Monoclonal C5 anti-body eculizumab, approved for aHUS treatment, is reported as efficient and safe. The long-term safety of eculizumab is promising but remains uncertain. Although hepatic side effects have been reported in pediatric cases after eculizumab treatment,1,2 hepatotoxicity in association with eculizumab has not been reported in adults previously. Herein, we presented an adult case of aHUS in whom hepatotoxicity was observed following eculizumab treatment.
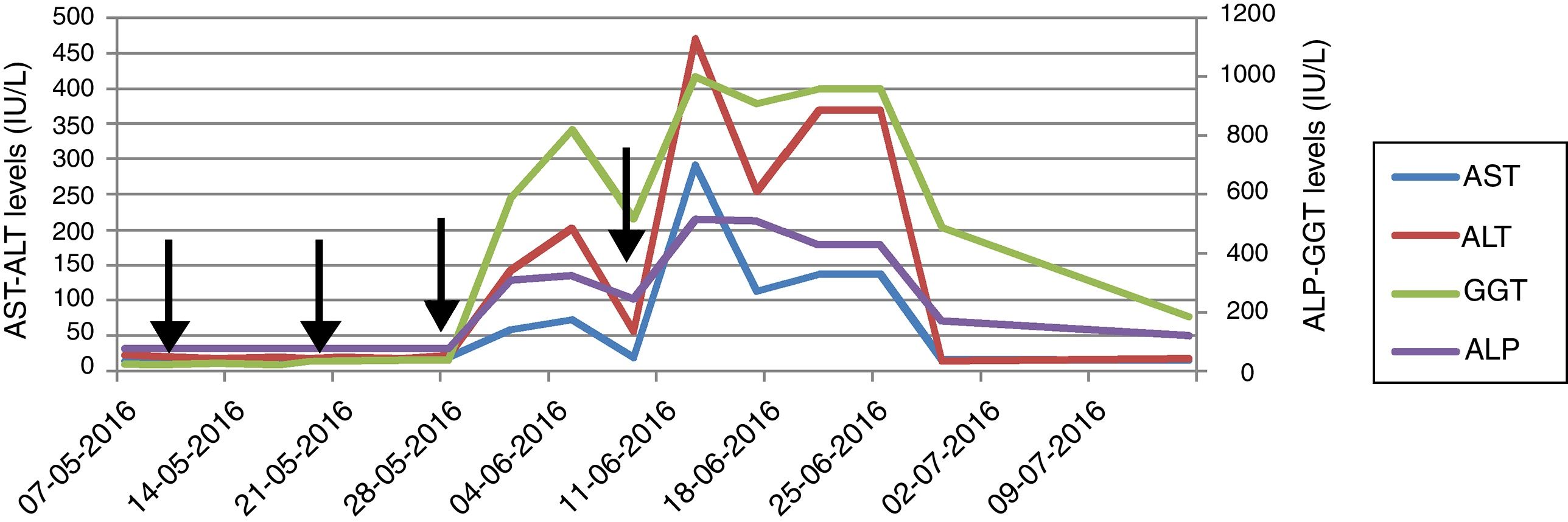
A 39-year-old male with blurred vision and headache for three days was referred to our center with the diagnosis of thrombotic microangiopathy (TMA). On his physical examination blood pressure was 220/110mmHg and grade 3 hypertensive retinopathy was determined. Laboratory tests revealed elevated serum creatinine and lactate dehydrogenase (LDH) levels, thrombocytopenia, schistocytes on peripheral blood smear, normal hepatic enzyme levels, negative direct coombs test, mildly decreased C3 (88.8mg/dL) and normal C4 level (9.5mg/dL). Serological tests were negative. Kidneys were normal on ultrasonography. He had varicocele operation a month ago, and creatinine was 0.9mg/dL. His mother was on hemodialysis with unknown etiology for 8 years. Plasma exchange (PE) was initiated for TMA. Blood pressure was controlled with intensive anti-hypertensive treatment consisted of amlodipine, carvedilol, ramipril and alpha-methyldopa. There was no left ventricular hypertrophy finding on echocardiography, and we excluded malignant hypertension in our case who was previously normotensive. On 7th day, ADAMTS13 activity was reported as normal (59%, reference range 40–130% activity). A total of 19 PE sessions were performed with the diagnosis of aHUS. Serum LDH level and thrombocytopenia were improved whereas kidney function was not. Renal biopsy, performed because of kidney dysfunction, revealed fibrin deposits in capillary lumina of glomerulus and mesangiolysis that confirmed TMA. He did not require hemodialysis during follow-ups. We decided to start eculizumab with the diagnosis of aHUS and persistent renal dysfunction. Eculizumab was initiated 900mg weekly for 4 weeks with appropriate administration under antibiotic prophylaxis, after immunization with vaccines. Transaminase levels increased at 3rd day after the third dose and the subsequent dose was delayed (Fig. 1). Further investigations excluded other causes of hepatocellular injury such as viral, autoimmune or toxic. Ultrasonography showed only mild hepatomegaly and hepatosteatosis. Serum bilirubin and INR levels were within normal ranges. Transaminase levels decreased after 12 day interval, and he received the 4th dose even though ALP and GGT levels were mildly elevated. Then, enzyme levels markedly re-elevated, and eculizumab was withdrawn because of fulminant hepatitis risk. Enzyme levels returned to normal ranges within 20 days. Laboratory changings were showed in Table 1. On follow-ups, he had no TMA relapse. However, his creatinine level remained high without any uremic symptoms. He continues to our outpatient clinic on anti-hypertensive treatment and free of TMA event with normal liver enzyme levels.
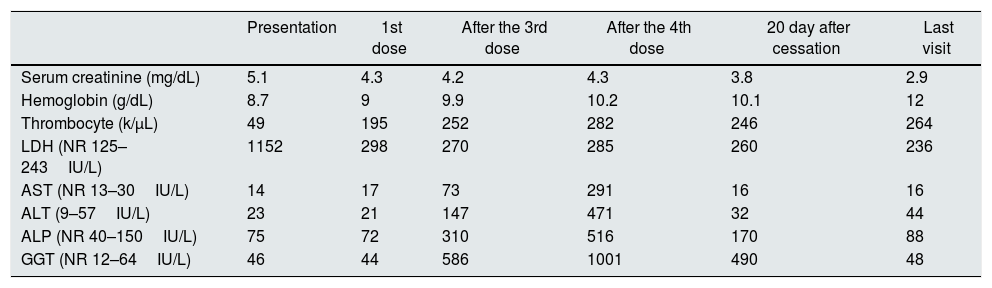
Laboratory findings at presentation and during eculizumab treatment.
| Presentation | 1st dose | After the 3rd dose | After the 4th dose | 20 day after cessation | Last visit | |
|---|---|---|---|---|---|---|
| Serum creatinine (mg/dL) | 5.1 | 4.3 | 4.2 | 4.3 | 3.8 | 2.9 |
| Hemoglobin (g/dL) | 8.7 | 9 | 9.9 | 10.2 | 10.1 | 12 |
| Thrombocyte (k/μL) | 49 | 195 | 252 | 282 | 246 | 264 |
| LDH (NR 125–243IU/L) | 1152 | 298 | 270 | 285 | 260 | 236 |
| AST (NR 13–30IU/L) | 14 | 17 | 73 | 291 | 16 | 16 |
| ALT (9–57IU/L) | 23 | 21 | 147 | 471 | 32 | 44 |
| ALP (NR 40–150IU/L) | 75 | 72 | 310 | 516 | 170 | 88 |
| GGT (NR 12–64IU/L) | 46 | 44 | 586 | 1001 | 490 | 48 |
NR, normal range; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase.
Drug-induced liver injury (DILI) is a leading cause of acute liver failure and has clinical manifestations ranging from asymptomatic liver enzyme derangement to fulminant liver failure. The pattern of injury is classified as hepatocellular, cholestatic and mixed and R-ratio is used to identify the pattern.3,4 Although withdrawal of suspected drug is recommended, the importance of the therapy might be considered before cessation. The drug should be stopped in the course of elevation in ALT more than 8 times of upper normal limit (UNL), with or without derangement in bilirubin, albumin, INR.5 The vast majority of the subjects recover after discontinuing; however, knowledge about using the suspected drug again is limited. We considered discontinuing because of marked elevation in ALT more than 8 times of UNL after the subsequent dose. Liver enzymes recovered within days after cessation.
Knowledge is limited among aHUS patients and there were no reports of hepatotoxicity with eculizumab in PNH studies.6–8 A recent study reported DILI in 5 of 11 children treated with eculizumab for aHUS in a single center.1 Elevation was seen on 10–29 days following eculizumab and resolution was within 14–21 days. Neither of presented cases had elevated enzyme levels prior to treatment. One patient discontinued because of marked elevation with subsequent infusion and clinical symptoms of tender hepatomegaly and jaundice. Transient elevation in transaminase levels were reported under eculizumab treatment.2,9 These studies suggested that hepatocellular injury might be related to pre-existing ongoing TMA before treatment initiation, and enzymes tended to normalization after treatment with resolving TMA. Opposite to findings of these studies, Hayes’ study and our patient had normal liver enzyme levels at the time of diagnosis and before eculizumab treatment. Hepatotoxicity was observed on the 3rd day after the 3rd dose of eculizumab and because it was the only alternative option we had to administer the consecutive dose that caused markedly elevation. These findings in the present case supported hepatotoxicity and diagnosis of mixed type DILI was considered. We did not consider other medications including prophylactic antibiotic were associated with DILI because similar pattern of injury was occurred with consecutive eculizumab administration. Due to fulminant hepatitis risk and insufficient knowledge about hepatotoxicity in adults in the literature, we stopped eculizumab, and decided to initiate PE if TMA relapse. Liver enzymes normalized within 20 days after cessation.
Mechanism of hepatic injury associated with biologic agents like eculizumab is not fully understood. There are several explanations like anti-body mediated injury, blockage of C5 which is effective in hepatic regeneration and defense reactions.1
Although reports are signed out that eculizumab is a safe and efficient treatment option for aHUS, hepatotoxicity risk should be taken into consideration as a potential adverse effect. To our knowledge, this is the first adult case report of severe hepatotoxicity associated with eculizumab. Hepatic enzyme levels should be controlled in patients receiving eculizumab. Further reports including large data are required to evaluate hepatotoxicity risk of this novel agent.
Conflict of interestThe authors declare that they have no conflict of interest.