Dear Editor,
Hypokalemia is one of the most common electrolyte abnormalities which etiology can be unclear and the incorrect diagnosis can result in the wrong treatment.
Gitelman syndrome (GS) is an autosomal recessive disorder of the thiazide-sensitive sodium chloride cotransporter, expressed at the distal convoluted tubule (DCT). The mutation is found in the SLC12A3 gene, but there are also others. Its prevalence is estimated to range 25 cases per 1 million.1 Acquired GS is rarer and usually associated with autoimmune diseases or after renal transplantation.2
GS phenotype is characterized by hypokalemic alkalosis, hypomagnesemia, hypocalciuria, and secondary aldosteronism without hypertension. Hyponatraemia is not a recognised feature of GS.3
CASE REPORT
A 34 year old caucasian woman with no prior medical presented with severe hypokalemia; hypomagnesemia and mild hyponatremia. Her past medical and family history were unremarkable. She was on no medication and denied any symptoms, unless for occasionally muscle cramps. Water intake ≥3L/day. She was normotensive, no edemas and normal urine output. The review of systems was otherwise negative.
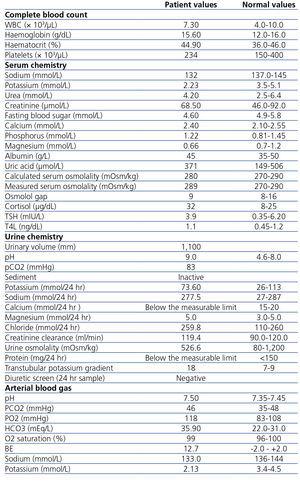
Table 1 summarizes laboratory investigation.
Patient was managed with oral magnesium and spironolactone 50mg/day. Her condition improved significantly and her last routine lab control showed serum potassium 3.78mmol/L, magnesium 0.79mmol/L and sodium 136.0mmol/L, without any other changes.
DISCUSSION
Potassium excretion is mostly derived from secretion in the distal nephron, driven by an electrochemical gradient increased by aldosterone-induced sodium reabsorption; and by an electroneutral K+ Cl- secretory mechanism.4
Hypokalemia may result from decreased intake, increased translocation into the cells, or, most often, increased losses in the urine, gastrointestinal tract, or sweat.
Although these causes were sought by history taking and clinical examination, we needed to exclude surreptitious vomiting or drugs abuse because high urinary potassium, metabolic alkalosis and alkaline urine can also be present in these two disorders. Differential diagnosis with vomiting was made through urinary chloride which is low in hypovolemia due to hyperaldosteronism, opposing to patient normal values poiting to a renal disorder. Diuretic abuse was excluded through a negative urinary screen.
Because the patient was normotensive, we stood with Gitelman or Bartter’s.
Magnesium excretion rate is regulated by distal reabsorption that depends on epithelial TRPM6 channels, which gene suffers a downregulation mutation in GS, inducing urinary magnesium wasting leading to hypomagnesemia,5 opposing to Bartter syndrome.
Calcium is absorbed in proximal nephron driven by an electronegative transcellular gradient induced by chloride-sodium transport; and in DCT driven by parathyroid hormone and Vitamin D. Hypocalciuria pathogenesis still remains debated but an important role is played by metabolic alkalosis.6
At the end, our patient fulfilled the diagnostic criteria for GS, although few unusual aspects. First, the inappropriately high urine pH and pCO2 (directly proportional to bicarbonate (HCO3-) concentration) could be explain through high chloride delivery that enhances HCO3- secretion in type A intercalated cells (to maintain electroneutrality). Adding to this, hypokalemia suppresses aldosterone secretion, which reduces sodium reabsorption (no hypovolemia) increasing back-diffusion of hydrogen, allowing the urine to become more alkaline than plasma.6 Aldosterone activity degree could be accessed through tubular fluid potassium concentration at distal cortical collecting tubule, estimated from transtubular potassium gradient but this would only be usefull in hyperkalemia settings. Additionaly, recente publications found that its assumptions were not valid.7 Finally, patients with metabolic alkalosis have a respiratory compensation but the beneficial pH effect is blunted when arterial pCO2 elevation increases renal acid excretion stimulating renal ammoniagenesis, raising urine pH.6
Mild hyponatraemia was present and, opposing to diuretic use (excluded), is not a typical feature of GS. After excluding hypothyroidism, adrenal insufficiency and renal failure, hyponatraemia associated to normal volemia, inappropriately high urine osmolality and urinary sodium concentration suggested the presence of a syndrome of inappropriate secretion of antidiuretic hormone (SIADH). The stimulus for this secretion is unclear. Although chronic hypokalemia could be characterized by some resistance of tube collector cells to ADH, GS has a urinary diluting capacity disturbed creating a SIADH like effect. Opposing to Bartters, GS has a preserved concentrating ability,6 because medullary thick ascending limb is intact. Since there is no hypovolemia, urinary sodium excretion is high to maintain electroneutrality as HCO3- is being excreted. Adding to this, in severe hypokalemia, via unknown mechanism, distal chloride excretion is increased causing a paralell sodium waste.
There are only a few cases of GS with hyponatraemia reported and in two of them a combination of high water intake with an impaired urinary dilution capacity caused by GS was described as a possible explanation for SIADH-like biochemical features.8,9 Adding to water, our patient must had a high intake of salt which raised the sodium delivery to the collecting duct and could explain both sodium and HCO3- excretion increase, with water free of electrolytes retention. To the best of our knowledge it was what happened to our patient.
A genetical study would confirm the diagnosis and clarify about additional anomalies but we didn’t performe it because of economic issues. However, we still don’t have many doubts about the diagnosis because there is a large phenotype variability without relationship between the clinical severity and type of mutations. Furthermore, heterozygotes have a higher rate of sodium excretion than wild-type individuals, probably due to higher salt intake.10
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Results of the laboratory investigation.