FGF23 is a recently identified hormone regulating mineral and vitamin D metabolism. In patients with chronic kidney disease (CKD), circulating FGF23 levels are progressively elevated to compensate for persistent phosphate retention, which result in reduced renal production of 1,25-dihydroxyvitamin D and thereby stimulate secretion of parathyroid hormone, suggesting its critical role in the pathogenesis of altered mineral homeostasis in CKD. Furthermore, it has recently been shown that FGF23 directly acts on parathyroid gland and mediate secretion of parathyroid hormone in the presence of Klotho as a cofactor, although such effects are not yet confirmed in patients with CKD. FGF23 can also be used as a predictor of mortality as well as future development of refractory hyperparathyroidism in patients undergoing dialysis therapy, where FGF23 levels are markedly elevated in response to hyperphosphatemia and active vitamin D treatment. This brief review summarizes recent insights into the role of FGF23 in the pathogenesis of mineral and bone disorders in CKD.
INTRODUCTION
Disorders of mineral and bone metabolism are common complications of chronic kidney disease (CKD)1. Abnormal mineral metabolism occurs early in the course of CKD, which can result in significant consequences even in patients not yet on dialysis2-4. Traditionally, these abnormalities have been investigated mainly in association with the development of secondary hyperparathyroidism, where phosphate retention, hypocalcemia, and a progressive decline in 1,25-dihydroxyvitamin D [1,25(OH)2D], have been considered to be the main factors for abnormal parathyroid hormone (PTH) secretion5-7.
Recently, a novel phosphaturic hormone FGF23 has been identified, initially as a pathogenic factor in rare hypophosphatemic syndromes disorders8,9. Studies since then have shown that this hormone plays an important role in normal physiology10 as well as in the pathogenesis of alterations in mineral metabolism such as that seen in patients with CKD11-13. In this brief review, we summarize recent findings about the role of FGF23 in the pathogenesis of mineral and bone disorders in CKD.
ROLE OF FGF23 IN PHOSPHATE AND VITAMIN D HOMEOSTASIS
FGF23 is a 32-kDa protein with 251 amino acids that is secreted mainly by osteocytes in bone14,15. FGF23 was first cloned in mice as a new member of FGF family16 and identified as a causative humoral factor for autosomal dominant hypophosphatemic rickets/osteomalacia (ADHR)8 and tumor-induced osteomalacia (TIO)9 that are characterized by severe hypophosphatemia, inappropriate phosphaturia, low levels of 1,25(OH)2D, and rickets or osteomalacia. Dysregulated secretion of FGF23 is also involved in a number of other diseases with abnormal phosphate and vitamin D homeostasis, such as X-linked hypophosphatemia (XLH)17, autosomal recessive hypophosphatemic rickets/osteomalacia (ARHR)18, and McCune-Albright syndrome19.
In accordance with human diseases, functional in vivo studies have shown that FGF23 is one of the most potent phosphatonins that induces renal phosphate wasting and reduction of 1,25(OH)2D. Administration of recombinant FGF23 results in phosphaturia and hypophosphatemia by suppressing the expression of sodium-phosphate cotransporter that mediates physiological phosphate uptake in proximal tubular epithelial cells20. Excess FGF23 also suppresses 1,25(OH)2D via inhibition of 1α-hydroxylase (CYP27B1) which converts 25-hydroxyvitamin D [25(OH)D] to 1,25(OH)2D and stimulation of 24-hydroxylase (CYP24) which converts 1,25(OH)2D to more hydrophilic metabolites with lesser biological activity20.
In keeping with these observations, transgenic mice that overexpress either wild-type or a mutant form of FGF23 that is resistant to cleavage developed hypophosphatemia, low serum 1,25(OH)2D levels, and rickets and osteomalacia21,22. Conversely, targeted ablation of FGF23 leads to the opposite renal phenotype, consisting of hyperphosphatemia and elevated production of 1,25(OH)2D23. Subsequent studies highlighted the physiologic role of FGF23 in maintaining normal serum phosphate levels in the setting of dietary phosphate variation10, although the precise mechanism by which phosphate loading mediates FGF23 production remains unknown.
FGF23-KLOTHO AXIS
Another unique characteristic of FGF23 is that this molecule derives from bone and exerts its hormonal effects in the kidney despite the ubiquitous presence of its receptors (FGFRs). This is in sharp contrast to other FGF family members that are thought to regulate various cell functions at a local level24. This mystery has been progressively unraveled by a recent major breakthrough that FGF23 requires Klotho, an anti-aging protein, as a cofactor in FGF23-FGFR1c interaction25,26. This fact clearly explains why Klotho mutant mice27 display a phenotype identical to that of FGF23 null mice23, both of which are characterized by premature agingrelated phenotypes associated with hyperphosphatemia and paradoxically high 1,25(OH)2D levels.
Of note, Klotho is expressed in limited tissues such as the kidney, parathyroid gland, and pituitary gland. It is intriguing that such limited expression pattern corresponds to the target tissues for FGF23 as functionally defined by the induction of early growth-responsive 1 (Egr-1) expression after intravenous administration of recombinant FGF23 to rats25. It is, however, still unclear how in the kidney FGF23 exerts its physiological effects on the proximal tubule despite the highest expression of Klotho-FGFR complexes in the distal tubule. Several investigators hypothesize that FGF23 actions on the proximal tubule may be indirectly mediated by FGF23 stimulation of the distal tubule and subsequent release of paracrine factors28, but further researches are needed to confirm this attracting hypothesis.
ELEVATED LEVELS OF FGF23 IN CKD
Insights into the role of FGF23 in mineral homeostasis have launched a new field of clinical research in CKD patients. Several studies have measured circulating FGF23 levels in predialysis 11 and dialysis 29 patients using an enzyme-linked immunosorbent assay (ELISA) that detects the carboxylterminal portion of FGF23, and reported progressively elevated FGF23 levels as serum creatinine or phosphate levels increase, suggesting its physiologic response to chronic phosphate retention. However, the possibility of accumulation of carboxyl-terminal fragments due to decreased renal function cannot be excluded in these studies.
Accordingly, a subsequent study measured intact FGF23 levels in CKD patients using a sandwich ELISA system that exclusively detects full-length human FGF23, and found similar increase in FGF23 levels along with decline in glomerular filtration rate (GFR)12. Furthermore, serum FGF23 levels were negatively associated with 1,25(OH)2D levels and maximal tubular reabsorption of phosphate (TmP/GFR) correlated negatively with serum FGF23 levels, consistent with the physiological action of FGF23 to inhibit phosphate reabsorption in the proximal tubule. However, patients with more advanced CKD exhibited impaired urinary phosphate excretion despite extremely high FGF23 levels.
Taken together, it is suggested that in early stage of CKD, serum FGF23 is elevated to maintain normal serum phosphate levels by promoting urinary phosphate excretion, but in advanced stage, overt phosphate loading may overcome such compensation for decreased GFR despite markedly elevated FGF23 levels, which in turn results in decreased renal production of 1,25(OH)2D, possibly thereby worsening secondary hyperparathyroidism30. Another group further supported this hypothesis by elegantly showing that increased FGF23 was an independent predictor of decreased 1,25(OH)2D levels and the effects of renal function and hyperphosphatemia on serum 1,25(OH)2D levels were completely extinguished by adjusting for FGF23, suggesting that FGF23 is a central factor in the early pathogenesis of secondary hyperparathyroidism13.
Of note, a recent observational study have shown that in patients who are beginning hemodialysis treatment high FGF23 levels were associated with mortality independently of serum phosphate levels and other known risk factors31. Supposing that FGF23 may indicate phosphate retention even in patients with normophosphatemia, its measurements may be useful to identify which of those patients might benefit from more aggressive phosphate management. Whether such strategies for the control of phosphate homeostasis would prolong survival of CKD patients is worthy of further investigation.
FGF23 AND SECONDARY HYPERPARATHYROIDISM IN CKD
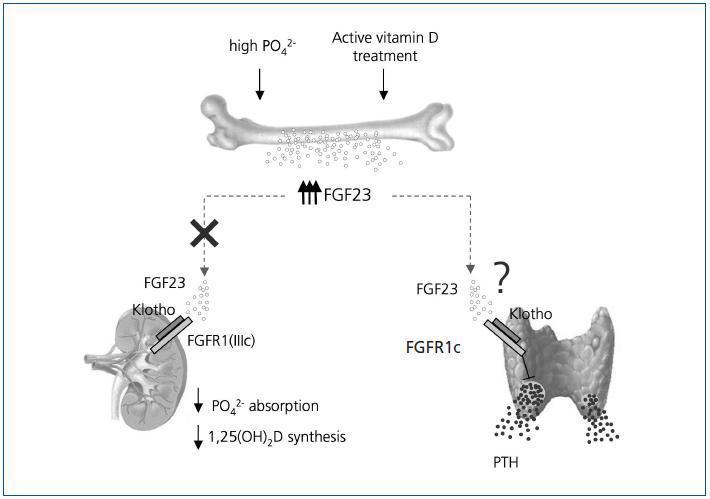
Serum FGF23 levels are progressively increased as kidney function declines and are markedly elevated once on dialysis therapy32,33. Such high levels of FGF23 may be due to persistent phosphate retention or hyperphosphatemia, while active vitamin D therapy has also been shown to increase serum FGF23 levels in dialysis patients34. This observation was further supported by in vivo and in vitro studies showing that 1,25(OH)2D directly increases the production of FGF23 by osteoblasts through the vitamin D-responsive elements present in the FGF23 promoter35. (Figure 1)
In this context, it is an interesting finding that in dialysis patients with secondary hyperparathyroidism high FGF23 levels may predict the future development of refractory hyperparathyroidism32,33. Although the mechanism of this finding remains unclear, it is possible that chronic phosphate retention as reflected by elevated FGF23 levels maycontribute to further progression of parathyroid hyperplasia, because high phosphate directly stimulates PTH secretion and parathyroid cell proliferation. Another possibility is that high levels of FGF23 at baseline may be a consequence of prolonged active vitamin D administration for severe hyperparathyroidism35, which may be related to future resistance to vitamin D therapy.
Besides the above-mentioned indirect effect of FGF23 on parathyroid function via inhibition of 1,25(OH)2D production, the abundant expression of Klotho in the parathyroid suggests that FGF23 may directly affect parathyroid function25. In fact, a recent study using rats with normal renal function has shown that FGF23 suppresses secretion of PTH in vivo and in vitro36. FGF23 also increases parathyroid 1α-hydroxylase expression and partly thereby decreases secretion of PTH in primary cultures of bovine parathyroid cells37. Thus, it is likely that FGF23 is a negative regulator of parathyroid function, at least in normal physiology. However, in CKD patients with secondary hyperparathyroidism, PTH secretion remains stimulated despite extremely high FGF23 levels32,33. Such resistance of the parathyroid to high FGF23 levels in uremia should be investigated in future studies.
Another issue of concern is a recent finding that PTH secretion is regulated in a Klotho- and Na+,K+-ATPasedependent manner38. It is proposed that when extracellular calcium is low, Na+,K+-ATPase is quickly recruited to the plasma membrane and an electrochemical gradient created by increased Na+,K+-ATPase may cause PTH release. However, it remains unclear how such a Klotho- and Na+,K+-ATPasedependent PTH regulation interacts with the inhibitory effect of FGF23 on PTH secretion through Klotho-FGFR complexes. Furthermore, it is also a matter of concern whether such a complex mechanism is modulated in dialysis patients in whom phosphate retention is prevalent and hypocalcemic state is rare due to treatment with calcium-based phosphate binders and active vitamin D analogs. Future studies should investigate the possible complex interaction between FGF23-, Klotho- and Na+,K+-ATPase-dependent pathways regulating PTH secretion and whether these complex mechanisms are modulated in the setting of CKD.
CONCLUSION
The recent identification of FGF23 and Klotho as a physiological regulator of phosphate and vitamin D metabolism has considerably advanced our understanding of the mineral and bone disorder in CKD. It is now clear that FGF23 plays a central role in the pathogenesis of altered mineral metabolism and secondary hyperparathyroidism in CKD patients. FGF23 can be used not only as a biomarker for assessing phosphate retention but also as a predictor of mortality and future development of refractory hyperparathyroidism. However, the precise role of extremely elevated FGF23 levels in uremia still emains unclear, especially as to its direct effect on parathyroid function. Further elucidation of the FGF23- Klotho axis will help us to establish a more rational approach for the management of mineral and bone disorder that is associated with high burden of morbidity and mortality in CKD patients39,40.
Figure 1.