ABO-incompatible (ABO-i) kidney transplantation (KT) has achieved remarkable clinical success in the last decades, survival rates of ABO-compatible and ABO-i KT have been shown to be similar.1,2 However, there is a higher incidence of humoral rejection and early graft loss, especially in patients with high pretreatment anti-ABO antibody (Ab) titers.3,4
Current strategies for desensitization are variable among centers, consisting of extracorporeal removal of anti-ABO Ab, B-cell depletion and intensified immunosuppression. The initial goal is to decrease anti-ABO Ab titers before surgery and most centers have adhered to the guideline that titers should be ≤1:16 before transplantation, a limit which has been based on empirical evidence. The proportion of cases that do not respond to desensitization and therefore cannot be transplanted under current strategies is unknown, and there is no establish limit titer (pre-treatment) above which desensitization may not be successful. A previous report from Gloor et al. showed graft loss to occur when the baseline titer was 1:512 despite intensive therapy.5
In our center, since 2006 we have performed 67 ABOi KT. In our protocol high anti-ABO Ab titers (defined as ≥1:512) are consider to preclude transplantation. For those cases, we offer the paired exchange program and the cadaveric donor waiting list. However, there are some reports of successful high titer ABOi KT in the literature 6–9 and it is plausible that high anti ABO Ab titers may be decreased using a more potent immunosuppressive approach.
Our standard protocol includes rituximab, plasma exchanges (PE) and intravenous immunoglobulins (IVIG). However, our current regimen has no significant effect on mature plasma cells, which are the major source of antibodies. Bortezomib, a proteasome inhibitor, used in the treatment of multiple myeloma (MM), causes apoptosis of plasma cells and have been successfully used for desensitization in HLA incompatible KT,10,11 thus bortezomib could be useful as well for desensitization in ABO-i KT.
Here in, we present a case of high titer ABO incompatible KT attempt using bortezomib in addition to our standard protocol.
We report the case a 32 years old female with chronic kidney disease secondary to ANCA vasculitis started hemodialysis in February 2012. A spousal living donor was studied; ABO incompatible A1 to O with anti-A Ab titers of IgM 128: IgG 512. In 2012, due to high titers transplantation was not advised. After three years on the cadaveric donor waiting list and in the paired exchange Spanish program, with no success in achieving a suitable donor, an attempt of performing an ABO-i KT was initiated.
We decided to use bortezomib in addition to the standard protocol with the goal to decrease the anti-A antibody to a titer of ≤8.
Bortezomib dose was adjusted to 1mg/m2 in concordance with our previous experience of moderate to severe adverse events in KT patients treated with bortezomib for acute humoral rejection (personal communication).
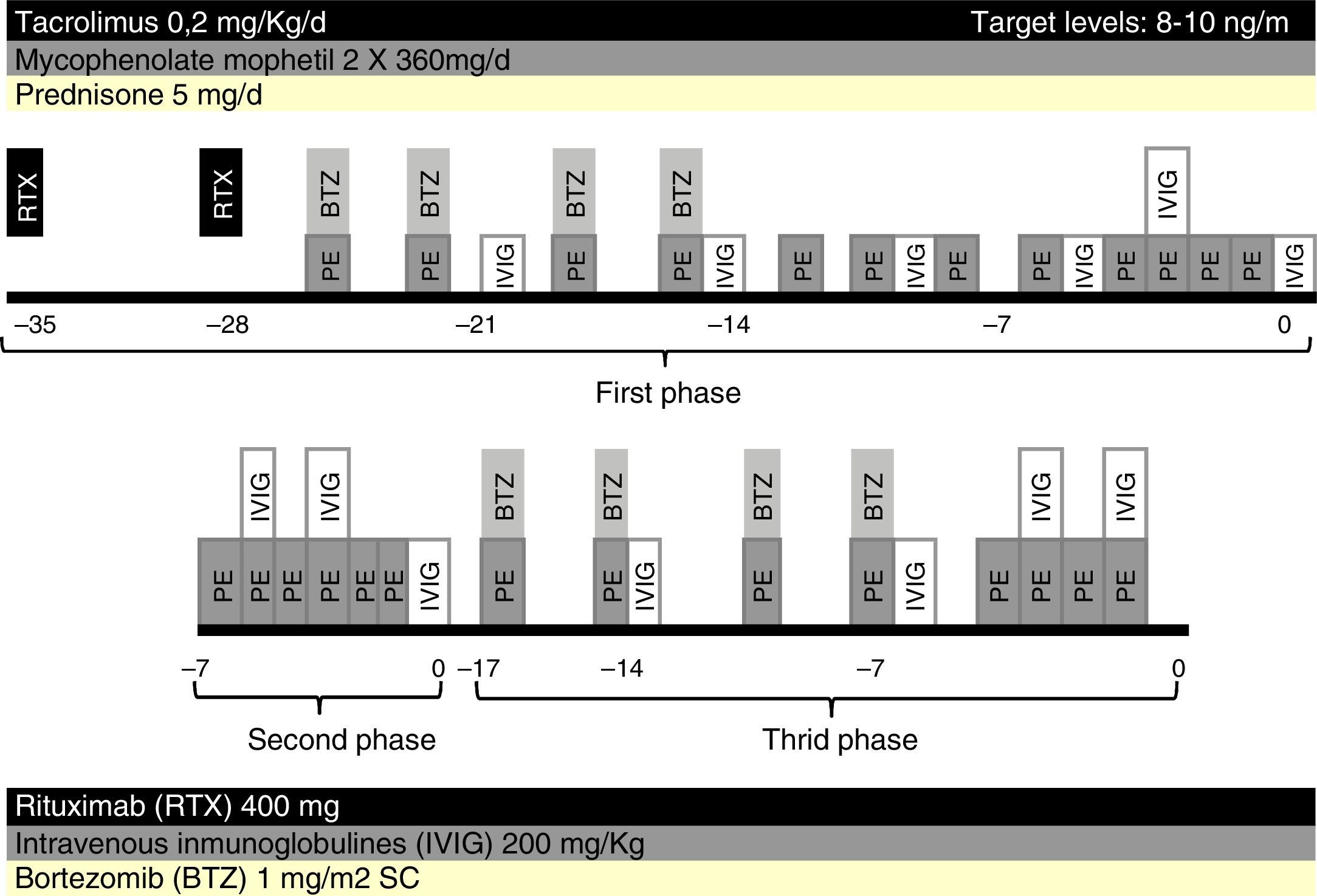
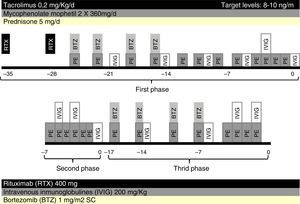
Five weeks prior to surgery, the patient received two weekly doses of rituximab 400mg each, one cycle of bortezomib, consisting of four doses of 1mg/m2 administered intravenous post-PE in days 1, 4, 8 and 11, twelve sessions of PE and low dose IVIG (200mg/kg every two PE). Maintenance immunosuppression was initiated with tacrolimus, mycophenolate mofetil and prednisone (Fig. 1).
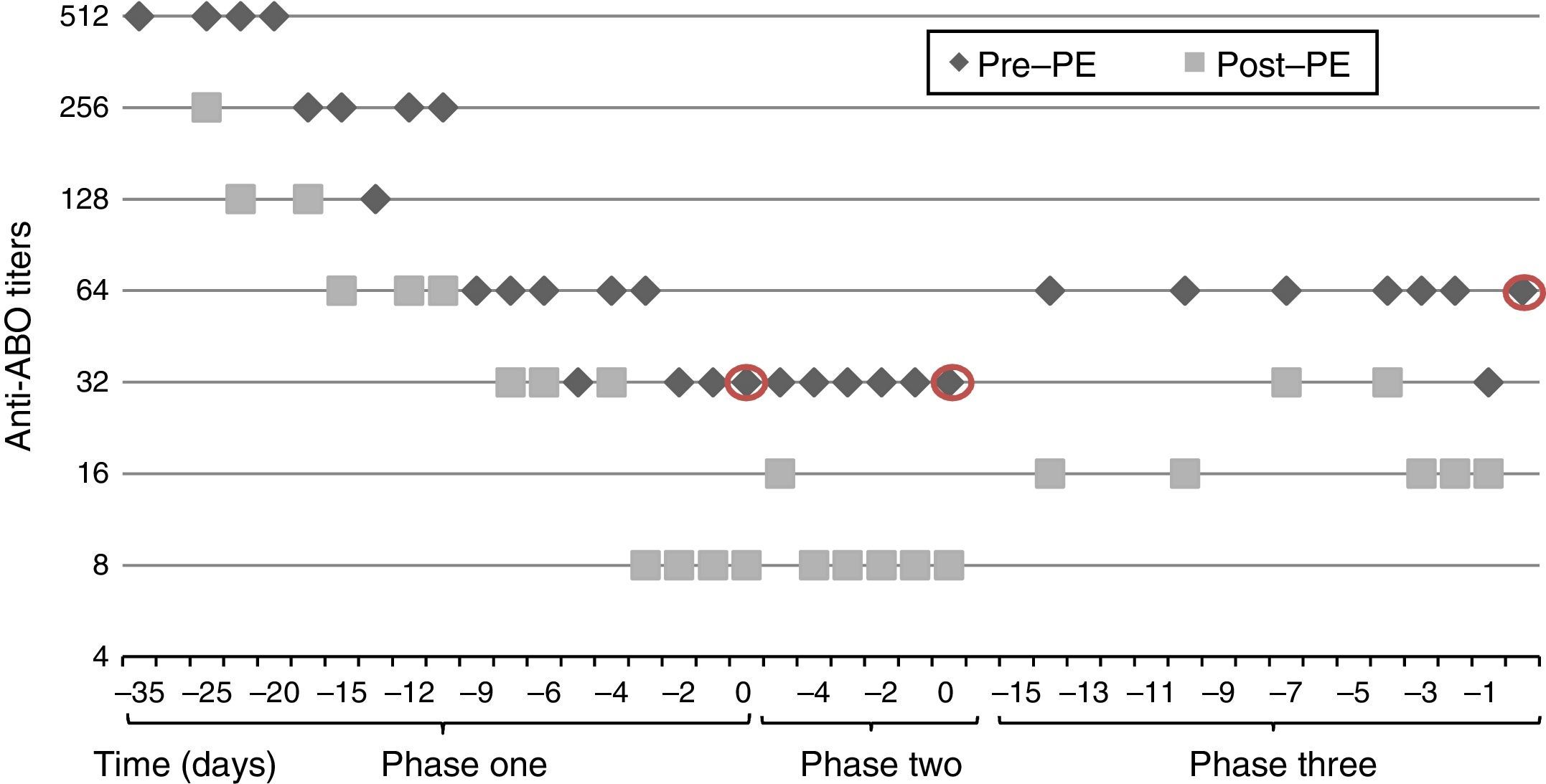
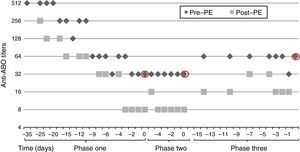
After five weeks of treatment, IgG titer post-PE was 8, but we observed a rebound of IgG titer to 32 in the morning before surgery, thus transplantation was postponed. A second attempt of transplantation was made a week later, after six sessions of daily PE and IVIG but titers remained unchanged, with IgG titer post-PE of 8 and pre-surgery of 32.
An additional cycle of bortezomib plus 8 PE and IVIG was given. Finally, nine weeks after initiation of treatment, anti-A IgG titers post-PE were 16 and there was a rebound to 64, which precluded transplantation (Fig. 2).
The treatment was well tolerated, except for a severe allergic reaction to fresh frozen plasma presented in the last PE session that required treatment with dexchlorpheniramine, ranitidine, corticosteroids and adrenaline. Six months after failed desensitization, the patient remains well in hemodialysis, without any infectious complication.
In this letter, we report our first experience in ABO-i KT with anti-ABO IgG titers ≥512, which was unsuccessful despite a very intensive immunosuppressive treatment including bortezomib, rituximab, plasma exchanges and IVIG.
Baseline high anti-ABO Ab have been shown to be the strongest predictor of ABO-i KT outcomes, and are associated with higher incidence of humoral rejection and early graft loss.3,4 Previous experience suggest that although pre-transplant treatment can reduce anti-ABO Ab to low levels at the time of transplantation, many patients rebound to high levels in the early post-transplant period, resulting in antibody-mediated damage to the allograft.4 A previous report showed graft loss to occur when the baseline titer was 1:512 despite intensive post transplantation PE/IVIG therapy and patients with elevated baseline antibody titer (≥1:256) appear to be at high risk for humoral rejection.5 However, the limit for patients with high anti-ABO Ab titers that can safely undergo transplantation is unclear.
Some centers have reported successful ABO-i KT in patients with anti-ABO Ab titers up to 2048, using a regimen including rituximab, PE on doubled filtration plasmapheresis, with or without splenectomy, reporting rates of failed desensitization ranging from 0 to 16%, although the number of cases is limited.6–8 A major problem is the lack of standardization in anti ABO Ab measurement. There are various methods to measure titers, the most common is the saline tube technique, although this technique yields significant intercenter variation.12 New techniques, such as gel card technique and flow cytometry, have shown better improved reproducibility,12,13 but still anti-ABO Ab measurements among centers may not be comparable. In our institution, we measure anti-ABO antibodies trough haemagglutination method using donor erythrocytes in gel cards and the isoagglutin titer is defined as the last dilution that gives in a gel card an agglutination intensity of +2 or bigger.
There is little data on the use of proteasome inhibitors for ABO-i KT. Benken et al., reported seven cases of ABO-i KT using bortezomib, including one patient with high anti-ABO titer (1:1024), but details on treatment administered (dosing, number of cycles and outcomes) are lacking.14 Also, Wong et al., reported one case of ABO-i and positive crossmatch KT performed using bortezomib, but in this particular case, anti ABO titers decrease after PE and IVIG, and bortezomib was given later due to positive crossmatch.15
There is no data regarding the appropriate dosing and the number of cycles of bortezomib to be used for ABO-i KT, more over current treatment schedules used in nephrology for other indications (treatment of acute mediated rejection or desensitization) were copied from the experience in MM. An important concern for treating patients with bortezomib may be the presence of renal failure. Formal studies are under way to define the pharmacokinetics and safety of bortezomib with varying renal impairment, but until now experience in MM patients with renal failure, showed that dose modification and treatment discontinuation rates were higher and the incidence of serious adverse effects (≥ grade 3) was increased.16 Current recommendations for MM patients with renal impairment advised no dose adjustment but careful assessment, and in cases of significant toxicities, dose reduction is advised.17
So far, evidence to support a potential beneficial effect of bortezomib in ABO incompatible kidney transplantation is lacking. In our experience, adding bortezomib to the standard desensitization protocol was not useful in a patient with high anti-ABO antibodies titer, but more research in this field will be needed.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
“This article does not contain any studies with animals performed by any of the authors.”
Informed consent“Informed consent was obtained from all individual participants included in the study.”
Conflict of interestThe authors declare that they have no conflict of interest.
This study is included in the framework of Redes Tematicas De Investigacion Cooperativa En Salud, REDINREN (RD06/0016/1002 and RD12/0021/0028), from Instituto de Salud Carlos III - Ministerio de Ciencia e Innovación and cofounded by Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa”.