Patients who are candidates for a second kidney transplant (SKT) frequently have a higher level of panel reactive antibodies (PRA). We assessed the allosensitisation change after a first graft failure (GF), its predictors and impact on retransplantat outcomes.
We retrospectively selected 140 adult patients who received a SKT. Recipient and donor characteristics were analyzed. We defined the delta PRA (dPRA) as the difference between peak PRA before the SKT and first one (cohort median value=+10%). Logistic regression analysis was used to determine risk factors for dPRA≥10% and acute rejection (AR) in the SKT. Univariable and multivariable Cox analysis was applied to assess independent predictors of second GF.
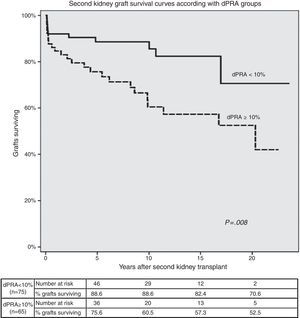
Risk factors for dPRA≥10% at SKT were AR (OR=2.57; P=0.022), first graft survival <1 year (OR=2.47; P=0.030) and ABDR HLA mismatch (OR=1.38 per each mismatch; P=0.038). AR in the SKT was associated with dPRA≥10% (OR=2.79; P=0.047). Induction with a lymphocyte-depleting agent had a protective effect (OR=0.23; P=0.010). SKT survival was lower (P=0.008) in patients with a dPRA≥10% (75.6%, 60.5% in dPRA≥10%; 88.6%, 88.6% in dPRA<10% patients at 5 and 10 years, post-transplant respectively).
Multivariable Cox regression showed that dPRA≥10% (HR=2.38, P=0.042), delayed graft function (HR=2.82, P=0.006) and AR (HR=3.30, P=0.001) in the SKT were independent predictors of retransplant failure.
This study shows that an increased allosensitisation at retransplant was associated with the degree of HLA mismatch and led to poorer outcomes. De-emphasis of HLA matching in current allocation policies may be undesirable, particularly in patients with a higher chance of needing a SKT.
Los pacientes candidatos a un segundo trasplante de riñón (STR) tienen a menudo un nivel superior de panel reactivo de anticuerpos (PRA). Evaluamos el cambio de alosensibilización después de un primer fracaso del injerto, sus predictores y repercusión en los resultados del retrasplante.
Elegimos retrospectivamente a 140 pacientes adultos que recibieron un STR. Analizamos las características de los receptores y de los donantes. Definimos el delta PRA (dPRA) como la diferencia entre el pico de PRA antes del STR y el primero (cohorte mediana=+10%). Se aplicó análisis de regresión logística para determinar los factores de riesgo para dPRA≥10% y rechazo agudo (RA) en STR y análisis univariado y multivariado de Cox para evaluar predictores independientes de fallo del retrasplante.
Los factores de riesgo para dPRA≥10% fueron: RA (OR=2,57; p=0,022), supervivencia del primero injerto menor a un año (OR=2,47; p=0,030) e incompatibilidad HLA ABDR (OR=1,38 por cada mismatch; p=0,038). El RA en el STR fue asociado con dPRA≥10% (OR=2,79; p=0,047). La inducción con un agente depletor de linfocitos tuvo un efecto protector (OR=0,23; p=0,010). La supervivencia del STR fue menor en pacientes con dPRA≥10% (75,6; 60,5% en dPRA≥10%; y 88,6; 88,6% en dPRA<10%; a los 5 y 10 años).
La regresión multivariada de Cox mostró que dPRA≥10% (HR=2,38; p=0,042), función retardada de injerto(HR=2,82; p=0.006) y RA (HR=3,30; p=0,001) en STR fueron factores predictores independientes de fracaso en el retrasplante.
Este estudio muestra que un incremento en la alosensibilización de retrasplante se ha asociado con el grado de incompatibilidad HLA y puede conducir a resultados más pobres en STR. La falta de énfasis en la compatibilidad de HLA en las políticas actuales de asignación puede no ser deseable, especialmente en pacientes con una mayor probabilidad de necesitar un STR.
Renal transplantation is the best renal replacement therapy for end-stage renal disease (ESRD).1 In general, a successful kidney transplant (KT) provides substantially longer survival and better quality of life than dialysis.2 Renal allograft survival has increased over past decades due to technical and pharmaceutical progresses, namely with advances in immunosuppression. So, acute rejection (AR) rates during the first year after KT have decreased over the last 15 years to incidences of 10–15%.3 The late rejection rates have also improved over the last years.4 The average survival of a KT is 8 years for deceased and 12 years for living donor transplants.5 Long-term kidney graft survival rates are lower in the United States when compared with Europe (both for deceased and living donor grafts). Between 2005 and 2008, overall 5 and 10 year graft survival rates for deceased donor grafts in Europe were 77 and 56% respectively when compared to American population (Whites, 71 and 46%, Hispanic, 73 and 48%, and African American, 62 and 34%). The reasons why that happens are unknown.6
HLA antibodies are a well-known and serious barrier to successful transplantation and HLA matching is a predictor of allograft and patient survival.7 HLA antibodies usually develop in association with contact to non-self HLA molecules through the exposure to blood products, foreign tissue during transplantation or during pregnancy. It can also happen spontaneously through cross-sensitization from infection and pro-inflammatory events.8,9
Although these antibodies may be donor-specific or non-donor-specific, their presence may increase the risk for acute and chronic rejection. Patients who are highly sensitized face longer waiting times, more graft rejection and consequently poorer graft outcomes.7,10–13
Historically, cytotoxic PRA (panel reactive antibodies) was the main tool to define immunologically high-risk KT recipients.14 An increased PRA means a higher allosensitization and leads to longer waiting times for a second graft. Moreover, it is a strong risk factor for AR and consequently for graft loss after retransplantation.13,15 A few years ago, a study from John Hopkins transplant center suggested, that a more mismatched kidney transplant leads to a higher degree of allosensitization after transplant loss.16 The third most frequent cause for being in the waiting list in United States is a previous failed graft.17 Importantly, over the past decade, the numbers of candidates with PRA≥20%, and particularly ≥80%, have increased18 and this trend particularly affects candidates to a second transplant.19
The authors aimed to evaluate the net change of allosensitization after a first graft failure, its risk factors and impact on retransplantation outcomes.
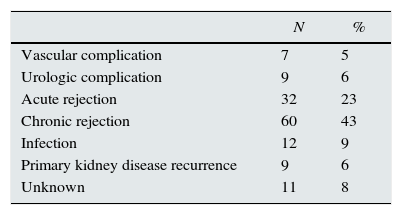
Material and methodsSubjectsFrom our unit database, we retrospectively selected 140 adult patients who received a first and second KT. First and second KT median year was 1992 (range: 1983–2009) and 2000 (range: 1986–2012), respectively. Causes of first KT failure are presented in Table 1. All patients were transplanted with a negative pre-transplant T and B-lymphocyte cytotoxicity crossmatch. The Institutional Review Board at Centro Hospitalar do Porto approved this study.
Cytotoxic panel reactive antibodies (PRA)Cytotoxic PRA test was performed before transplant in all patients with sera collected every 3 months while in waiting list, using total peripheral blood lymphocytes collected from a HLA-typed representative donor population. It was considered positive if cell lyses remained present after dithiothreitol (DTT) treatment, identifying only IgG anti-HLA isotypes positive cases.
ImmunosuppressionGiven the retrospective nature of this analysis, spanning 30 years of kidney transplantation, multiple practices in immunosuppression were in place: before 1990, only azathioprine and cyclosporine (no microemulsion) were available; between 1990 and 2000, cyclosporine microemulsion and mycophenolate mofetil (MMF) were introduced and wider use of antithymocyte globulin was seen; after 2000, tacrolimus was largely the calcineurin inhibitor of choice and the use of IL-2 receptor antagonists was current for induction therapy. Data on the strategy of immunosuppression withdrawal after first kidney graft failure was not available for analysis.
Data analysis and outcomesData regarding recipient and donor characteristics and pre- and post-transplantation variables were collected retrospectively in all patients (Table 2). Delayed graft function was defined as dialysis requirement in the first week after transplantation. All AR episodes were biopsy proven. Graft survival was analyzed considering graft failure censored for death with a functioning graft. Given the wide period of KT analyzed, we defined an era variable according with the year of transplant as follows: KT era 1 (1983–1999) and era 2 (2000–2012). PRA levels considered in this analysis were the peak values observed before the first and second KT (in the second KT, only the values obtained after failure of first graft were considered). We defined the delta PRA (dPRA) as the difference between peak PRA before the second and first KT. In order to clarify, data analyzed in Tables 3 and 4 refers to variables associated with the first KT, while data from Tables 5 and 6 reports information from the second KT.
Comparison of baseline characteristics between the first and second transplant.
| At first KT | At second KT | P | |
|---|---|---|---|
| N=140 | N=140 | ||
| Recipient age (years), mean±SD | 30.0±11.8 | 38.1±12.3 | – |
| Female recipient, n (%) | 61 (43.6) | – | |
| Donor age (years), mean±SD | 31.4±14.1 | 35.1±15.4 | 0.027 |
| Female donor, n (%) | 41 (30.6) | 38 (29.2) | 0.892 |
| Living donor, n (%) | 0 | 15 (10.7) | <0.001 |
| Transfusions before 1st transplant, n (%) | 90 (64.3) | – | |
| Transfusions after 1st transplant, n (%)# | 48 (34.3) | – | |
| Pregnancies before 1st transplant, n (%)* | 29 (47.5) | – | |
| Dialysis vintage (months), median (IQR)§ | 18 (3–40) | 37 (12–73) | <0.001 |
| Transplant year, median (range) | 1992 (1983–2009) | 2000 (1986–2012) | – |
| KT era 2, n (%) | 22 (16) | 76 (54) | – |
| ABDR HLA mismatch, mean±SD | 2.91±1.36 | 2.99±1.27 | 0.390 |
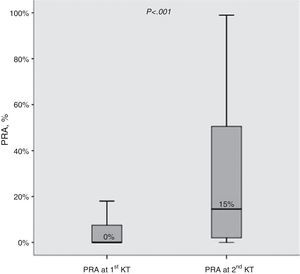
| PRA (%), median (IQR) | 0 (0–8) | 15 (2–51) | <0.001 |
| PRA>0%, n (%) | 60 (42.9) | 111 (79.3) | <0.001 |
| dPRA (%), median (IQR) | 10 (0–38) | – | |
| ATG induction, n (%) | 43 (30.7) | 80 (57.1) | <0.001 |
| Antiproliferative, n (%) | <0.001 | ||
| None | 88 (62.9) | 18 (12.9) | |
| Azathioprine | 29 (20.7) | 40 (28.6) | |
| Mycophenolate mofetil | 23 (16.4) | 82 (58.6) | |
| Calcineurin inhibitors, n (%) | <0.001 | ||
| None | 15 (10.7) | 0 | |
| Cyclosporine | 119 (85) | 81 (57.9) | |
| Tacrolimus | 6 (4.3) | 59 (42.1) | |
| Delayed graft function, n (%) | 68 (48.6) | 42 (30.0) | 0.002 |
| Acute rejection, n (%) | 76 (54.3) | 30 (21.4) | <0.001 |
| Kidney graft failure, n (%) | 140 (100) | 35 (25.0) | – |
| Follow-up time (months) after KT, median (IQR) | 32 (1–106) | 86 (27–161) | – |
KT, kidney transplant; SD, standard deviation; IQR, interquartile range; PRA, panel reactive antigen; dPRA, delta panel reactive antigen; ATG, anti-thymocyte globulin.
Comparison of characteristics from the first transplant according to delta PRA (dPRA) groups.
| dPRA<10% n=75 | dPRA≥10% n=65 | P | |
|---|---|---|---|
| Recipient age (years), mean±SD | 30.9±11.8 | 29.9±11.7 | 0.305 |
| Female recipient, n (%) | 37 (49.3) | 24 (36.9) | 0.140 |
| Donor age (years), mean±SD | 32.8±16.9 | 29.8±17.1 | 0.302 |
| Female donor, n (%) | 23 (30.7) | 20 (30.8) | 0.990 |
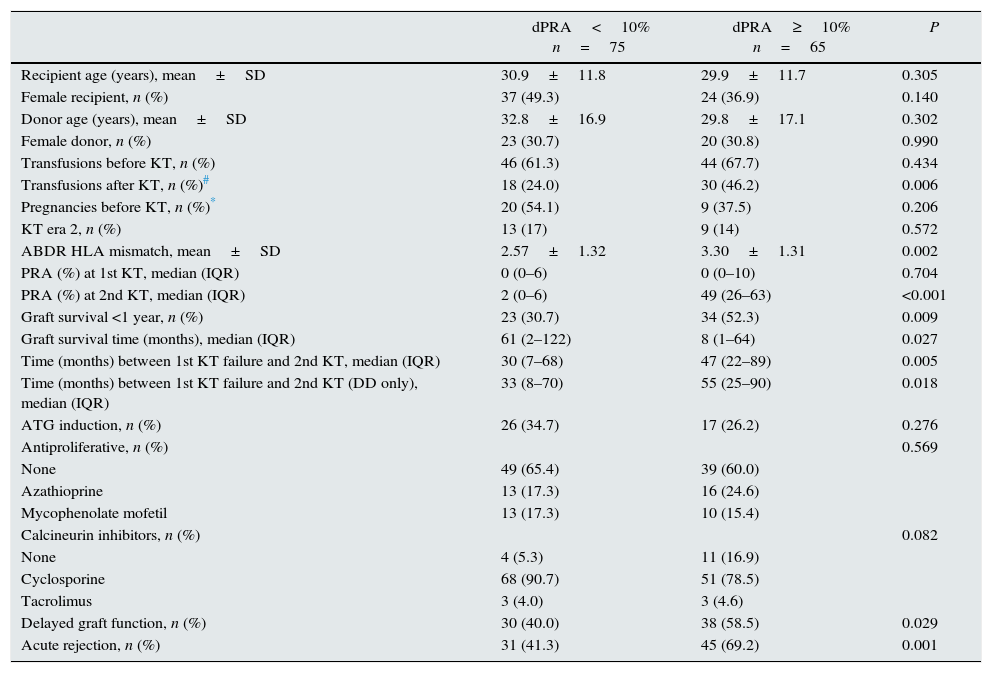
| Transfusions before KT, n (%) | 46 (61.3) | 44 (67.7) | 0.434 |
| Transfusions after KT, n (%)# | 18 (24.0) | 30 (46.2) | 0.006 |
| Pregnancies before KT, n (%)* | 20 (54.1) | 9 (37.5) | 0.206 |
| KT era 2, n (%) | 13 (17) | 9 (14) | 0.572 |
| ABDR HLA mismatch, mean±SD | 2.57±1.32 | 3.30±1.31 | 0.002 |
| PRA (%) at 1st KT, median (IQR) | 0 (0–6) | 0 (0–10) | 0.704 |
| PRA (%) at 2nd KT, median (IQR) | 2 (0–6) | 49 (26–63) | <0.001 |
| Graft survival <1 year, n (%) | 23 (30.7) | 34 (52.3) | 0.009 |
| Graft survival time (months), median (IQR) | 61 (2–122) | 8 (1–64) | 0.027 |
| Time (months) between 1st KT failure and 2nd KT, median (IQR) | 30 (7–68) | 47 (22–89) | 0.005 |
| Time (months) between 1st KT failure and 2nd KT (DD only), median (IQR) | 33 (8–70) | 55 (25–90) | 0.018 |
| ATG induction, n (%) | 26 (34.7) | 17 (26.2) | 0.276 |
| Antiproliferative, n (%) | 0.569 | ||
| None | 49 (65.4) | 39 (60.0) | |
| Azathioprine | 13 (17.3) | 16 (24.6) | |
| Mycophenolate mofetil | 13 (17.3) | 10 (15.4) | |
| Calcineurin inhibitors, n (%) | 0.082 | ||
| None | 4 (5.3) | 11 (16.9) | |
| Cyclosporine | 68 (90.7) | 51 (78.5) | |
| Tacrolimus | 3 (4.0) | 3 (4.6) | |
| Delayed graft function, n (%) | 30 (40.0) | 38 (58.5) | 0.029 |
| Acute rejection, n (%) | 31 (41.3) | 45 (69.2) | 0.001 |
PRA, panel reactive antigen; SD, standard deviation; KT, kidney transplant; IQR, interquartile range; DD, deceased donor; ATG, anti-thymocyte globulin.
Significant risk factors for increased allosensitization (dPRA≥10%) before second kidney transplant, by a multivariable logistic regression analysis.
| OR | 95% CI | P | |
|---|---|---|---|
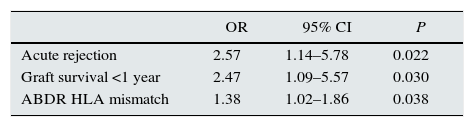
| Acute rejection | 2.57 | 1.14–5.78 | 0.022 |
| Graft survival <1 year | 2.47 | 1.09–5.57 | 0.030 |
| ABDR HLA mismatch | 1.38 | 1.02–1.86 | 0.038 |
Model: Transfusions after transplant; ABDR HLA mismatch, 1st graft survival <1 year, acute rejection, recipient gender, calcineurin inhibitors use.
Significant risk factors for acute rejection in the second graft, by a multivariable logistic regression analysis (variables considering second KT only).
| OR | 95% CI | P | |
|---|---|---|---|
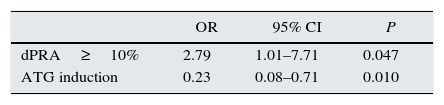
| dPRA≥10% | 2.79 | 1.01–7.71 | 0.047 |
| ATG induction | 0.23 | 0.08–0.71 | 0.010 |
KT, kidney transplant; dPRA, delta panel reactive antigen; ATG, anti-thymocyte globulin.
Model: dPRA≥10%, ATG induction, KT era, delayed graft function, ABDR HLA mismatch, recipient age and gender, calcineurin inhibitors and antiproliferative use.
Analysis of predictors of second graft failure by Cox regression analysis (variables considering second KT only).
| Univariable HR | 95% CI | P | Multivariable HR | 95% CI | P | |
|---|---|---|---|---|---|---|
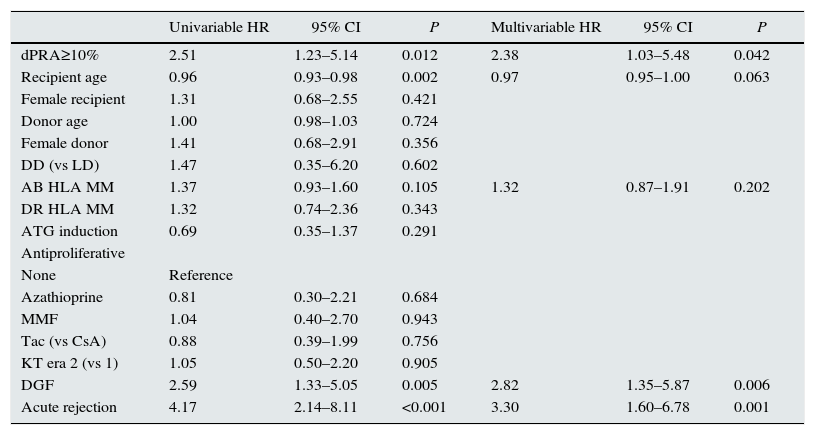
| dPRA≥10% | 2.51 | 1.23–5.14 | 0.012 | 2.38 | 1.03–5.48 | 0.042 |
| Recipient age | 0.96 | 0.93–0.98 | 0.002 | 0.97 | 0.95–1.00 | 0.063 |
| Female recipient | 1.31 | 0.68–2.55 | 0.421 | |||
| Donor age | 1.00 | 0.98–1.03 | 0.724 | |||
| Female donor | 1.41 | 0.68–2.91 | 0.356 | |||
| DD (vs LD) | 1.47 | 0.35–6.20 | 0.602 | |||
| AB HLA MM | 1.37 | 0.93–1.60 | 0.105 | 1.32 | 0.87–1.91 | 0.202 |
| DR HLA MM | 1.32 | 0.74–2.36 | 0.343 | |||
| ATG induction | 0.69 | 0.35–1.37 | 0.291 | |||
| Antiproliferative | ||||||
| None | Reference | |||||
| Azathioprine | 0.81 | 0.30–2.21 | 0.684 | |||
| MMF | 1.04 | 0.40–2.70 | 0.943 | |||
| Tac (vs CsA) | 0.88 | 0.39–1.99 | 0.756 | |||
| KT era 2 (vs 1) | 1.05 | 0.50–2.20 | 0.905 | |||
| DGF | 2.59 | 1.33–5.05 | 0.005 | 2.82 | 1.35–5.87 | 0.006 |
| Acute rejection | 4.17 | 2.14–8.11 | <0.001 | 3.30 | 1.60–6.78 | 0.001 |
KT, kidney transplant; dPRA, delta panel reactive antigen; DD, deceased donor; LD, living donor; MM, mismatches; ATG, anti-thymocyte globulin; MMF, mycophenolate mofetil; Tac, tacrolimus; CsA, cyclosporine; DGF, delayed graft function.
Continuous data was described using mean (standard deviation) or median (interquartile range, IQR) and categorical data was expressed as number (frequencies). Demographic and clinical data were compared using Pearson chi-square test or Fisher's exact test for categorical data and Student's t-test or Mann–Whitney U test for continuous data, as appropriate. Logistic regression analysis was used to determine significant associations between studied variables and the following outcomes: dPRA≥10% and AR in the second graft. Multivariable models were used and included variables presenting P≤0.1 in univariable analysis (outcome dPRA≥10%: transfusions after transplant; ABDR HLA mismatch, graft survival <1 year, AR, recipient gender, calcineurin inhibitors use; outcome AR in the second graft: dPRA≥10%, ATG induction, KT era, delayed graft function, ABDR HLA mismatch, recipient age and gender, calcineurin inhibitors and antiproliferative use) (data not shown). Graft survival curves were visualized using Kaplan–Meier method, with comparison between groups being done by log-rank test. Univariable and multivariable Cox proportional hazards analysis was applied to assess independent predictors of second graft failure; a multivariable model including variables presenting P≤0.1 in univariable analysis was constructed to adjust for potential confounders.
A two-sided P value <0.05 was considered as statistically significant. Statistical calculations were performed using SPSS for Mac, version 23.0 (SPSS Inc., Chicago, IL, USA).
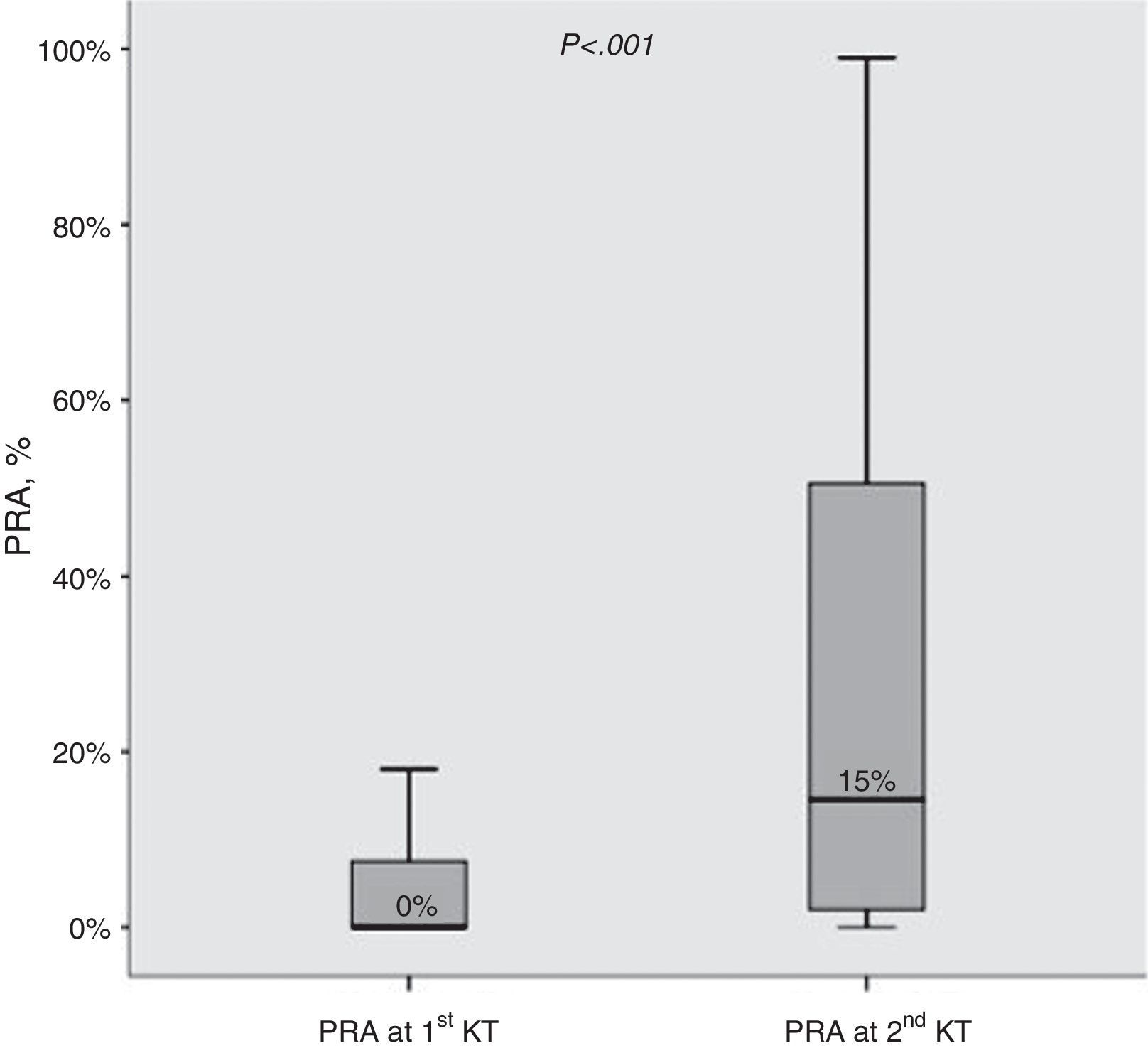
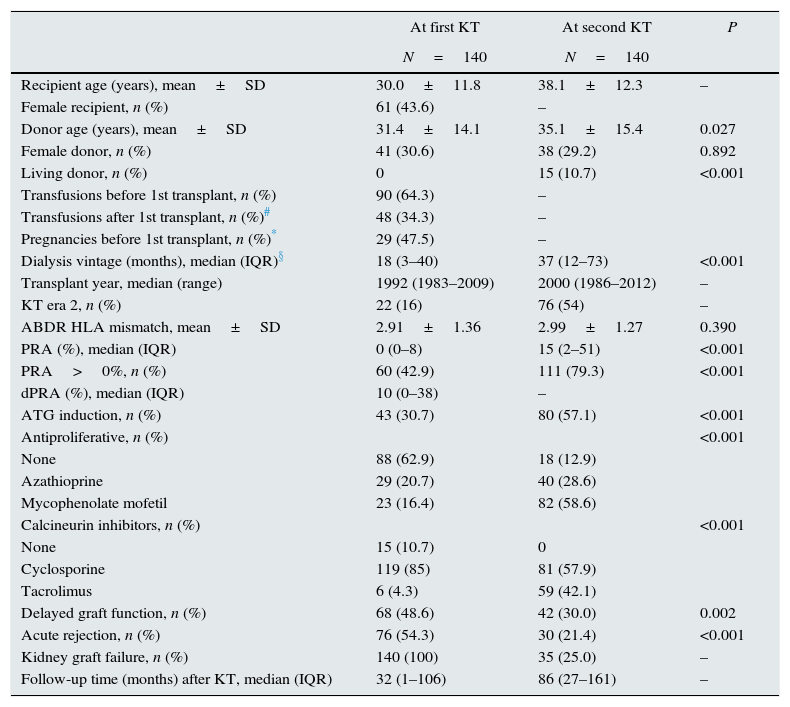
ResultsBaseline characteristics at the first and second transplant (Table 2)Median change in PRA observed before the first and second KT was +10% (IQR 0–38%). Moreover, the proportion of allosensitized patients (PRA>0%) was clearly higher at the second KT (79.3% vs 42.9%; P<0.001). The distribution of PRA levels at first and second KT is shown in Fig. 1.
PRA levels (%) before 1st and 2nd kidney transplant. Median values are shown. Boxes display the interquartile range of the values; whiskers display the lowest and the highest value within 1.5 times below or above the interquartile range, respectively. PARA, panel reactive antibody; KT, kidney transplant.
Patients at the second KT, more frequently, received a graft from a living (P<0.001) and an older (P=0.027) donor, had a longer time on dialysis (P<0.001) and were induced with ATG (P<0.001). No difference was observed in HLA mismatches (P=0.390) between the two KT. Differences in post-transplant outcomes and maintenance immunosuppression regimen reflect known temporal trends, with significantly less patients experiencing acute rejection (P<0.001) and delayed graft function (P=0.002), and more patients receiving MMF (P<0.001) and tacrolimus (P<0.001) at the second KT.
Median time between first KT and failure was 32 months. Median time between second KT and failure (35 patients lost their second graft) or end of follow-up was 86 months.
Analysis of factors associated with higher allosensitization after first failure (Tables 3 and 4)We defined two groups given the median PRA change (dPRA=+10%) before the first and second KT. Patients with dPRA≥10%, at first KT, received a graft with higher HLA mismatch (P=0.002), were more frequently transfused after transplantation (P=0.006), and experienced more delayed graft function (P=0.029) and acute rejection (P=0.001). A trend toward lower use of calcineurin inhibitors in patients with a dPRA≥10% was noticeable (P=0.082). Importantly, patients with shorter first graft survival (<1 year) were more common in dPRA≥10% group (52.3% vs 30.7%; P=0.009). Furthermore, waiting time for retransplantation in this group was higher (47 vs 30 months; P=0.005), even after exclusion of patients receiving a graft from a living donor (55 vs 33 months; P=0.018).
Risk factors for higher allosensitization (dPRA≥10%) after first graft failure were AR (OR=2.57; P=0.022), graft survival <1 year (OR=2.47; P=0.030) and ABDR HLA mismatches (OR=1.38 per each mismatch; P=0.038).
Analysis of factors associated retransplantation outcomes (Tables 5 and 6)Acute rejection occurred in 30 patients (21.4%) in the second KT. Higher allosensitization increase (dPRA≥10%) after first graft failure was a significant risk factor for acute rejection (OR=2.93; P=0.036), and, instead, ATG induction was a protective factor (OR=0.22; P=0.031).
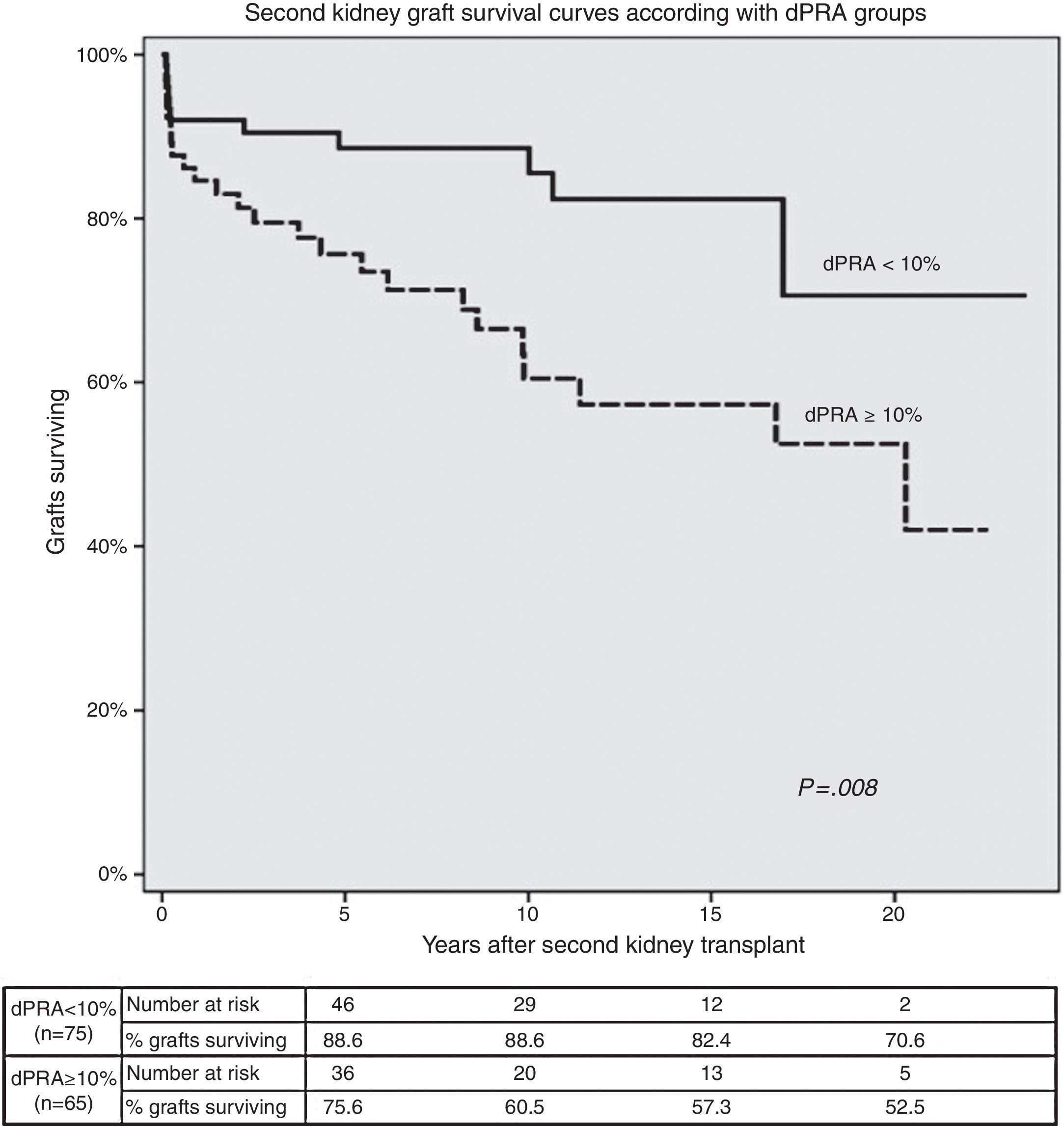
Second KT survival curves according with allosensitization status are shown in Fig. 2. Second graft survival was lower (P=0.008) in patients with higher allosensitization after first graft failure (75.6%, 60.5%, 57.3% and 52.5% in dPRA≥10%; 88.6%, 88.6%, 82.4% and 70.6% in dPRA<10% patients); at 5, 10, 15 and 20 years post-transplant, respectively).
The analysis by multivariable Cox regression showed as independent predictors of second KT failure dPRA≥10% (HR=2.38, P=0.042), delayed graft function (HR=2.82, P=0.006) and AR (HR=3.30, P=0.001).
DiscussionRenal transplantation is the best renal replacement therapy, however, many recipients face graft loss and must return to dialysis treatment.1 Retransplantation provides the best chance for long-term survival and improved quality of life in these patients as compared to those remaining on dialysis after a graft loss.20,21 It was described that retransplant patients with higher PRA face longer waiting times and have poor retransplantation outcomes.22,23 The impact of HLA matching can be particularly more important in young than older recipients, because the former have a higher likelihood of needing more than one KT during their lifetime.
In our study the proportion of allosensitized patients (PRA>0%) was clearly higher at the second KT and median change in PRA observed between the first and second KT was +10%.
Magee et al. compared a large cohort of kidney graft recipients (more than 2000 second KT versus more than 20,000 first KT), from the Organ Procurement and Transplantation Network registry, and described that retransplant recipients are more allosensitized, even with adjustment for donor and recipient factors (recipient age, race, body mass index, diagnosis, PRA, length of ESRD treatment and level of human leukocyte antigen mismatch) at time of relisting.24
Treébern-Launay et al. compared the long-term patient and graft survival between second and first KT in more than 3000 patients. The transplant patients faced longer time in the waiting list and were also more sensitized when compared with patients at first transplantation. They were more frequently exposed to induction therapy with a lymphocyte-depleting agent,22 as were patients in our study at retransplant. We showed that higher HLA mismatches (A, B, DR HLA), acute rejection in first KT and first graft survival <1 year were risk factors for higher allosensitization (dPRA≥10%) after first graft failure. Some other studies show also that poorer HLA matching at first transplant was associated with higher PRA at retransplant.7,8,23 In contrast, Gritsch et al. found no association between HLA A, B or DR MM at first transplant and PRA at listing for second transplant in a group of 313 pediatric, but they only considered the effect of each of the A, B and DR alleles separately and not the effect of overall HLA mismatching.25
The presence of PRA >0% has been a widely accepted marker for acute rejection risk after kidney transplantation based on retrospective and prospective data.24–27 In our cohort, an increased allosensitization (dPRA>10%) after a first KT failure was associated with higher AR occurrence in the second KT.
Heaphy et al. showed that patients who experienced AR during their first KT were significantly more likely to experience AR during their retransplant with poor graft survival. A higher serum PRA and more A, B or DR HLA mismatches in the second graft were associated with an increased likelihood of AR.28 Coupel et al. analyzed 1174 kidney transplantations, with 233 being second grafts; when long-term second graft survival was examined, they concluded that the number of acute rejections episodes in the first transplant are a risk factor for poor outcomes in the second.26
Magee et al. showed that second KT had more acute rejections in the first graft, with poor outcomes24 which is in agreement with our results. Contrary to our results, Trébern-Launay et al. did not show significant differences in the occurrence of AR between patients who received a first or a second KT; importantly, second KT recipients received better HLA-matched transplants, a finding not observed in our cohort.22
Our results indicated also that shorter time to graft failure in the primary transplant is a risk factor for dPRA>10%, which was associated with retransplant failure. Some other studies showed that graft survival time from the primary transplant significantly predicted graft survival time in the retransplant.28–31
Acute rejection, dPRA≥10% and delayed graft function were independent predictors of retransplant failure. A German study showed that the number of the HLA mismatches and the occurrence of acute rejections episodes, the type of immunosuppression and delayed graft function in the first graft were associated with poor graft survival after second or subsequent renal transplantation.32
Some studies have demonstrated that the higher risk of second graft failure was mainly related to increased levels of preformed HLA antibodies.22,24,27,30,33,34 Others reported a similar graft survival between second and first KT with no differences in the occurrence of AR.35–39 However, these studies have several limitations: the limited number of cohorts analyzed with low statistical power, the single-center design, and the number of adjustment covariates or the short follow-up period.
Kousoulas et al. evaluated patient and graft survival of the 61 renal retransplant recipients with the aim to identify risk factors associated with inferior outcomes and loss of renal graft. They concluded that the number of previous renal transplantations had no effect on graft survival, but it was shown that immunological aspects such as the number of the HLA mismatches and the occurrence of acute rejections episodes were associated with poor graft survival after second or subsequent renal transplantation and on graft survival.37
Our results reflect the major difficulties faced by allosensitized retransplant patients. In order to prevent allosensitization, it is necessary to avoid additional sensitizing events as blood transfusions through the optimization of erythropoietin and iron supplementation. Moreover, immunosuppression withdrawal after kidney graft failure with or without transplantectomy should be cautiously considered, given that it has been shown to be a predictor of allosensitization.38 Donor-specific antibodies (DSAs) appeared frequently when immunosuppressive therapy was discontinued after graft loss. Unfortunately, the information about immunosuppression withdrawal and DSA after a graft failure was not available in our study. Some other limitations of this study must be acknowledged. This is a single center retrospective analysis, which may account for confounding factors. Adjustment for long-term immunosuppression regimens was not done, as it reflects the therapeutic advances over the last 30 years. Our study also failed to consider the effects of some confounding factors such as medication compliance. Historically, the detection of HLA antibodies was based on complement-dependent cytotoxicity (CDC). Its sensitivity for detecting antibodies is low, but the positive predictive value for early antibody mediated rejection is high.15 Currently, HLA antibody screening is carried out on solid phase assays using HLA molecules bound to polystyrene beads using the Luminex platform18. So, the use of cytotoxic PRA as an allosensitization marker is a limitation but it was necessary given the historical nature of our cohort.
In conclusion, this study shows that there is a significant increase in allosensitization after the first kidney failure, particularly in patients experiencing AR and in those transplanted with a higher HLA mismatched graft. Retransplant patients with higher net change of allosensitization, had poorer outcomes after a second KT. We found that AR and higher HLA mismatch were major predictors of increased allosensitization after graft failure. With the advances in immunosuppression therapies, AR has diminished in the last decades, reducing its impact on allosensitization at retransplant nowadays. Differently, reduced HLA mismatching at a first KT remains an important factor for an allosensitization increase at retransplant. So, de-emphasis of HLA matching in current allocation policies may be undesirable, particularly in younger patients, since they have a higher likelihood of needing a second transplant during their lifetime.
Conflicts of interestNothing to declare.