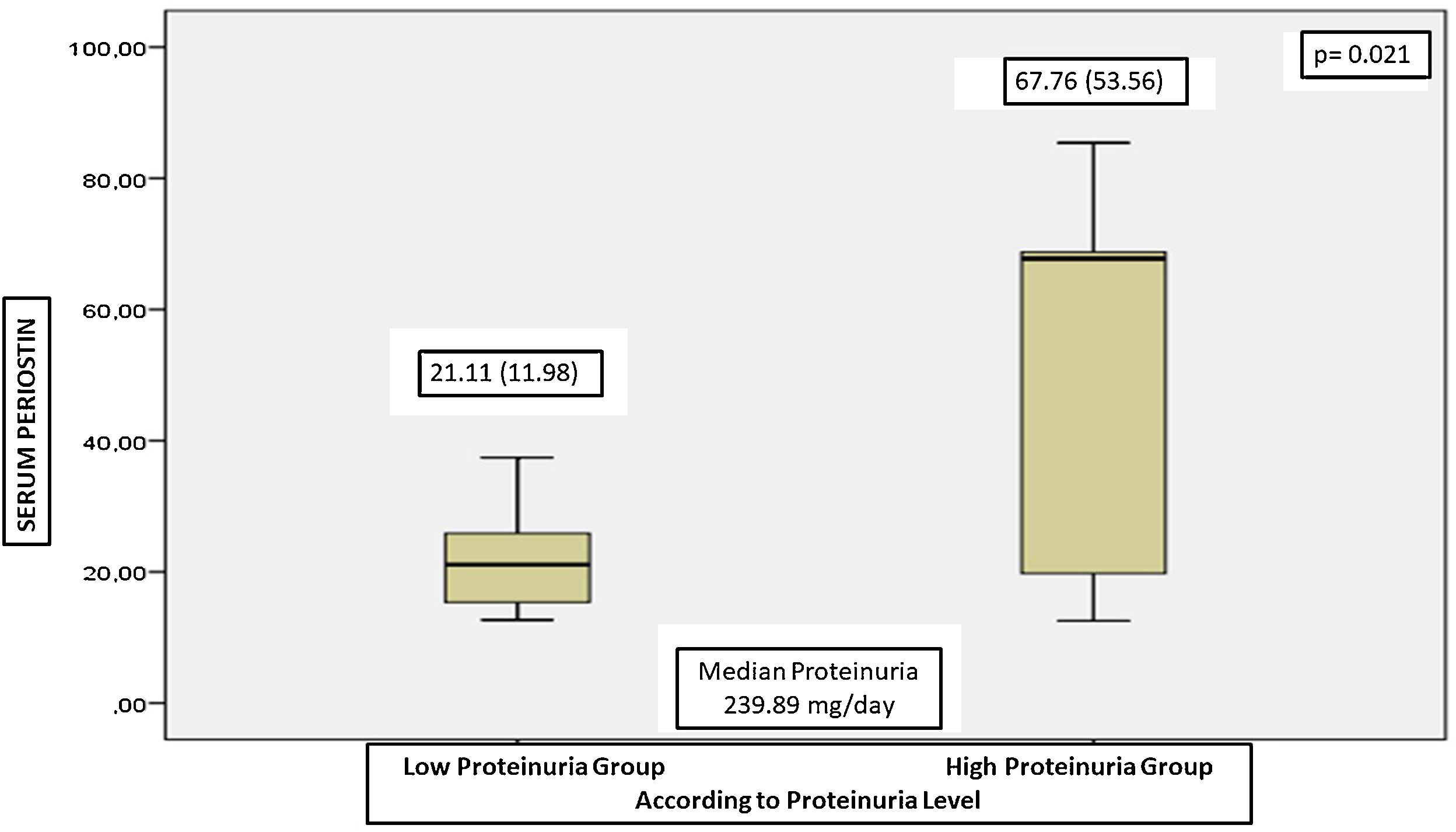
ResultsIn FD patients, serum periostin was negatively correlated with age of first symptom and GFR; and positively correlated with proteinuria and lyso-Gb3. In regression analysis, we found that serum periostin was the only independent determinant of proteinuria in patients with Fabry disease. The serum periostin levels were significantly lower in patients with low proteinuria, and the serum periostin levels were correlated with proteinuria.
DiscussionPeriostin may be a valuable marker of Fabry nephropathy and proteinuria. Periostin seems to be one of the molecules that may have an important role in the management of the fibrotic process in Fabry nephropathy. We think that the role of periostin among these mechanisms is worth investigating. In addition to standard ERTs, periostin-reducing therapies may contribute to better kidney survival in Fabry disease. Progressive fibrosis processes caused by periostin in patients with Fabry disease are still a hidden issue waiting to be clarified. Progressive fibrosis processes caused by periostin in Fabry patients are still a hidden issue waiting to be clarified.
En la enfermedad de Fabry (EF), son diferentes los factores primarios tales como el depósito de glicoesfingolípidos que inicia el daño renal, y los factores secundarios que progresan de daño renal a fibrosis. Periostina es una molécula de importancia probada en la inflamación renal y la fibrosis. Se ha demostrado previamente que periostina juega un papel esencial en el proceso que causa la fibrosis renal, y que su expresión se incrementa en muchas enfermedades renales. En el presente estudio, nuestro objetivo fue revelar la relación entre la periostina y la nefropatía de Fabry.
Material y métodoEste estudio transversal incluyó 18 pacientes con EF (10 varones y 8 mujeres) con indicación de terapia enzimática (ERT) y 22 controles sanos con edad y sexo similares. En el momento del diagnóstico se escanearon del sistema hospitalario las pruebas de alfa-galactosidasa A (α-gal-A) plasmática y globotriaosilsfingosina (lyso-Gb3), proteinuria y función renal de todos los pacientes con EF antes de la ERT. Se analizó el nivel de periostina en las muestras séricas recogidas y almacenadas antes de realizar la ERT. Se investigaron los parámetros relacionados con los niveles séricos de periostina en la enfermedad de Fabry.
ResultadosEn los pacientes con EF, el nivel de periostina sérica se correlacionó negativamente con la edad del primer síntoma y la GFR, y positivamente con proteinuria y lyso-Gb3. En el análisis de regresión, encontramos que el nivel de periostina sérico fue el único determinante independiente de proteinuria en los pacientes con EF. Los niveles séricos de periostina fueron significativamente menores en los pacientes con baja proteinuria, correlacionándose los niveles séricos de periostina con proteinuria.
DiscusiónLa periostina puede ser un marcador valioso de nefropatìa de Fabry y proteinuria. Periostina parece ser una de las moléculas que pueden jugar un papel importante en el manejo del proceso fibrótico en la nefropatía de Fabry. Creemos que merece investigar el papel de periostina entre estos mecanismos. Además de las ERT estándar, las terapias reductoras de periostina pueden contribuir a una mejor supervivencia del riñón en la EF. Los procesos de fibrosis progresiva causados por periostina en los pacientes con EF siguen constituyendo una cuestión poco conocida que debe esclarecerse.
Fabry disease (FD) is a X-linked systemic disease characterized by the accumulation of globotriaosylceramide (Gb-3) in the extracellular space secondary to decreased activity of the lysosomal enzyme alpha-galactosidase-A (α-gal-A).1 Inflammation and subsequent fibrosis play an essential role in developing and progressing nephropathy in FD. The main culprits of the fibrotic process in Fabry nephropathy are still unclear. Previous studies showed that the deposition of Gb-3 and globotriaosylsphingosine (lyso-Gb3) may be responsible for these undesirable results.2 While glycosphingolipid accumulation is initially reversible, as the accumulation increases over time, it causes irreversible tissue injury and organ dysfunction. In Fabry nephropathy, glycosphingolipid accumulate in tubular and vascular structures, mainly glomerular structures, and progress to the fibrosis process which leads to chronic kidney injury over time.3 Early enzyme replacement therapy (ERT) slows the deterioration of kidney function, but ERT cannot equally affect the progression of glomerulosclerosis or proteinuria in all patients and often cannot prevent progressive kidney injury, especially in patients with proteinuria greater than 1g/day.4 To prevent kidney injury in FD, the search for new treatments continues.
Periostin (osteoblast-specific factor 2) is an extracellular matrix protein expressed in various tissues during embryonic development and mostly synthesized from collagen-rich connective tissues in adults.5 While it is expressed extensively in renal tissues during nephrogenesis, its expression is very low in healthy renal tissues in adults and increases significantly after kidney injury.6 Previously, it has been shown that there is periostin expression from cyst epithelium in autosomal dominant polycystic kidney patients, periostin expression in interstitial areas and tubule epithelium, especially in fibrotic areas in glomerulopathies, and increased periostin expression in chronic kidney disease patients.7 The proposed mechanism in the relationship between periostin and kidney injury is that periostin mediates and amplifies kidney inflammation and fibrosis in the kidney epithelium. As a result of all studies, it was concluded that increased expression of periostin is associated with kidney injury, and deficiency or inhibition of periostin is associated with better preservation of kidney structure and function.
In FD, primary factors that initiate kidney injury and secondary factors that continue and advance the process to fibrosis are different. Therefore, replacing only the deficient enzyme may not be able to stop kidney injury in all patients. We planned this study based on our hypothesis that periostin is one of the perpetuating factors causing kidney injury. If we know the secondary factors that cause kidney injury, we can reach new treatment options and be more successful in treating Fabry nephropathy.
Material-methodsThis is a cross-sectional study. Ethics committee approval was obtained from our institution (2018/1266). Written informed consent was obtained from FD patients and control patients.
The study included eighteen FD patients (10 males, 8 females) diagnosed between 2014 and 2020. Twenty-two healthy adults (12 males, 10 females) with demographic characteristics similar to the FD patient group were included in the study as the control group. At the time of diagnosis, 5 cc venous blood samples were taken from all FD patients, centrifuged, and serum samples were stored at −80°C. All FD patients included in the study had an indication for ERT. When the target number of patients was reached (June 2022), serum periostin levels were studied in serum samples stored at −80°C. Plasma α-gal-A and lyso-Gb3 levels at the time of diagnosis were recorded from the hospitals’ system. In addition, a review of medical records (including information on age, sex, weight, height, disease duration, medications, history of other diseases, proteinuria and kidney function tests at the time of diagnosis) was undertaken from our hospitals’ system.
Inclusion criteria were (1) 18–70 years of age (2) decreased (<2.5nmol/ml/h) α-gal-A activity in dried blood spots (DBS) in male patients, (3) presence of GLA gene mutation associated with FD in female patients, (4) patients with an indication for initiation of ERT due to FD. Exclusion criteria were (1) Active infection, (2) presence of diabetes mellitus, (3) presence of glomerulonephritis, lupus nephritis, systemic vasculitis proven by kidney biopsy. Inclusion criteria as a control group were 18–70 years of age, non-smoker, and no history of chronic disease. Having a FD family history, smoking, a history of chronic disease, and having signs of acute infection at the time of blood tests were considered exclusion criteria for control groups.
Diagnosis of Fabry diseaseThe screening of FD was performed by assessing α-gal-A activity<2.5nmol/ml/h in DBS and was confirmed by GLA gene mutation analysis. The criteria for the diagnosis of FD were α-gal-A activity<2.5nmol/ml/h in male patients and the presence of a genetic mutation associated with FD in female patients.
GLA gene sequencingGLA gene was sequenced using the MiSeq next generation sequencing (NGS) platform, a FDA approved diagnostic system (Illumina, San Diego, CA, USA).
Measurement of plasma Lyso-Gb3 levelsPlasma lyso-Gb3 levels were measured via tandem mass spectrometry method from DBS before ERT at the diagnosis. The reference range for the plasma lyso-Gb3 level was accepted as below 1.3ng/ml.
Biochemical analysesVenous blood samples were drawn after an overnight fast and stored at −80°C for biochemical analyses in FD and control patients. All biochemical analyses were undertaken in the Central Biochemistry Laboratory of our hospital. Serum creatinine was measured with Jaffe Method. Serum c-reactive protein (CRP) levels were measured with an immunoturbidimetric assay (Diasis Diagnostic System) using an automated clinical chemistry analyzer. Others biochemical analyses were undertaken using an oxidase-based technique at Roche/Hitachi Modular System (Mannheim, Germany) in the biochemistry laboratory. The 24-h urinary proteinuria levels detected within the first one week of diagnosis. Total protein concentration levels were measured by a turbidometric assay using benzethonium chloride. The results were expressed as mg/L. The estimated glomerular filtration ratio (e-GFR) was estimated using the abbreviated MDRD equation in all patients.
Measurement of serum periostin levelsFor the measurement of periostin level, 5ml of venous blood was taken into biochemistry tubes. The sample was placed in flat plastic tubes containing a preservative and centrifuged for 5min at 4000rpm at 5°C. Results were evaluated by the enzyme-linked immunosorbent assay (ELISA) method using serum periostin kits (Biont, China). Reference ranges for periostin were 6.25–400ng/ml.
Statistical analysesThe data obtained were evaluated using the Statistical Package for Social Sciences for Windows 21.0 (SPSS Inc., Chicago, Illinois, USA) statistical program. Descriptive statistics were determined for each variable. Data were expressed as mean±standard deviation. A statistically significant difference between the groups was determined by the χ2 test for categorical variables. Nonparametric statistics (Mann–Whitney U) and parametric statistics (independent sample t test) were all used for continuous variables. Associations between the variables were ex-plored using Spearman's rho test. Lineer regression analysis was performed to determine independent predictors for proteinuria. Factors with a p value of <0.2 were included in the univariate analysis in the regression test, while those that were significant in the univariate analysis were included in the multivariable evaluation. A statistically significant difference was considered when the p-value <0.05.
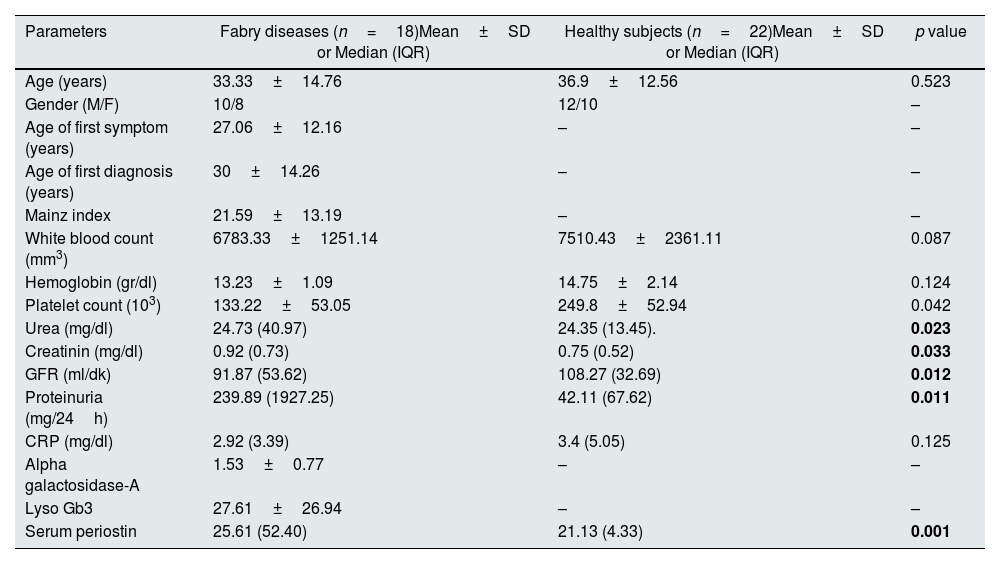
ResultsCharacteristics of the patient and control groupA total of 18 FD patients (10 male, 8 female) with ERT indication and 22 healthy controls (12 males 10 females) of similar age and gender were included in the study. The mean age of the patients was 33.33±14.76. The mean age of first symptom was 27.06±12.16, and the mean age of first diagnosis was 30±14.26. There were significant differences in urea, creatinine, GFR, and proteinuria between the FD patient and the healthy control group (Table 1).
Demographic characteristics and laboratory results of Fabry patients.
| Parameters | Fabry diseases (n=18)Mean±SD or Median (IQR) | Healthy subjects (n=22)Mean±SD or Median (IQR) | p value |
|---|---|---|---|
| Age (years) | 33.33±14.76 | 36.9±12.56 | 0.523 |
| Gender (M/F) | 10/8 | 12/10 | – |
| Age of first symptom (years) | 27.06±12.16 | – | – |
| Age of first diagnosis (years) | 30±14.26 | – | – |
| Mainz index | 21.59±13.19 | – | – |
| White blood count (mm3) | 6783.33±1251.14 | 7510.43±2361.11 | 0.087 |
| Hemoglobin (gr/dl) | 13.23±1.09 | 14.75±2.14 | 0.124 |
| Platelet count (103) | 133.22±53.05 | 249.8±52.94 | 0.042 |
| Urea (mg/dl) | 24.73 (40.97) | 24.35 (13.45). | 0.023 |
| Creatinin (mg/dl) | 0.92 (0.73) | 0.75 (0.52) | 0.033 |
| GFR (ml/dk) | 91.87 (53.62) | 108.27 (32.69) | 0.012 |
| Proteinuria (mg/24h) | 239.89 (1927.25) | 42.11 (67.62) | 0.011 |
| CRP (mg/dl) | 2.92 (3.39) | 3.4 (5.05) | 0.125 |
| Alpha galactosidase-A | 1.53±0.77 | – | – |
| Lyso Gb3 | 27.61±26.94 | – | – |
| Serum periostin | 25.61 (52.40) | 21.13 (4.33) | 0.001 |
M/F: male/female, GFR: glomerular filtration rate, CRP: c-reactive protein, lyso-Gb3: globotriaosylsphingosine.
Serum periostin in the FD patient group were statistically significantly higher than in the control group (p=0.001). Demographic characteristics and laboratory findings of our study and control groups are shown in Table 1.
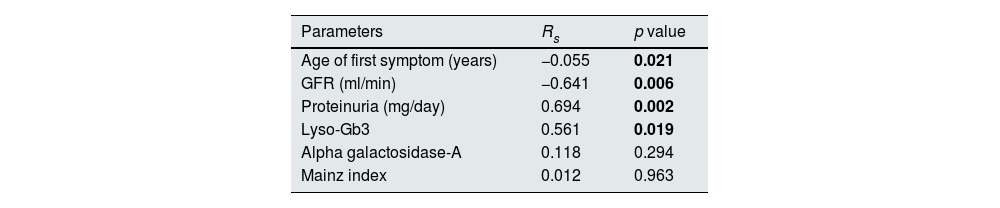
Parameters correlating with serum periostin in fabry patientsWhen the bivariate correlation analysis of serum periostin levels with other parameters in FD patients was performed, serum periostin was found to be negatively correlated with age of first symptom and GFR (rs: −0.055, −0.641, respectively) and positively correlated with proteinuria and lyso-Gb3 (rs: 0.694, 0.561, respectively). There was no correlation between α-gal-A and Mainz index with serum periostin (Table 2).
Bivariate correlation analysis between serum periostin and other parameters in Fabry patients.
| Parameters | Rs | p value |
|---|---|---|
| Age of first symptom (years) | −0.055 | 0.021 |
| GFR (ml/min) | −0.641 | 0.006 |
| Proteinuria (mg/day) | 0.694 | 0.002 |
| Lyso-Gb3 | 0.561 | 0.019 |
| Alpha galactosidase-A | 0.118 | 0.294 |
| Mainz index | 0.012 | 0.963 |
GFR: glomerular filtration rate, lyso-Gb3: globotriaosylsphingosine.
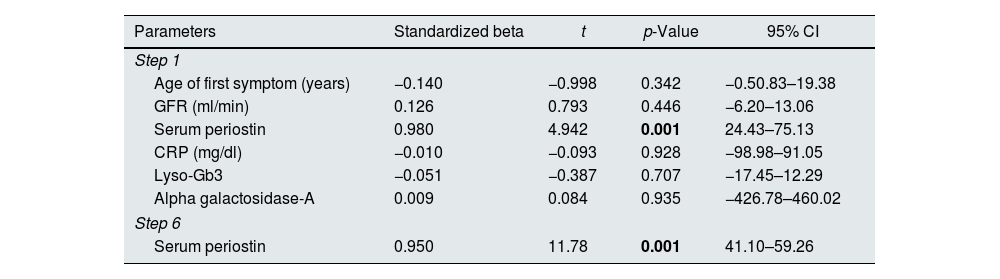
Proteinuria is one of the earliest and most essential determinants of kidney failure in FD patients. In our study, serum periostin was found to be the only independent predictor of proteinuria in Fabry patients (p=0.001) (Table 3). The serum periostin was positively correlated with proteinuria (rs: 0.694, p=0.002).
Independent variable of serum proteinuri in patients with Fabry disease.
| Parameters | Standardized beta | t | p-Value | 95% CI |
|---|---|---|---|---|
| Step 1 | ||||
| Age of first symptom (years) | −0.140 | −0.998 | 0.342 | −0.50.83–19.38 |
| GFR (ml/min) | 0.126 | 0.793 | 0.446 | −6.20–13.06 |
| Serum periostin | 0.980 | 4.942 | 0.001 | 24.43–75.13 |
| CRP (mg/dl) | −0.010 | −0.093 | 0.928 | −98.98–91.05 |
| Lyso-Gb3 | −0.051 | −0.387 | 0.707 | −17.45–12.29 |
| Alpha galactosidase-A | 0.009 | 0.084 | 0.935 | −426.78–460.02 |
| Step 6 | ||||
| Serum periostin | 0.950 | 11.78 | 0.001 | 41.10–59.26 |
GFR: glomerular filtration rate, CRP: c-reactive protein, lyso-Gb3: globotriaosylsphingosine.
The patients were divided into two groups according to their initial proteinuria levels. Those with baseline proteinuria below the median proteinuria level were divided into the low proteinuria group, and those above the median proteinuria level were divided into the high proteinuria group, and subgroup analyses were performed. There were 10 patients in low proteinuria group and 8 patients in high proteinuria group. The serum periostin of the patients in the group with high proteinuria group were significantly higher than low proteinuria group (Fig. 1).
DiscussionWe found some important results in the study in which serum periostin levels were evaluated in Fabry patients. First, serum periostin was negatively correlated with age of the first symptom and GFR; and positive correlated with proteinuria and lyso-Gb3. The other significant result, the only independent predictor of proteinuria, one of the most critical kidney failure progression indicators, was serum periostin, and we found that patients with high proteinuria had high serum periostin levels. To the best of our knowledge, our study is the first to examine the relationship between serum periostin and FD, and for the first time in our research, it was shown that serum periostin is higher in FD patients than in healthy individuals.
We found that age of the first symptom and GFR were negatively correlated with serum periostin. Renal expression of periostin increases in correlation with increasing age and decreased kidney function.8 FD patients are divided into classical and atypical FD patients according to their clinical findings, and symptom onset and organ dysfunctions are earlier in patients with classical FD.9 The mean age of symptom onset in our FD patient group is 27, and we can consider that all of our patients are late-onset. Periostin is a marker that increases in inflammatory processes; we may think that inflammation is more in FD patients with early onset of symptoms, and therefore periostin increases.10 Periostin levels increase in chronic kidney disease.11 GFR and periostin are expected to be negatively correlated in FD patients, like in our study. However, only 5 (27.7%) of the patients in our study had a GFR below 60ml/min. Most of our patients consisted of patients with normal/near-normal GFR and whose kidney functions were not severely reduced shows us that a process independent of chronic kidney disease is observed in the relationship between serum periostin and FD.
The expression of periostin in healthy and damaged kidneys and its role on kindey function are not yet fully understood. While its expression in healthy kidney tissues is extremely low in adults, periostin, which is re-expressed in renal tissues in case of inflammatory injury, has an important role in the process of renal inflammation and fibrosis.12,13 Many studies have shown that renal periostin expression increases in different patient groups, and renal inflammation and fibrosis decrease with measures to reduce the level of periostin.12–16 Our study showed for the first time that periostin levels are higher in FD patients than in the healthy population.
There are similarities between the pathogenesis of diabetic nephropathy and Fabry nephropathy. Annual GFR losses are similar, kidney injury begins as proteinuria in both diseases, and the presence of secondary factors that continue the injury after the primary metabolic components that initiate renal impairment are similar in the two diseases. Even after the metabolic components that initiated the injury are eliminated, the injury mechanism may continue to progress in both disorders. Following early podocyte injury in both diseases, the fibrotic process by extracellular matrix elements begins.17 In FD, levels of lyso-Gb3, an active lipid metabolite, are elevated, and lyso-Gb3 induces the proliferation of vascular smooth muscle cells.2 Previously, the secondary pathway inducing renal fibrosis in FD was shown to be TGF-β activation following lyso-Gb3 deposition.17,18 ERTs reduce endothelial lyso-Gb3 levels, but sphingolipid deposits and their clinical repercussions are not completely normalized.2 In addition, starting ERT after the onset of tissue fibrosis does not regress the fibrosis and does not completely prevent organ dysfunction.4 While all these data confirm the role of sphingolipid deposits as the primary trigger causing FD nephropathy, the secondary pathways that still sustain the injury are not clearly known. The fact that both sphingolipid depositions in Fabry nephropathy and periostin expression during kidney injury begin first in the same kidney areas (glomerular areas) raises doubts about the possible role of periostin in Fabry nephropathy.13 The idea that periostin may be a secondary mechanism that induces TGF-β and perpetuates kidney injury caused by sphingolipid accumulation in FD patients seems to be a plausible explanation for the mechanism of worsening kidney function in these patients. We think that periostin may also have an essential role in answering the question of why ERTs do not prevent progression to end-stage kidney disease in some patients.
Although no correlation can be established between serum or urine Gb3 levels and kidney dysfunction, there is a relationship between heart and brain lesions and serum free sphingolipid levels.18 These circulating molecules, primarily lyso-Gb3, are the main responsible factors for initiating the process of kidney dysfunction that causes vasculopathy and vascular remodeling in FD, thus resulting in renal fibrosis.17 In our study, we found that lyso-Gb3 and serum periostin was correlated. In patients with high Lyso-Gb3 levels, an already elevated inflammatory state is expected. Lyso-Gb3 increases the expression of TGF-β and macrophage inhibitory factor receptor CD74, which causes fibrosis in podocytes.17 Periostin-induced TGF-β-mediated fibrosis process following the inflammatory pathway initiated by lyso-Gb3 may be a new hypothesis for the development of Fabry nephropathy.
In Fabry nephropathy, glomerular sclerosis and fibrosis process has already begun in the kidney before significant proteinuria or kidney dysfunction is reflected in the clinic.19 Considering the lack of correlation of circulating Gb3 accumulations with kidney dysfunction, there is a need for valuable markers to predict renal survival. It is essential to identify predictors of poor clinical outcomes in Fabry disease. Proteinuria greater than 1g/day, GFR less than 45ml/min, or the presence of glomerulosclerosis demonstrated by kidney biopsy is important indicators of inadequate response to ERT.4 The presence of multiple factors influencing proteinuria or GFR makes it unreliable to estimate renal survival from these parameters. Therefore, there is a need for reliable, non-invasive, and easy-to-predict biomarkers of renal survival and fibrosis in Fabry patients. In our study, we found that serum periostin was higher in patients whose has high proteinuria levels, and serum periostin was correlated with lyso-Gb3 and proteinuria. Previously, it has been shown that proteinuria levels and urinary periostin levels are correlated in diseases such as diabetic and hypertensive nephropathy, and lupus nephritis.12,20,21 However, this study revealed this relationship for the first time in Fabry nephropathy. Considering that serum periostin levels are an important indicator of the progression to kidney fibrosis, this correlation between serum periostin levels and proteinuria in Fabry patients is important both for the indicator of renal survival and a possible future treatment target.
While beneficial effects of ERT alone on GFR have been demonstrated, it has no proteinuria-reducing effect alone.22,23 Proteinuria significantly regresses in patients who receive RAS blockade in addition to ERT. This ensures long-term preservation of kidney functions.24 One of the mechanisms by which periostin plays a role in kidney injury is the RAS activation pathway. While periostin can activate the RAS, RAS activation causes increased periostin expression.11 Reducing periostin also reduces kidney injury via integrin-linked kinase, integrin-B3, and oxidative stress pathways.11 Given the reducing effect of RAS inhibition on periostin, RAS blocker therapy may also inhibit the inflammatory and fibrotic process induced by periostin in FD patients, resulting in a more significant reduction in proteinuria in addition to ERT.15
Our study has some limitations. First, the study sample is relatively small. Second, the results of our study consisted of families that included only Turkish Fabry patients. Therefore, applying these results to different races would not be correct. Although many factors that could affect serum periostin levels were excluded during the inclusion process, we think that much more valuable information can be obtained in the development of nephropathy in FD about periostin by looking at the expression of renal periostin. And finally, since our study was a cross-sectional study, it is impossible to draw clear conclusions about the role of periostin in the pathogenesis of Fabry nephropathy.
ConclusionIn the development of Fabry nephropathy, some secondary mechanisms may be in addition to the primary inflammatory process that starts with the accumulation of lyso-Gb3, and continue the kidney injury processes after ERT. Periostin seems to be one of the molecules that may have an essential role in the management of the fibrotic process in Fabry nephropathy. We think that the role of periostin among these mechanisms is worth investigating. In addition to standard ERTs, periostin-reducing therapies may contribute to better kidney survival in Fabry disease. Progressive fibrosis processes caused by periostin in patients with Fabry disease are still a hidden issue waiting to be clarified.
Informed consentEthics committee approval was obtained from the institution for the study and written consent was obtained from all patients.
Conflicts of interestAll authors declare that there is no conflict of interest in this study.