The lack of adherence to phosphate -binders (PB) is the most important factor in not achieving the objectives of serum phosphorus (sP). Studies in the real-world population are needed to understand the influence of PBs on adherence and how to modify it.
MethodsProspective study conducted during 3 months in usual clinical practice. Out of 105 hemodialysis patients, 57 were switched to SFOH and 48 maintained their baseline treatment (control group). sP levels and the percentage of patients with sP levels <5mg/dl were compared. Adherence before and after introduction of SFOH, number of pills of PB, preferences in the administration mode and side effects were analyzed.
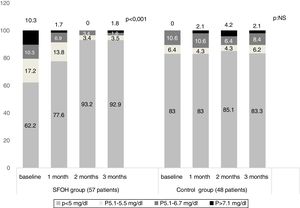
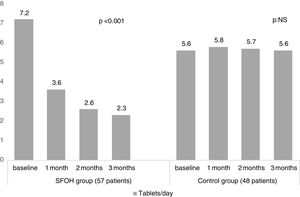
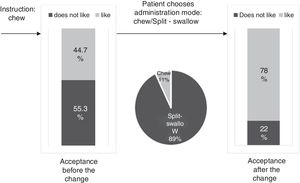
ResultsThe percentage of patients with controlled sP (<5mg/dl) increased significantly in the SFOH users’ group (62.1–92.9%, p<0.001), but not in the control group (83–83.3%, p=NS). The average of daily tablets decreased significantly in the SFOH group (7.2–2.3 comp, p<0.001), but not in the control group (5.6–5.6, p=NS) and 100% of the patients used only one PB in SFOH group. The use of SFOH increased the adherence according to the SMAQ questionnaire (57.8–84.3%; OR 13.1, p<0.001). The possibility to choose the preferred mode of administration (split-swallowing 89% compared to chewing 11%), improved the acceptance (44.7–78%). 14% of the patients experienced side effects and in 5.2% SFOH was discontinued for this reason.
ConclusionsSFOH controlled serum sP in 93% of patients, 100% in monotherapy, and with fewer tablets. The exploration and adaptation of preferences in the mode of administration influenced the acceptance of the drug by the patient and, probably, the future adherence.
La falta de adherencia a los captores del fósforo es el factor más importante para no lograr los objetivos del fósforo sérico (Ps). Se necesitan estudios en la población del mundo real para comprender la influencia de los CP sobre la adherencia y como modificarla.
Objetivos Evaluar la eficacia y la adherencia de un nuevo CP, oxihidróxido sucroférrico (OHSF) en pacientes en hemodiálisis y la influencia de un cambio en el modo de administración del fármaco sobre la aceptación del mismo.
MétodosEstudio prospectivo realizado durante 3 meses en práctica clínica habitual. De 105 pacientes de hemodiálisis, 57 pacientes con P mal controlado (p<5mg/dl) fueron cambiados a OHSF y 48 mantuvieron su tratamiento inicial (grupo control). Se compararon los niveles de Ps y el porcentaje de pacientes con niveles de Ps<5mg/dl. Se analizó la adherencia antes y después de la introducción de OHSF, el número de comprimidos de captores del P, los efectos secundarios y el grado de aceptación del fármaco tras ofrecer varias alternativas en el modo de administración.
ResultadosEl porcentaje de pacientes con P controlado (<5mg/dl) aumentó significativamente a los 3 meses de seguimiento en el grupo de pacientes con OHSF (62,1 al 92,9%; p<0,001), pero no en el grupo de control (83 al 83,3%; p=NS). El promedio de comprimidos diarios disminuyó significativamente en el grupo OHSF (7,2 a 2,3 comprimidos; p<0,001), pero no en el grupo control (5,6 a 5,6; p=NS) y todos los pacientes en tratamiento con OHSF se controlaron con monoterapia. El uso de OHSF aumentó la adherencia según el cuestionario SMAQ (57,8 al 84,3%; OR: 13,1; p<0,001). La posibilidad de elegir el modo de administración preferido (cortar-tragar 89% en comparación con masticar 11%) mejoró la aceptación (44,7 al 78%) de los pacientes. El 14% de los pacientes experimentaron efectos secundarios y en 5,2% se suspendió el OHSF por esta razón.
ConclusionesOHSF controló el P sérico en el 93% de los pacientes, siendo la totalidad de ellos en monoterapia, y con menor número de comprimidos a corto plazo. La exploración y adaptación de las preferencias en el modo de administración influyó en la aceptación del fármaco por parte del paciente y, probablemente en la adherencia futura.
The search for a phosphate binder (PB) that is effective, well-tolerated, overcomes the problem of non-adherence, and controls serum phosphorus (sP) in patients on hemodialysis (HD) in a stable and sustained manner, remains an open question. In controlled clinical trials, all currently available PB have demonstrated their effectiveness in lowering the sP,1 and some of them have additional advantages in other aspects such as vascular calcification,2 pleiotropic effects on cholesterol3 or decrease in FGF 23.4
However, in actual clinical practice, results are not as favorable, and despite having sufficient tools, a large number of patients treated with PB fail to maintain sP within the ranges recommended by the different clinical guidelines.5,6
Sucroferric oxyhydroxide (SFOH) is a non-calcium, iron-based PB that compared to sevelamer carbonate (“sevelamer®”), has demonstrated sustained Ps control, good tolerability, and lower pill burden in a Phase 3 study conducted in dialysis patients with hyperphosphatemia.7
The main drawback of the treatment of hyperphosphatemia is that it must be controlled with drugs that depend tightly on adequate compliance of the patient: adherence to the drug is essential to obtain the expected results.8 PB probably are the group of drugs prescribed in HD with a higher non-adherence rate.8,9
The causes of this low adherence are related to PB pill burden,10 the complexity of the regimen (they must be taken in association with the intake),11 their interference with the habits of the patient8 and the high prevalence of gastrointestinal symptoms.12 Studies in real clinical practice are necessary to understand the influence of patients’ preferences in the acceptance of PB13 and to design strategies associated with better compliance.14
The development of the new PB must include not only the analysis of its clinical efficacy in achieving the phosphorus objective, but also to deepen in those aspects that may influence adherence in the medium-long term. In this sense, it is important to approach the opinions of patients regarding the new PB, their preferences and their degree of acceptance.
The objective of this study is to evaluate the efficacy of SFOH in hemodialysis patients, as well as adherence and acceptance by the patient and the strategies that favor a good compliance in real clinical practice.
Material and methodsThis was a prospective study carry out during 3 months in usual clinical practice at the Hemodialysis unit of Vithas Hospital Perpetuo Internacional, Alicante (Spain).
Study populationAll HD patients being currently treated at our unit with PB were included in the study. Eligible patients were between 18 and 90 years of age, and on renal replacement HD therapy (4h/session, 3 days a week, with single pool dialyzer clearance time/volume of water in patient's body (Kt/V)>1.4) for at least 6 months before inclusion in the study.
Adherence, Ps levels, pill burden and the percentage of patients with sP levels <5mg/dl were compared between the two groups of treatment (SFOH and control) at baseline and 1, 2 and 3 months. In SFOH group, preferences in the administration mode and side effects were recorded.
All patients participating in the study signed an informed consent.
Study design57 patients were assigned to SFOH (SFHO group) and 48 patients maintained their baseline treatment (association of low doses of Ca acetate plus Mg carbonate (Osvaren®) (less than 440mg of calcium element) with lanthanum carbonate (Fosrenol®) or carbonate of sevelamer (Renagel® or Renvela®) (control group).
The inclusion criteria for assigning patients to the SFOH group were: sP higher than 5mg/dl (46 patients) or sP lower than 5mg/dl with a high number of pills (3 patients) or poor tolerance to PB and suspected non-adherence to PB (8 patients). Initially, all patients were asked through a one-to-one interview if they agreed to switch to this new alternative treatment. Those patients who did not wish to change maintained the same treatment even if they had sP poorly controlled.
All patients received the instruction to split the tablets and distribute then during intakes, starting with one or two tablets daily.
Sampling for Ps levels was performed immediately before HD session the second day of the week (Wednesday or Thursday).
Self-reported non-adherence to treatment to PB was estimated using the Simplified Medication Adherence Questionnaire (SMAQ), which has been validated in the Spanish population with Acquired Immune Deficiency Syndrome15 and has shown sufficient internal consistency in HD patients.8,13,14 Patients were considered non-adherent when answering: 1: “Do you always take your medication at the appropriate time?” NO; 2: “When you feel bad, have you ever discontinued taking your medication? YES; 3: “Have you ever forgotten to take your medication?” YES; 4: “Have you ever forgotten to take your medications during the weekend? “YES; 5: “In the LAST WEEK, HOW MANY TIMES did you fail to take your prescribed dose?” C: 3–5 times, D: 6–0 times or E: more than 10 times; 6: “Since your last visit how many whole days have gone by in which you did not take your medication?” more than two days. The questionnaire is dichotomous; any response in the sense of non compliance is considered non adherent. SMAQ was collected baseline and at 2 months after starting the new treatment.
Study variablesPs levels and the percentage of patients with sP levels <5mg/dl were the primary study variable. Secondary study variables were number of pills of PB, adherence, preferences in the administration mode and side effects.
Additional information included age; gender and HD vintage.
Statistical methodsData were analyzed using SPSS software, version 17.0. First, we obtained a descriptive analysis of all variables collected. The qualitative variables are described as absolute frequencies and percentages, while quantitative variables were analyzed using the mean, standard deviation (SD), and range (maximum and minimum values). Independent t-tests or chi-square tests were used. ANOVA was performed for repeated measurements, and the Friedman Test was used to evaluate the differences observed in quantitative variables at different study stages. The level of significance was established at p<0.05.
ResultsPopulation characteristicsA total of 105 patients were enrolled. Mean (DS) age of patients was 63.39 (13.8), being 63.7% males. Mean (SD) time on HD replacement was 7.13 (7.17) years. Mean time of follow-up 3 (SD 1.4) months. The baseline patient's characteristics in the 2 groups are in Table 1.
Baseline patient characteristics.
| SFOH group (N: 57) | Control group (N:48) | p | |
|---|---|---|---|
| Age, mean years (SD) | 65.8 (13.5) | 72.6 (12.8) | <0.05 |
| Female, % | 35.3% | 33.5% | NS |
| Dialysis vintage, mean years (SD) | 10 (16) | 11 (9.8) | NS |
| Primary cause of ESRD, diabetes mellitus % | 23.7% | 25% | NS |
| Baseline P (mg/dL), mean (SD) | 5.0 (1.6) | 4.3 (1.3) | <0.05 |
| Baseline albumin cCa (mg/dL), mean (SD) | 8.9 (0.8) | 8.7 (0.6) | NS |
| Baseline PTH (pg/mL), mean (SD) | 423.0 (325.6) | 334.5 (432.1) | <0.05 |
The percentage of patients with controlled sP (<5mg/dl) increased significantly in the SFOH users’ group (62.1–92.9%, p<0.001), but not in the control group (83–83.3%, p=NS) at 3 months (Fig. 1). The average of daily tablets decreased significantly in the SFOH group (7.2–2.3 comp, p<0.001), but not in the control group (5.6 to 5.6, p=NS) (Fig. 2) and 100% of the patients used only one PB in SFOH group. The use of SFOH increased the adherence according to the SMAQ questionnaire (57.8% to 84.3%; OR 13.1, p<0.001) at 3 months. Adherence in the control group did not change (81–82.3%; p: NS) Patients were informed about the various modes of administration (swallow/chew), and adapting it to the preferences of the patient (split-swallowing 89% compared to chewing 11%) improved the acceptance (44.7–78%) (Fig. 3). No changes in efficacy between the two modes of administration was observed. Eight of the patients (14.0%) experienced side effects: 4 diarrhea, 1 constipation and 3 thirst. 3 patients (5.2%) discontinued SFOH because they did not like the medication even after changing the way of administration, and 4 patients (7%) SFOH were discontinued as a consequence of gastrointestinal adverse events
The main findings of this study are that the introduction of a new PB (SFOH) achieves an improvement in the control of phosphorus (it goes from 62% to 93% of controlled patients) with a lower number of tablets per day (of 7.2–2.3 tablets daily), while the group that was kept receiving basal treatment did not show significant differences in the control of phosphorus or in the number of daily tablets during the observation period. These results are consistent with those previously seen in controlled clinical trials7 and in other real-world studies16 in which SFOH was able to control a greater number of patients with a lower number of tablets.
It is important to highlight the fact that, in our study, patients in the group that changed to SFOH were worse controlled and less adherent to the PB than those in the control group and the results might affected by a selection bias. In spite of this, a greater percentage of these patients had their Ps controlled at the end of the observation period, what still shows a more striking result, being the group worse controlled at baseline. Even patients who had the worst controlled phosphorus get better results (92.9%) than patients who did not change treatment (83%).
Lack of adherence to PB is probably the most important factor for not achieving the objectives of P recommended in clinical guidelines, and it is estimated that it affects up to 50% of hemodialysis patients treated.8–12 In this study, the non-adherence rate measured by the SMAQ questionnaire decreased significantly after the introduction of SFOH. It is important to use tools to measure therapeutic adherence. Some studies show that the assessment of adherence directly and subjectively underestimates the non-adherence of patients.17,18 This does not allow identifying many non-adherent patients, which means that the opportunity to intervene on them is lost and therapeutic decisions can be adopted with a high degree of empiricism that lead us to unnecessarily increase the doses of the drugs.
Several studies have shown an association between larger number of tablets and non-adherence.10,12 SFOH has been shown to be able to control sP with a lower number of tablets per day (less than 1 tablet per meal, average 2.3 tablets/day) in patients who previously were taking an average of 7.2 tablets per day (more than 2 tablets per intake). This lower need for tablets may influence the better adherence observed in our study when switching to SFOH, but also can mean better compliance with less need for pills.
The effectiveness of a PB relies not only on its ability to bind Ps but also on its ability to be accepted by the patient who makes the final decision to take it or not. Patient's preferences and adherence to the prescribed PB are, at least, as important as their effectiveness. In clinical practice, the least effective PB is the one that the patient does not want to take: “Drugs do not work in patients who do not take them.”19 SFOH was well accepted initially, but when we explore the degree of acceptance of “chew” instruction as a mode of intake, more than half of the patients admitted that they did not like it. However, when they were offered another possibility of administration, Split-swallow, the percentage of acceptance increased from 44% to 78%, and 89% of patients chose this option as the mode of administration. Good short-term results in the control of phosphorus and adherence are not enough, as they may be motivated by the novelty of the new PB itself, but it is important to know the degree of acceptance of the prescribed PB to ensure long-term compliance. This will allow us to adapt to difficulties and anticipate the possibility of future non-adherence. Physicians may be able to improve adherence by selecting a PB or the mode of intake according to patient's preferences.13 Assessing patient beliefs and preferences about medications (form, size, unpalatable taste, number of tablets) is a reliable way of finding out intentional non-adherence to treatment with PB and may help to identify ways in which adherence rates can be improved.13 In a previous study, the percentage of patients prescribed PB that they did not like was 54.5%, and those patients had a greater risk of not meeting the goal of sP <5.5mg/dl.8 The change in the mode of administration (chew/Split-swallow) according to the preference of the patient could help us to maintain the treatment and control of phosphorus in the long term. In this study, 3 patients (5.2%) discontinued SFOH because they did not like the medication even after changing the way of administration. Individualization of treatment could be rewarded with increased medication adherence and, potentially, improved outcomes.
Intolerance to drugs and their side-effects are frequent causes of non-adherence.20,21 14% of the patients treated with SFOH experienced side effects (none serious): diarrhea, constipation and thirst and in 7% SFOH was discontinued for this reason. Intentional non-adherence to therapy may be modified with a closer consideration of the side effects of any particular binder. In a previous study, initial changes of PB, considering patient's preferences, were due to immediate intolerance to the new PB, while long-term changes were caused by fatigue and boredom with the PB they were taking.13
In our experience, starting with a slow and progressive increase of the dose of SFOH minimizes initial side effects. As we have seen in this study, most of the patients are controlled with less than 1 tablet per intake. The instruction to split the tablets and distribute then during intakes, starting with one or two tablets daily, favors a lower occurrence of side effects, and, therefore, reduces the abandonment of SFOH by the patient.
The type of relationship/communication established between doctor and patient is essential in intentional non-adherent patients and influences the improvement of self-motivation.22,23
In conclusion, SFOH is a potent BP that controlled sP in monotherapy in 93% of our patients short term. It improves adherence and has good acceptance due to its tolerability, the lower number of tablets it requires and its intake flexibility that allows it to be adapted to the patient's preferences.
Combination between good tools (well tolerated PB, well accepted, flexible in the taking, etc.) together with a close doctor and patient relationship with respect to the patient's preferences and opinions manages to improve the results in the control of sP.
Conflict of interestsDra Arenas and Navarro F have given conferences sponsored by Abbott (Abbvie), Amgen, Genzyme, Shire and Vifor Fresenius. They had also participated in national advisory committees of Abbott (Abbvie), Amgen and Vifor Fresenius.
To all the patients who with their collaboration contribute daily to improve our knowledge.