Anti-glomerular basement membrane disease (anti-GBM) is a rare disease usually mediated by IgG autoantibodies. It usually presents as rapidly progressive glomerulonephritis, often accompanied by pulmonary hemorrhage.1 The hallmark of anti-GBM disease are the blood circulating and tissue-bound autoantibodies that target antigenic sites within the glomerular basement membrane (GBM) and sometimes alveolar basement membranes.2
We present a rare case of anti-GBM disease mediated by IgA autoantibody. A 65-year-old-man presented with gross haematuria and nephrotic range proteinuria and was admitted to the hospital.
His medical history included meningitis in childhood, obesity (BMI 47.53kg/m2), glucose intolerance, hypertension, hypercholesterolemia, duodenal ulcers, liver hemangioma and rectal polyps. He lost 12kg during the last 3 months and suffered from dysuria, pollakiuria and nocturia. Medications were: amlodipine and valsartan, moxonidine, torasemide, nebivolol, atorvastatin, meloxicam and tramadol with paracetamol.
Upon admission his blood pressure was 150/80mmHg. He had pretibial edemas. Ultrasound examinations showed normal kidneys. Laboratory investigation revealed negative cANCA, pANCA, ANA, ENA, anti-dsDNA and antiphospholipid antibodies along with normal C3 and C4 levels. Urine and serum electrophoresis showed no monoclonal IgA, kappa or lambda light chains. Routine enzyme-linked immunosorbent assay (ELISA) targeting IgG circulating anti-GBM autoantibodies were negative. Urine examination revealed proteinuria of nephrotic range (11.8g/day), haematuria with dysmorphic erythrocytes and leukocyturia. His serum creatinine level raised from 98 to 313μmol/L in two months and serum proteins were low. Chest X-ray showed bilateral pleural effusion and voluminous hiluses. He had partial respiratory insufficiency with SO2 94%.
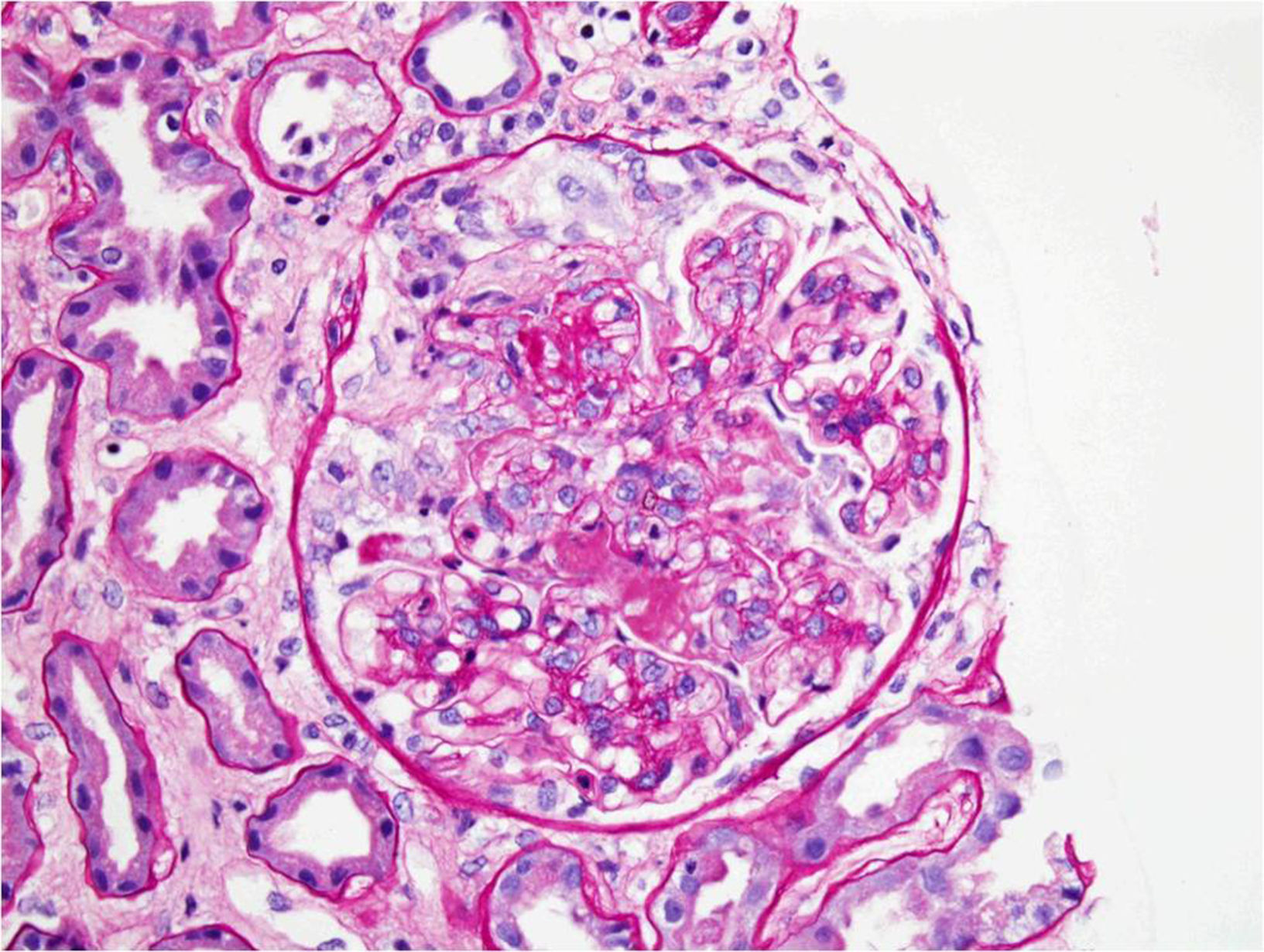
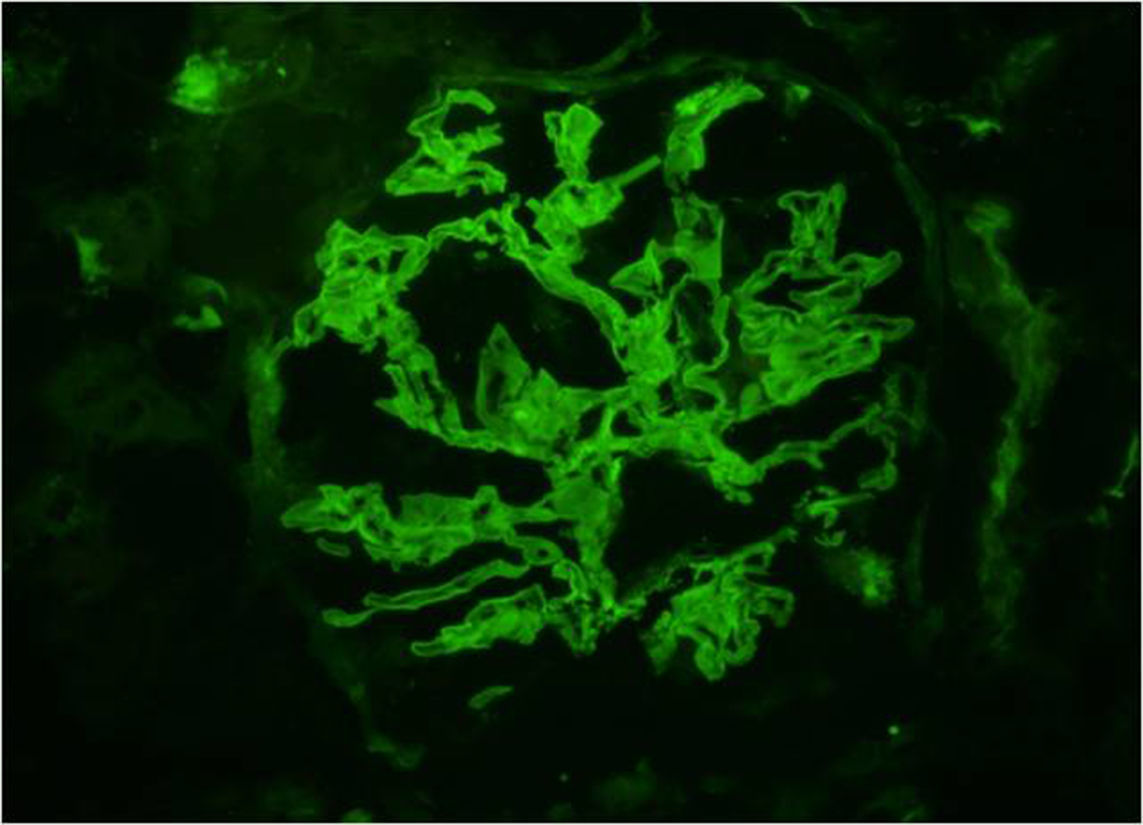
Renal biopsy was performed. Light microscopy demonstrated focal necrotizing crescentic glomerulonephritis with cellular and fibrous crescents (Fig. 1). 40% of glomeruli were globally sclerotic. Immunofluorescence microscopy demonstrated linear (2+) staining along the glomerular capillary loops for IgA (Fig. 2) along with weak linear staining for IgG, anti-kappa and anti-lambda antibodies. Interstitial fibrosis and tubular atrophy occupied 40% of renal parenchyma. By electron microscopy there were no immune complex-type deposits. The findings were consistent with IgA-mediated anti-GBM disease.
The patient had 12 plasma exchanges during a 1 month time, along with albumin substitution. He was given intravenous cyclophosphamide (CYC) (1g the first day) and steroids (methylprednisolone, 1g/day) during 3 days, and continued with oral prednisone 60mg/day.
Two months after the diagnosis of IgA-mediated anti-GBM disease the patient had serum creatinine level 258μmol/L and proteinuria of 4.6g/day. He continued albumin substitution and oral prednisone (60mg/day) therapy. He was given another cycle of CYC, but the therapy was stopped because of sepsis. He started chronic hemodialysis 3 months after the onset of the disease.
As our case demonstrated, standard assays used for detecting circulating IgG anti-GBM antibodies can perceive only IgG and they will fail to detect IgA anti-GBM antibodies. Thus the recognition of the disease depends on linear staining of IgA on glomerular basement membranes detected using immunofluorescence microscopy on the kidney biopsy.3,4
In IgA-mediated anti-GBM disease antigens belong to α5 and α6 chains of type IV collagen. That differs from antingens belong to α3 or α5 chains of type IV collagen found in IgG-mediated anti-GBM disease.5
The patient we presented did not had signs of alveolar hemorrhage. In previously described cases of IgA-mediated anti-GBM disease pulmonary involvement was found in 5 cases.1,4,6–8
Major predictors of piteous renal outcome in anti-GBM disease are high serum creatinine level (≥5.7mg/dL, 503.9μmol/L) and many circumferential crescents.9 Comparing to the IgG-mediated anti-GBM disease, prognosis of IgA-mediated anti-GBM disease is poor.3,4 Renal function usually does never improve and in most cases the disease leads to the end-stage renal failure.1,3,4 Also, 2 patients described in previous reports died because of uncontrolled alveolar hemorrhage6 and pneumonia.10
Classically, IgA-mediated anti-GBM disease is treated in the same way as its IgG counterpart, with the triple regimen of plasmapheresis, steroids and oral CYC.1,3,4 Whether this intensive treatment is the best option in IgA-mediated anti-GBM disease is unknown as this approach was used in only few cases and in all described cases the patients developed ESRD.1,3–5
Kidney transplantation is the treatment of choice for patients who develop ESRD caused by anti-GBM disease. In 2 previously described cases of IgA-mediated anti-GBM disease the patients underwent renal transplantation. In the report by Moulis et al.3 there was no recurrence of the disease 5 months after transplantation. In other case1 the disease was secondary to a plasma cell dyscrasia. The disease recurred and caused the loss of the allograft 2 years after the transplantation.
In order to make the right diagnosis, performing the renal biopsy is essential in cases of rapidly progressive glomerulonephritis. Early diagnosis of IgA-mediated anti-GBM disease can ensure the treatment in earlier stages of the disease and ESRD can possibly be prevented or at least delayed. As current treatment regimens result in poor prognosis, the disease might require more specific treatment strategy, distinct from the therapy for IgG anti-GBM disease.
FundingThere was no extrainstitutional funding.