The impact of etelcalcetide on patients with chronic kidney disease (CKD) and secondary hyperparathyroidism (SHPT) has been studied since its introduction in 2016/2017. However, only a handful of studies reported clinically relevant outcomes. This narrative review aims to summarize the published data about etelcalcetide, focusing on biochemical, cardiovascular (CV) and bone endpoints, as well as adverse effects and all-cause mortality.
Materials and methodsA literature review of the use of etelcalcetide in hemodialysis patients with SHPT was conducted. Several sources were used, such as PubMed, Google Scholar and Cochrane Library.
ResultsRegarding bone and mineral metabolism, etelcalcetide is effective in reducing serum levels of parathormone (PTH), calcium, phosphate and fibroblast growth factor 23 (FGF23). Preliminary data have highlighted its role in reducing bone turnover and improving mineralization and preservation of bone structure, indicating a possible positive impact on renal osteodystrophy. From a CV perspective, etelcalcetide is associated with a significant reduction in left ventricular hypertrophy. In addition, etelcalcetide reduces FGF23 and increases sclerostin serum levels. This data suggests a possible CV beneficial effect.
ConclusionsEtelcalcetide is effective in controlling SHPT. Promising data is available for some bone and surrogate cardiovascular endpoints, suggesting a possible beneficial effect. There is a lack of studies specifically designed to evaluate its role in reducing fractures, CV and all-cause mortality.
El impacto de la etelcalcetida en los pacientes con enfermedad renal crónica (ERC) e hiperparatiroidismo secundario (HPTS) ha sido estudiado desde que se introdujo en 2016/1017. Sin embargo, sólo un puñado de estudios han publicado resultados clínicamente relevantes. Esta revisión tiene como objetivo resumir los datos publicados sobre etelcalcetida, enfocándose en marcadores de valoración bioquímicos, cardiovasculares (CV) y óseos, así como en sus efectos adversos y sobre la mortalidad por cualquier causa.
Materiales y métodosSe llevó a cabo una revisión de la literatura sobre el uso de la etelcalcetida en pacientes en hemodiálisis con HPTS. Se usaron varias fuentes, tales como PubMed, Google Scholar and Cochrane Library.
ResultadosCon respecto al metabolismo óseo y mineral, la etelcalcetida es eficiente reduciendo los niveles séricos de hormona paratiroidea (PTH), calcio, fósforo y de factor de crecimiento fibroblástico 23 (FGF23). Los datos preliminares resaltan su papel en la reducción del remodelado óseo y la mejora de la mineralización, así como en la preservación de la estructura ósea, indicando un posible impacto positivo sobre la osteodistrofia renal. Desde una perspectiva cardiovascular, la etelcalcetida está asociada con una reducción significativa de la hipertrofia de ventrículo izquierdo. Además, la etelcalcetida reduce los niveles de FGF23 e incrementa los niveles séricos de esclerostina. Este dato sugiere un posible efecto beneficioso sobre el sistema CV.
ConclusionesEtelcalcetida es efectiva para controlar el HPTS. Datos prometedores están disponibles para algunos marcadores de valoración ósea y CV, sugiriendo un posible efecto beneficioso. Sin embargo, existe una falta de estudios específicamente diseñados para evaluar su papel en la reducción de fracturas, eventos CV y mortalidad por cualquier causa.
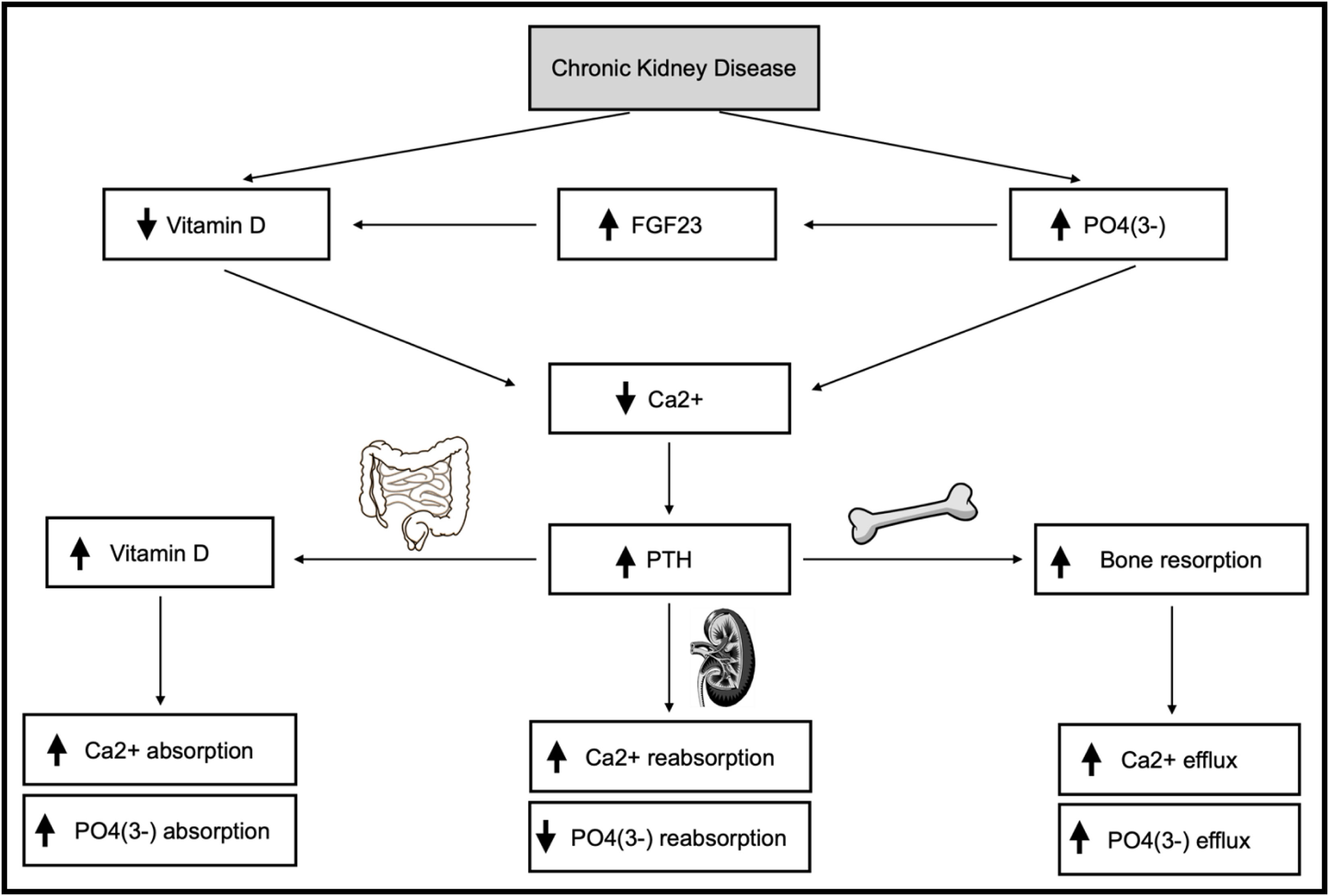
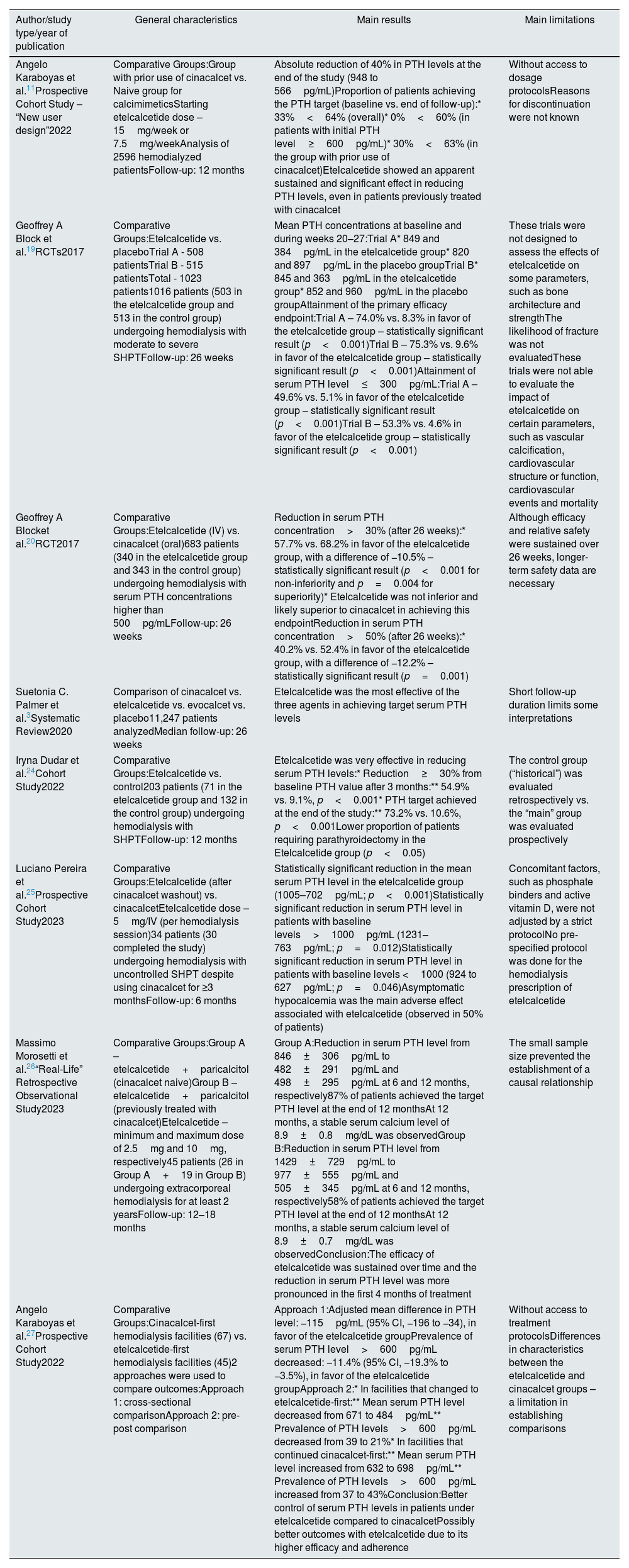
Secondary hyperparathyroidism (SHPT) is a serious and common complication of chronic kidney disease (CKD), particularly in hemodialysis patients/stage 5.1,2 Its pathophysiology focuses on hyperphosphatemia, hypocalcemia and vitamin D deficiency, representing an adaptive response to changes in phospho-calcium metabolism.3,4 Fibroblast growth factor 23 (FGF23), synthesized in the bone, acts as an additional driver of SHPT.3,4Fig. 1 illustrates the impact of CKD on phospho-calcium metabolism.
Impact of chronic kidney disease on phospho-calcium metabolism. Description: Demonstration of how chronic kidney disease interferes with phospho-calcium metabolism in different ways, involving several organs, such as kidney, bone and intestine. Abbreviations: FGF23, fibroblast growth factor 23; P04(3-), phosphate; Ca2+, calcium; PTH, parathormone; ↑, increase; ↓, decrease.
SHPT leads to the development of bone disease with an increased risk of fractures, as well as it is associated with a higher risk of left ventricular hypertrophy (LVH) and vascular calcification.5 SHPT is independently associated with cardiovascular (CV) and all-cause morbidity and mortality, especially for serum parathormone (PTH) values>600pg/mL.6,7
Treatment of SHPT includes phosphate-restricted diets, phosphate binders, vitamin D and analogs, calcimimetics and, in refractory cases, parathyroidectomy.3,8 The target range for serum PTH levels in patients with CKD stage 5 should be between 2 and 9 times the upper limit of normal (130–585pg/mL).8
Calcimimetics reduce serum PTH levels by acting on the calcium-sensing receptor (CaSR) present in the parathyroid glands and other locations (e.g., kidney, bone, blood vessels and intestine).9–11 Cinacalcet was the first calcimimetic introduced into clinical practice, being administered orally, daily.11 Despite having demonstrated efficacy,12–14 several studies report low adherence due to tolerability issues, especially in the gastrointestinal tract, with high discontinuation rates.15–18
Three randomized clinical trials (RCTs) were crucial for the approval of the second calcimimetic in 2017 by the Food and Drug Administration (FDA), etelcalcetide. Two RCTs, comparing etelcalcetide versus placebo, showed clear superiority of etelcalcetide in reducing serum PTH levels.19 The third, comparing etelcalcetide versus cinacalcet, demonstrated non-inferiority and even superiority of etelcalcetide in reducing serum PTH levels.20
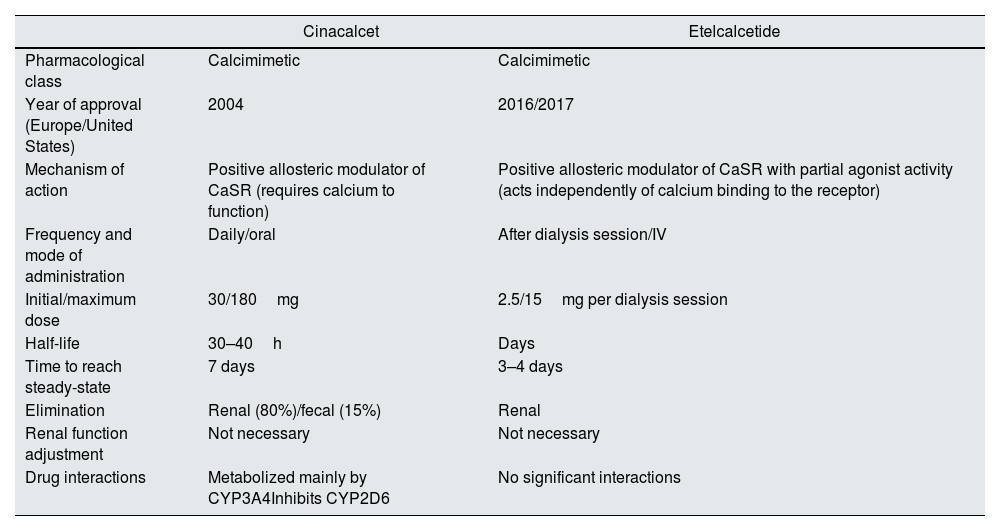
Etelcalcetide is administered three times per week, intravenously, after hemodialysis sessions, leading to better adherence.11,12,21 It is neither metabolized nor an inducer/inhibitor of cytochrome P450 (versus cinacalcet), making its pharmacological interactions negligible.12,22 Currently, etelcalcetide seems to play a role in SHPT control, especially in advanced cases or those refractory to cinacalcet.17 Poor adherence to cinacalcet makes etelcalcetide a good option in patients with lower tolerability/adherence to it.11,12,21Table 1 highlights the main characteristics of these two calcimimetics.
Key features of cinacalcet and etelcalcetide.
| Cinacalcet | Etelcalcetide | |
|---|---|---|
| Pharmacological class | Calcimimetic | Calcimimetic |
| Year of approval (Europe/United States) | 2004 | 2016/2017 |
| Mechanism of action | Positive allosteric modulator of CaSR (requires calcium to function) | Positive allosteric modulator of CaSR with partial agonist activity (acts independently of calcium binding to the receptor) |
| Frequency and mode of administration | Daily/oral | After dialysis session/IV |
| Initial/maximum dose | 30/180mg | 2.5/15mg per dialysis session |
| Half-life | 30–40h | Days |
| Time to reach steady-state | 7 days | 3–4 days |
| Elimination | Renal (80%)/fecal (15%) | Renal |
| Renal function adjustment | Not necessary | Not necessary |
| Drug interactions | Metabolized mainly by CYP3A4Inhibits CYP2D6 | No significant interactions |
Abbreviations: CaSR, calcium-sensing receptor; IV, intravenous; CYP, cytochrome P450.
As etelcalcetide is a relatively recent drug in clinical practice, data reporting biochemical and clinically relevant outcomes are scarce. This review article aims to summarize the published data about this drug since its introduction, focusing on its impact on biochemical, CV and bone endpoints. Adverse effects as well as all-cause mortality were also evaluated.
Material and methodsFor the development of this narrative review, carried out between 1/July/2023 and 1/March/2024, a set of articles was included, searched through several available sources, including PubMed, Google Scholar and Cochrane Library. Several keywords and Medical Subject Headings (MeSH) Terms were employed in this search, such as etelcalcetide hydrochloride; etelcalcetide; velcalcetide; Parsabiv; AMG-416; AMG 416; SHPT; chronic kidney disease; renal osteodystrophy; left ventricular hypertrophy; vascular calcification; “renal insufficiency, chronic” [MeSH Terms]; “kidney diseases” [MeSH Terms]; “kidney failure, chronic” [MeSH Terms]; “renal dialysis” [MeSH Terms]; “hyperparathyroidism, secondary” [MeSH Terms] and “chronic kidney disease-mineral and bone disorder” [MeSH Terms].
This research encompassed different types of studies, including systematic reviews, RCTs, observational studies and narrative reviews.
Regarding inclusion criteria, articles addressing the topic of etelcalcetide in adults were considered potentially eligible. As for exclusion criteria, studies in languages other than Spanish, English and Portuguese were considered ineligible. Studies focusing on pediatric age group were also considered ineligible.
Results and discussionEffect of etelcalcetide on serum PTH, calcium and phosphate levelsMany studies in this field have evaluated the efficacy and effectiveness of etelcalcetide, through RCTs and observational Studies (particularly in real-world setting).11,19,20,23–27
Two RCTs, involving 1023 patients, comparing etelcalcetide versus placebo, showed statistically significant results (p<0.001) in favor of etelcalcetide (RCT A – 74% versus 8.3%; RCT B – 75.3% versus 9.6%) in reducing >30% of baseline serum PTH levels.19 Another RCT, with 683 patients, comparing etelcalcetide versus cinacalcet, demonstrated not only non-inferiority (p<0.001) but also superiority (p<0.004) in favor of etelcalcetide for the aforementioned outcome (68.2% versus 57.7%).20 These three RCTs were crucial for its approval by FDA.19,20
After etelcalcetide approval, additional studies were conducted to further explore this topic. In a systematic review comparing three calcimimetics, including cinacalcet and etelcalcetide, 24 RCTs (6521 patients) were used to evaluated their efficacy in achieving target levels of serum PTH, with the result being statistically significant, considering etelcalcetide the most effective (etelcalcetide versus cinacalcet – odds ratio 2.78; 95% confidence interval (CI), 1.19–6.67).23
A prospective cohort study evaluated the effectiveness of etelcalcetide in 2596 hemodialysis patients with a 12-month follow-up, with and without prior use of cinacalcet.11 After one year of etelcalcetide therapy, the mean serum PTH level reached target values (according to KDIGO8), decreasing from 948 to 566pg/mL, with the proportion of patients within target values increasing from 33 to 64%.11 Also, serum calcium levels were substantially reduced at the end of the study (from 38 to 15–20% of patients with corrected calcium levels≥9.5mg/dl). Etelcalcetide appears to have good effectiveness regardless of prior use of calcimimetics.11 These findings are supported by another study where in an etelcalcetide-treated group versus control group (without calcimimetic), there was a reduction in PTH, calcium and phosphate levels in favor of the etelcalcetide group, effectively achieving target PTH levels in 73.2% versus 10.6% (p<0.0001) of patients in each group, respectively, after 12 months of follow-up.24
Superior efficacy/effectiveness of etelcalcetide versus cinacalcet was evident in three more studies.25–27 In a prospective cohort study by Pereira et al., in patients poorly controlled with cinacalcet, with a 6-month follow-up, statistically significant reductions in serum phosphate and PTH levels were observed, decreasing from 5.4 to 4.9mg/dl (p=0.01) and from 1005 to 702pg/mL (p<0.001), respectively.25 In a “real life” retrospective observational study by Morosetti et al., after a 12–18 month follow-up, 87% of patients in the vitamin D-only group (serum PTH level – 846±306pg/mL) and 58% of patients in the vitamin D and cinacalcet group (serum PTH level – 1429±729pg/mL) had PTH levels within recommended targets after the introduction of etelcalcetide, reinforcing its impact, even in patients poorly controlled with cinacalcet.26 Lastly, in a large registry study (prospective cohort study) involving 112 hemodialysis facilities in the United States, the cinacalcet-first hemodialysis facilities that switched to etelcalcetide-first showed that the mean serum PTH level decreased from 671 to 484pg/mL and the prevalence of patients with PTH levels>600pg/mL decreased from 39 to 21%. On the other hand, in hemodialysis facilities that stayed as cinacalcet-first, the mean serum PTH level increased from 632 to 698pg/mL and the prevalence of patients with PTH levels>600pg/mL increased from 37% to 43%.27 Also, when both types of facilities (cinacalcet-first versus etelcalcetide-first) were compared, there was an adjusted mean difference in PTH values of −115pg/mL (95% CI, −196 to −34), as well as a decrease of 11.4% (95% CI, −19.3% to −3.5%) in the prevalence of PTH level>600pg/mL, with both results statistically significant in favor of etelcalcetide.27
Therapeutic response of etelcalcetide is rapid and sustained.26,27 In Morosetti et al.’s study, the impact of etelcalcetide on reducing serum PTH level was primarily observed in the first months of treatment (first 4 months), demonstrating a rapid response.26 The studies by Morosetti et al. and Karaboyas et al. concluded that etelcalcetide had a sustained effect over time, which is particularly important in chronically hemodialyzed patients.26,27 Several referenced studies have a follow-up≥1 year, reinforcing this long-lasting effect of the drug.11,24,26
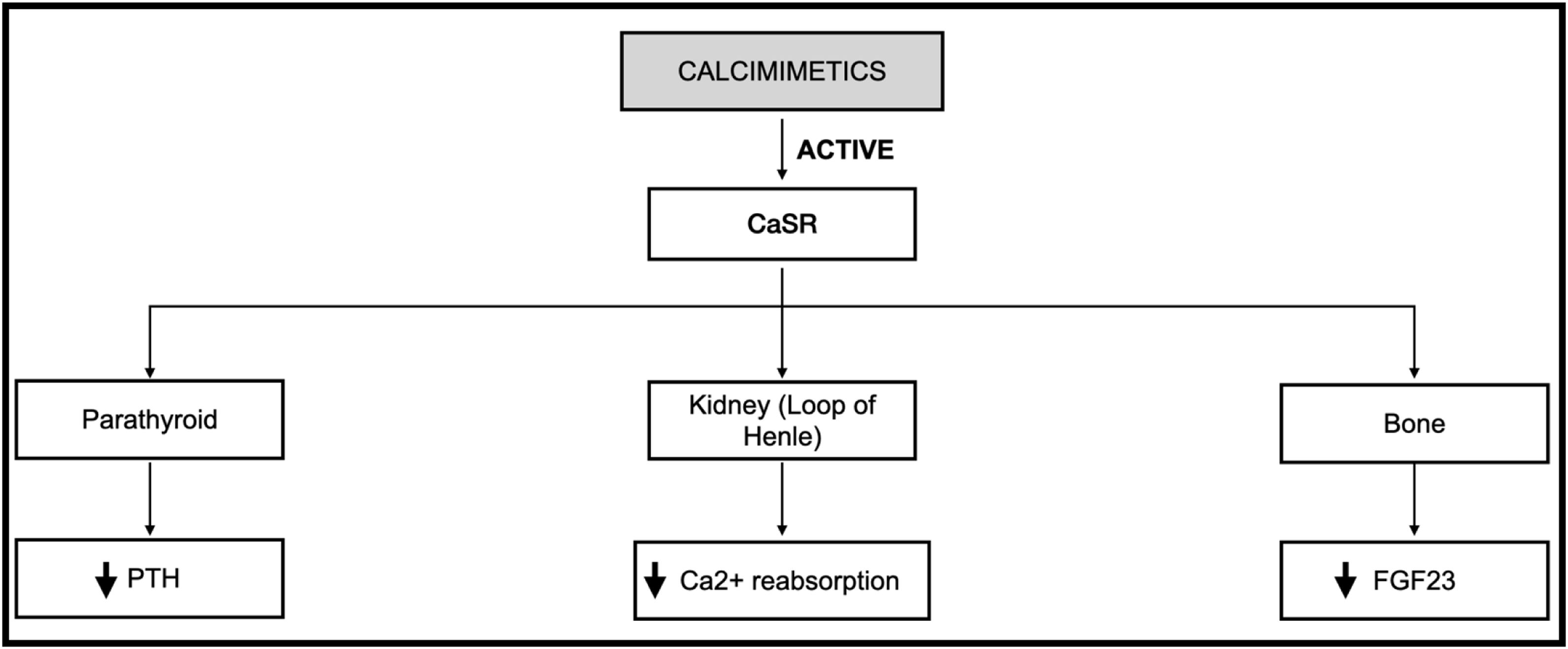
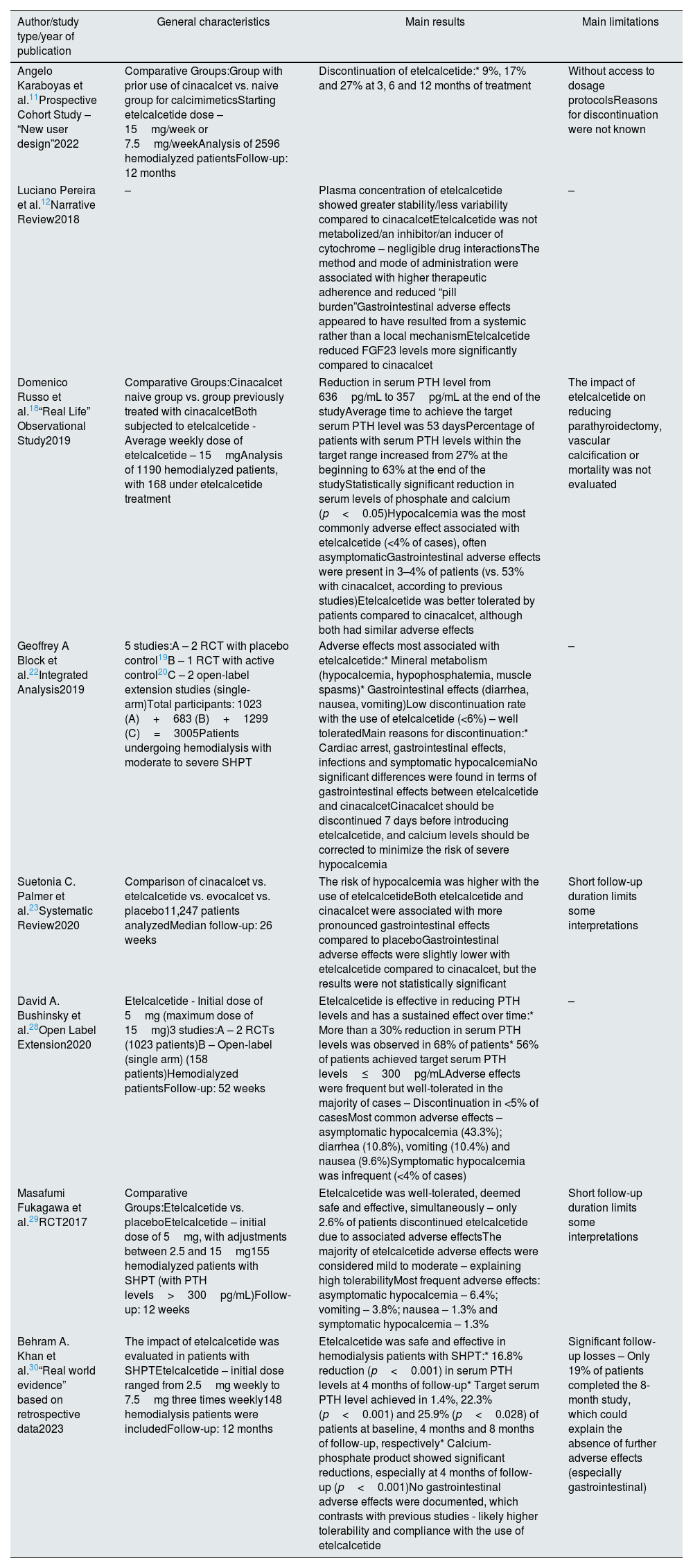
Thus, etelcalcetide appears to be effective in different biochemical parameters (PTH, calcium and phosphate), showing superiority to cinacalcet in this regard. Fig. 2 demonstrates the main actions of calcimimetics. Table 2 summarizes the main characteristics and conclusions of articles focusing on this topic.
Action of calcimimetics on the main target organs of the body. Description: Demonstration of how calcimimetics act on different organs that express CaSR, such as parathyroid, kidney and bone. Abbreviations: CaSR, calcium-sensing receptor; PTH, parathormone; Ca2+, calcium; FGF23, fibroblast growth factor 23; ↓, decrease.
Summary of key studies addressing the impact of etelcalcetide on phospho-calcium metabolism.
| Author/study type/year of publication | General characteristics | Main results | Main limitations |
|---|---|---|---|
| Angelo Karaboyas et al.11Prospective Cohort Study – “New user design”2022 | Comparative Groups:Group with prior use of cinacalcet vs. Naive group for calcimimeticsStarting etelcalcetide dose – 15mg/week or 7.5mg/weekAnalysis of 2596 hemodialyzed patientsFollow-up: 12 months | Absolute reduction of 40% in PTH levels at the end of the study (948 to 566pg/mL)Proportion of patients achieving the PTH target (baseline vs. end of follow-up):* 33%<64% (overall)* 0%<60% (in patients with initial PTH level≥600pg/mL)* 30%<63% (in the group with prior use of cinacalcet)Etelcalcetide showed an apparent sustained and significant effect in reducing PTH levels, even in patients previously treated with cinacalcet | Without access to dosage protocolsReasons for discontinuation were not known |
| Geoffrey A Block et al.19RCTs2017 | Comparative Groups:Etelcalcetide vs. placeboTrial A - 508 patientsTrial B - 515 patientsTotal - 1023 patients1016 patients (503 in the etelcalcetide group and 513 in the control group) undergoing hemodialysis with moderate to severe SHPTFollow-up: 26 weeks | Mean PTH concentrations at baseline and during weeks 20–27:Trial A* 849 and 384pg/mL in the etelcalcetide group* 820 and 897pg/mL in the placebo groupTrial B* 845 and 363pg/mL in the etelcalcetide group* 852 and 960pg/mL in the placebo groupAttainment of the primary efficacy endpoint:Trial A – 74.0% vs. 8.3% in favor of the etelcalcetide group – statistically significant result (p<0.001)Trial B – 75.3% vs. 9.6% in favor of the etelcalcetide group – statistically significant result (p<0.001)Attainment of serum PTH level≤300pg/mL:Trial A – 49.6% vs. 5.1% in favor of the etelcalcetide group – statistically significant result (p<0.001)Trial B – 53.3% vs. 4.6% in favor of the etelcalcetide group – statistically significant result (p<0.001) | These trials were not designed to assess the effects of etelcalcetide on some parameters, such as bone architecture and strengthThe likelihood of fracture was not evaluatedThese trials were not able to evaluate the impact of etelcalcetide on certain parameters, such as vascular calcification, cardiovascular structure or function, cardiovascular events and mortality |
| Geoffrey A Blocket al.20RCT2017 | Comparative Groups:Etelcalcetide (IV) vs. cinacalcet (oral)683 patients (340 in the etelcalcetide group and 343 in the control group) undergoing hemodialysis with serum PTH concentrations higher than 500pg/mLFollow-up: 26 weeks | Reduction in serum PTH concentration>30% (after 26 weeks):* 57.7% vs. 68.2% in favor of the etelcalcetide group, with a difference of −10.5% – statistically significant result (p<0.001 for non-inferiority and p=0.004 for superiority)* Etelcalcetide was not inferior and likely superior to cinacalcet in achieving this endpointReduction in serum PTH concentration>50% (after 26 weeks):* 40.2% vs. 52.4% in favor of the etelcalcetide group, with a difference of −12.2% – statistically significant result (p=0.001) | Although efficacy and relative safety were sustained over 26 weeks, longer-term safety data are necessary |
| Suetonia C. Palmer et al.3Systematic Review2020 | Comparison of cinacalcet vs. etelcalcetide vs. evocalcet vs. placebo11,247 patients analyzedMedian follow-up: 26 weeks | Etelcalcetide was the most effective of the three agents in achieving target serum PTH levels | Short follow-up duration limits some interpretations |
| Iryna Dudar et al.24Cohort Study2022 | Comparative Groups:Etelcalcetide vs. control203 patients (71 in the etelcalcetide group and 132 in the control group) undergoing hemodialysis with SHPTFollow-up: 12 months | Etelcalcetide was very effective in reducing serum PTH levels:* Reduction≥30% from baseline PTH value after 3 months:** 54.9% vs. 9.1%, p<0.001* PTH target achieved at the end of the study:** 73.2% vs. 10.6%, p<0.001Lower proportion of patients requiring parathyroidectomy in the Etelcalcetide group (p<0.05) | The control group (“historical”) was evaluated retrospectively vs. the “main” group was evaluated prospectively |
| Luciano Pereira et al.25Prospective Cohort Study2023 | Comparative Groups:Etelcalcetide (after cinacalcet washout) vs. cinacalcetEtelcalcetide dose – 5mg/IV (per hemodialysis session)34 patients (30 completed the study) undergoing hemodialysis with uncontrolled SHPT despite using cinacalcet for ≥3 monthsFollow-up: 6 months | Statistically significant reduction in the mean serum PTH level in the etelcalcetide group (1005–702pg/mL; p<0.001)Statistically significant reduction in serum PTH level in patients with baseline levels>1000pg/mL (1231–763pg/mL; p=0.012)Statistically significant reduction in serum PTH level in patients with baseline levels <1000 (924 to 627pg/mL; p=0.046)Asymptomatic hypocalcemia was the main adverse effect associated with etelcalcetide (observed in 50% of patients) | Concomitant factors, such as phosphate binders and active vitamin D, were not adjusted by a strict protocolNo pre-specified protocol was done for the hemodialysis prescription of etelcalcetide |
| Massimo Morosetti et al.26“Real-Life” Retrospective Observational Study2023 | Comparative Groups:Group A – etelcalcetide+paricalcitol (cinacalcet naive)Group B – etelcalcetide+paricalcitol (previously treated with cinacalcet)Etelcalcetide – minimum and maximum dose of 2.5mg and 10mg, respectively45 patients (26 in Group A+19 in Group B) undergoing extracorporeal hemodialysis for at least 2 yearsFollow-up: 12–18 months | Group A:Reduction in serum PTH level from 846±306pg/mL to 482±291pg/mL and 498±295pg/mL at 6 and 12 months, respectively87% of patients achieved the target PTH level at the end of 12 monthsAt 12 months, a stable serum calcium level of 8.9±0.8mg/dL was observedGroup B:Reduction in serum PTH level from 1429±729pg/mL to 977±555pg/mL and 505±345pg/mL at 6 and 12 months, respectively58% of patients achieved the target PTH level at the end of 12 monthsAt 12 months, a stable serum calcium level of 8.9±0.7mg/dL was observedConclusion:The efficacy of etelcalcetide was sustained over time and the reduction in serum PTH level was more pronounced in the first 4 months of treatment | The small sample size prevented the establishment of a causal relationship |
| Angelo Karaboyas et al.27Prospective Cohort Study2022 | Comparative Groups:Cinacalcet-first hemodialysis facilities (67) vs. etelcalcetide-first hemodialysis facilities (45)2 approaches were used to compare outcomes:Approach 1: cross-sectional comparisonApproach 2: pre-post comparison | Approach 1:Adjusted mean difference in PTH level: −115pg/mL (95% CI, −196 to −34), in favor of the etelcalcetide groupPrevalence of serum PTH level>600pg/mL decreased: −11.4% (95% CI, −19.3% to −3.5%), in favor of the etelcalcetide groupApproach 2:* In facilities that changed to etelcalcetide-first:** Mean serum PTH level decreased from 671 to 484pg/mL** Prevalence of PTH levels>600pg/mL decreased from 39 to 21%* In facilities that continued cinacalcet-first:** Mean serum PTH level increased from 632 to 698pg/mL** Prevalence of PTH levels>600pg/mL increased from 37 to 43%Conclusion:Better control of serum PTH levels in patients under etelcalcetide compared to cinacalcetPossibly better outcomes with etelcalcetide due to its higher efficacy and adherence | Without access to treatment protocolsDifferences in characteristics between the etelcalcetide and cinacalcet groups – a limitation in establishing comparisons |
Abbreviations: PTH, parathormone; RCT, randomized clinical trial; SHPT, secondary hyperparathyroidism; IV, intravenous; CI, confidence interval; vs., versus.
Regarding the adverse effects of etelcalcetide, this issue has been a subject of controversy. While it is true that studies with higher quality/evidence (systematic reviews/RCTs) did not find statistically significant differences in terms of adverse effects when comparing etelcalcetide versus cinacalcet,20,23 some recent studies in a real-world setting suggest that etelcalcetide appears to be better tolerated than cinacalcet.11,18,30
Hypocalcemia is an important concern. Etelcalcetide is more effective than cinacalcet in reducing PTH,11,19,20,23–27 so it tends to have a greater impact on reducing serum calcium levels.11,24 In fact, in the systematic review by Palmer et al., etelcalcetide had higher odds of hypocalcemia compared to cinacalcet (odds ratio 1.47; 95% CI, 1.08–2.00), which was statistically significant.23 In a prospective cohort study by Karaboyas et al., hypocalcemia was the most commonly adverse effect associated with etelcalcetide due to its potency, however, the frequency of severe hypocalcemia (<7.5mg/dl) after 1 year of treatment was low (<1–2%).11 The occurrence of symptomatic hypocalcemia or the discontinuation of etelcalcetide due to hypocalcemia were rarely reported.11 In another study, hypocalcemia was common, with 52.1%, 14.7% and 6.4% of patients having serum calcium levels of 7.5–8.3mg/dl, 7.0–7.5mg/dl and <7.0mg/dl, respectively.28 However, only 3.7% of patients experienced symptomatic hypocalcemia. Thus, although common, the clinical impact of hypocalcemia associated with etelcalcetide is minimal.28 To avoid severe and symptomatic hypocalcemia, an integrated review advised a pause of at least 7 days between discontinuing cinacalcet and starting etelcalcetide, with serum calcium levels within the normal range before its introduction.22
In relation to hypocalcemia in dialysis patients, it should be noted that the measurement of corrected calcium (cCa) levels may overestimate ionized calcium (iCa) (biologically active) levels and may erroneously consider that a patient has normocalcemia when, in fact, he has hidden hypocalcemia (normal cCa with low iCa).31 This is relevant because there are some studies, in particular the one by Yamaguchi et al., showed that when assessing the risk of cardiovascular disease and death from all causes, this risk was higher in patients with hidden hypocalcemia versus normocalcemia (normal iCa), with a statistically significant result (hazard ratio, 2.56; 95% CI, 1.11–5.94).31 However, when comparing patients with evident hypocalcemia (low cCa and iCa) versus normocalcemia, no statistically significant differences were found, probably because in this group (evident hypocalcemia) interventions (e.g., analogs of vitamin D and calcium carbonate) to correct hypocalcemia were more timely than in the group with hidden hypocalcemia.31 Thus, as highlighted by Yamaguchi et al., it is crucial to measure iCa (which is more expensive) in order to correctly and timely identify hidden hypocalcemia, with the aim of preventing/reducing the risk of associated serious adverse events.
Regarding the gastrointestinal effects, controversy prevails. In disagreement with several RCTs on this topic, a phase 3 RCT demonstrated that despite gastrointestinal effects being evident with both drugs, these effects were milder with etelcalcetide, leading to a lower discontinuation rate and higher tolerability.29 This lower severity may justify its preference in certain patients.
According to the systematic review by Palmer et al., concerning gastrointestinal effects (nausea and vomiting), etelcalcetide did not show statistically significant differences versus cinacalcet (odds ratio cinacalcet versus etelcalcetide 1.07; 95% CI, 0.63–1.80). However, the authors suggested that for healthcare professionals etelcalcetide was the preferred drug for minimizing gastrointestinal adverse effects.23
The integrated analysis by Block et al. highlighted adverse effects related to mineral-bone metabolism (particularly hypocalcemia) and gastrointestinal tract (diarrhea, nausea and vomiting) as the main adverse effects associated with etelcalcetide, with a low discontinuation rate versus cinacalcet.22 The study by Bushinsky et al. corroborated these findings, emphasizing asymptomatic hypocalcemia (43.3% of patients) and gastrointestinal symptoms (diarrhea (10.8%), vomiting (10.4%) and nausea (9.6%)) as the main adverse effects associated with etelcalcetide. Despite 89.8% of patients experienced at least one adverse effect related to this drug, only 4.6% had to discontinue it, indicating their reduced severity.28
Regarding the discontinuation of calcimimetics, in a prospective cohort study, individuals on etelcalcetide had discontinuation rates of 9%, 17% and 27% at 3, 6 and 12 months on this therapy, respectively, with a higher discontinuation rate observed in individuals who were not previously on cinacalcet.11 Patients who took cinacalcet in the previous 3 months tolerated etelcalcetide better, suggesting that these adverse effects may not be as pronounced as those of cinacalcet, justifying lower discontinuation rates associated with etelcalcetide.11 Patients with lower PTH values (<600pg/mL), younger age (<65 years), recently on dialysis (<3 years) and with lower starting doses of etelcalcetide (7.5mg/week) had higher discontinuation rates since, presumably, for younger patients and those with milder/early disease, the benefit gained from the medication is not worth it given these adverse effects.11 Previous studies reinforce higher discontinuation rates (lower tolerability) with the use of cinacalcet (40% and 73%).15,16 Finally, in a 52-week follow-up study, 23.2% of patients discontinued etelcalcetide, but only 4.6% discontinued it due to associated adverse effects. Cardiac arrest, nausea, vomiting, cellulitis, hypocalcemia and seizures were the main reasons for discontinuation.28
The “real-life” study by Russo et al. deserves particular relevance as the authors used self-reporting (instead of questionnaires) to identify the most bothersome adverse effects for patients.18 Patients’ submission to cinacalcet and then to etelcalcetide allowed understanding how the same patient evaluated the inconvenience of two different drugs, reducing interindividual subjectivity. In fact, the recording of gastrointestinal adverse events was clearly higher with cinacalcet (53%) versus etelcalcetide (3–4%), demonstrating that, at least for the studied population, the tolerability of etelcalcetide was much higher than cinacalcet, especially in terms of gastrointestinal effects. Thus, although both drugs reported similar adverse effects, tolerability was clearly superior with etelcalcetide.18
The results of Khan et al.’s study are even more surprising. In 148 hemodialyzed patients with SHPT treated with etelcalcetide, no gastrointestinal adverse effects were documented, greatly increasing the compliance of these patients with this treatment.30 However, it is important to realize that there were significant follow-up losses, which could explain the absence of some adverse effects and make some interpretations more difficult.30 Although previous studies have reported gastrointestinal adverse effects, making it difficult to believe that this drug does not cause any gastrointestinal adverse effects at all, this study at least demonstrated that the frequency of these adverse effects is probably much lower than that demonstrated with the use of cinacalcet.
Regarding the pathophysiology behind gastrointestinal complaints associated with etelcalcetide, this is still unclear and not universally accepted within the scientific community. Pereira et al. group point to nausea and vomiting as a likely systemic effect, independent of the administration route.12 Additionally, the study by Friedl et al. assumes that this effect is also related to the action on non-parathyroid organs that have the CaSR.32
Table 3 summarizes the main conclusions of the most relevant articles on this topic (adverse effects associated with etelcalcetide).
Summary of key studies addressing adverse effects associated with calcimimetics (particularly etelcalcetide).
| Author/study type/year of publication | General characteristics | Main results | Main limitations |
|---|---|---|---|
| Angelo Karaboyas et al.11Prospective Cohort Study – “New user design”2022 | Comparative Groups:Group with prior use of cinacalcet vs. naive group for calcimimeticsStarting etelcalcetide dose – 15mg/week or 7.5mg/weekAnalysis of 2596 hemodialyzed patientsFollow-up: 12 months | Discontinuation of etelcalcetide:* 9%, 17% and 27% at 3, 6 and 12 months of treatment | Without access to dosage protocolsReasons for discontinuation were not known |
| Luciano Pereira et al.12Narrative Review2018 | – | Plasma concentration of etelcalcetide showed greater stability/less variability compared to cinacalcetEtelcalcetide was not metabolized/an inhibitor/an inducer of cytochrome – negligible drug interactionsThe method and mode of administration were associated with higher therapeutic adherence and reduced “pill burden”Gastrointestinal adverse effects appeared to have resulted from a systemic rather than a local mechanismEtelcalcetide reduced FGF23 levels more significantly compared to cinacalcet | – |
| Domenico Russo et al.18“Real Life” Observational Study2019 | Comparative Groups:Cinacalcet naive group vs. group previously treated with cinacalcetBoth subjected to etelcalcetide - Average weekly dose of etelcalcetide – 15mgAnalysis of 1190 hemodialyzed patients, with 168 under etelcalcetide treatment | Reduction in serum PTH level from 636pg/mL to 357pg/mL at the end of the studyAverage time to achieve the target serum PTH level was 53 daysPercentage of patients with serum PTH levels within the target range increased from 27% at the beginning to 63% at the end of the studyStatistically significant reduction in serum levels of phosphate and calcium (p<0.05)Hypocalcemia was the most commonly adverse effect associated with etelcalcetide (<4% of cases), often asymptomaticGastrointestinal adverse effects were present in 3–4% of patients (vs. 53% with cinacalcet, according to previous studies)Etelcalcetide was better tolerated by patients compared to cinacalcet, although both had similar adverse effects | The impact of etelcalcetide on reducing parathyroidectomy, vascular calcification or mortality was not evaluated |
| Geoffrey A Block et al.22Integrated Analysis2019 | 5 studies:A – 2 RCT with placebo control19B – 1 RCT with active control20C – 2 open-label extension studies (single-arm)Total participants: 1023 (A)+683 (B)+1299 (C)=3005Patients undergoing hemodialysis with moderate to severe SHPT | Adverse effects most associated with etelcalcetide:* Mineral metabolism (hypocalcemia, hypophosphatemia, muscle spasms)* Gastrointestinal effects (diarrhea, nausea, vomiting)Low discontinuation rate with the use of etelcalcetide (<6%) – well toleratedMain reasons for discontinuation:* Cardiac arrest, gastrointestinal effects, infections and symptomatic hypocalcemiaNo significant differences were found in terms of gastrointestinal effects between etelcalcetide and cinacalcetCinacalcet should be discontinued 7 days before introducing etelcalcetide, and calcium levels should be corrected to minimize the risk of severe hypocalcemia | – |
| Suetonia C. Palmer et al.23Systematic Review2020 | Comparison of cinacalcet vs. etelcalcetide vs. evocalcet vs. placebo11,247 patients analyzedMedian follow-up: 26 weeks | The risk of hypocalcemia was higher with the use of etelcalcetideBoth etelcalcetide and cinacalcet were associated with more pronounced gastrointestinal effects compared to placeboGastrointestinal adverse effects were slightly lower with etelcalcetide compared to cinacalcet, but the results were not statistically significant | Short follow-up duration limits some interpretations |
| David A. Bushinsky et al.28Open Label Extension2020 | Etelcalcetide - Initial dose of 5mg (maximum dose of 15mg)3 studies:A – 2 RCTs (1023 patients)B – Open-label (single arm) (158 patients)Hemodialyzed patientsFollow-up: 52 weeks | Etelcalcetide is effective in reducing PTH levels and has a sustained effect over time:* More than a 30% reduction in serum PTH levels was observed in 68% of patients* 56% of patients achieved target serum PTH levels≤300pg/mLAdverse effects were frequent but well-tolerated in the majority of cases – Discontinuation in <5% of casesMost common adverse effects – asymptomatic hypocalcemia (43.3%); diarrhea (10.8%), vomiting (10.4%) and nausea (9.6%)Symptomatic hypocalcemia was infrequent (<4% of cases) | – |
| Masafumi Fukagawa et al.29RCT2017 | Comparative Groups:Etelcalcetide vs. placeboEtelcalcetide – initial dose of 5mg, with adjustments between 2.5 and 15mg155 hemodialyzed patients with SHPT (with PTH levels>300pg/mL)Follow-up: 12 weeks | Etelcalcetide was well-tolerated, deemed safe and effective, simultaneously – only 2.6% of patients discontinued etelcalcetide due to associated adverse effectsThe majority of etelcalcetide adverse effects were considered mild to moderate – explaining high tolerabilityMost frequent adverse effects: asymptomatic hypocalcemia – 6.4%; vomiting – 3.8%; nausea – 1.3% and symptomatic hypocalcemia – 1.3% | Short follow-up duration limits some interpretations |
| Behram A. Khan et al.30“Real world evidence” based on retrospective data2023 | The impact of etelcalcetide was evaluated in patients with SHPTEtelcalcetide – initial dose ranged from 2.5mg weekly to 7.5mg three times weekly148 hemodialysis patients were includedFollow-up: 12 months | Etelcalcetide was safe and effective in hemodialysis patients with SHPT:* 16.8% reduction (p<0.001) in serum PTH levels at 4 months of follow-up* Target serum PTH level achieved in 1.4%, 22.3% (p<0.001) and 25.9% (p<0.028) of patients at baseline, 4 months and 8 months of follow-up, respectively* Calcium-phosphate product showed significant reductions, especially at 4 months of follow-up (p<0.001)No gastrointestinal adverse effects were documented, which contrasts with previous studies - likely higher tolerability and compliance with the use of etelcalcetide | Significant follow-up losses – Only 19% of patients completed the 8-month study, which could explain the absence of further adverse effects (especially gastrointestinal) |
Abbreviations: PTH, parathormone; FGF23, fibroblast growth factor 23; RCT, randomized clinical trial; SHPT, secondary hyperparathyroidism; vs., versus.
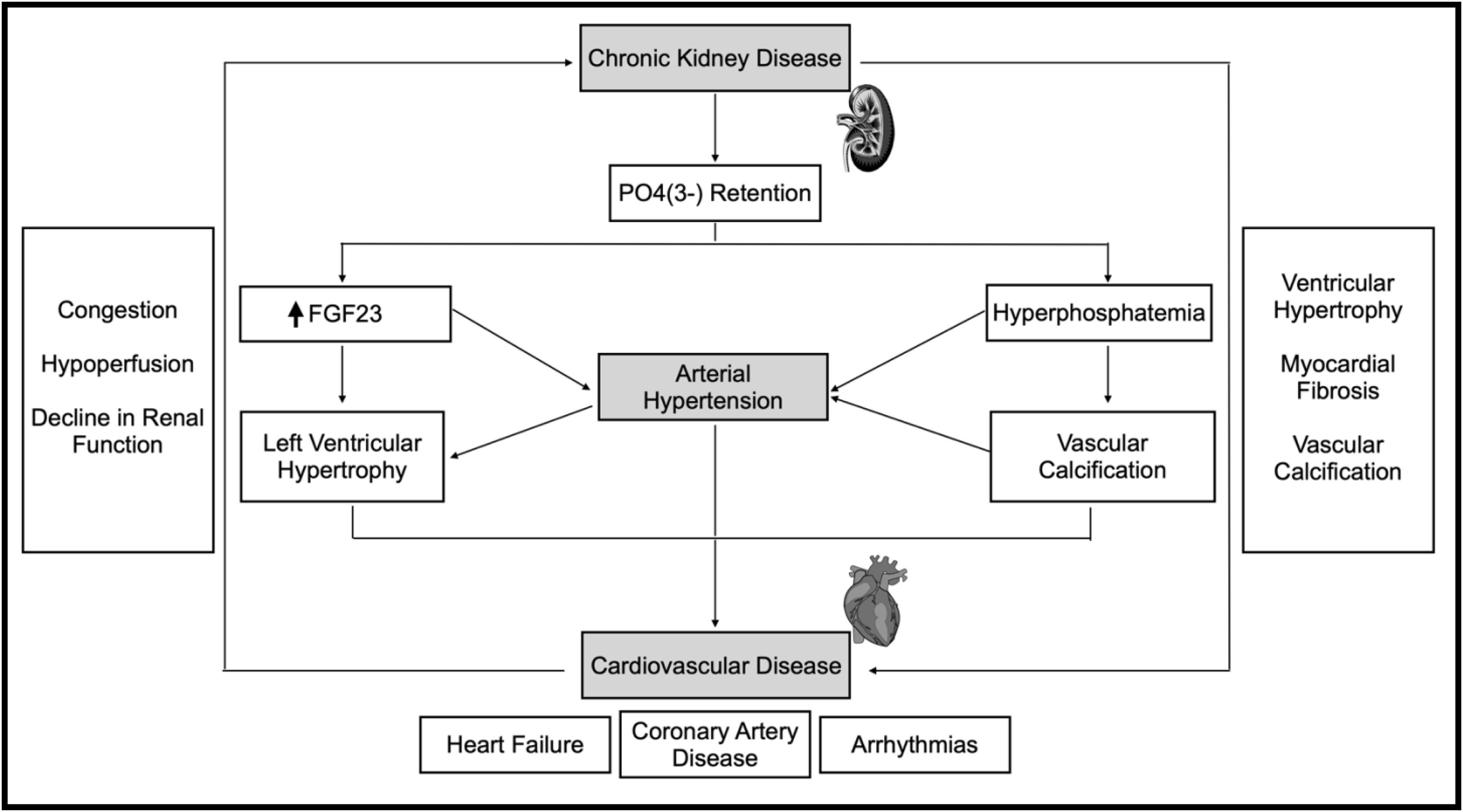
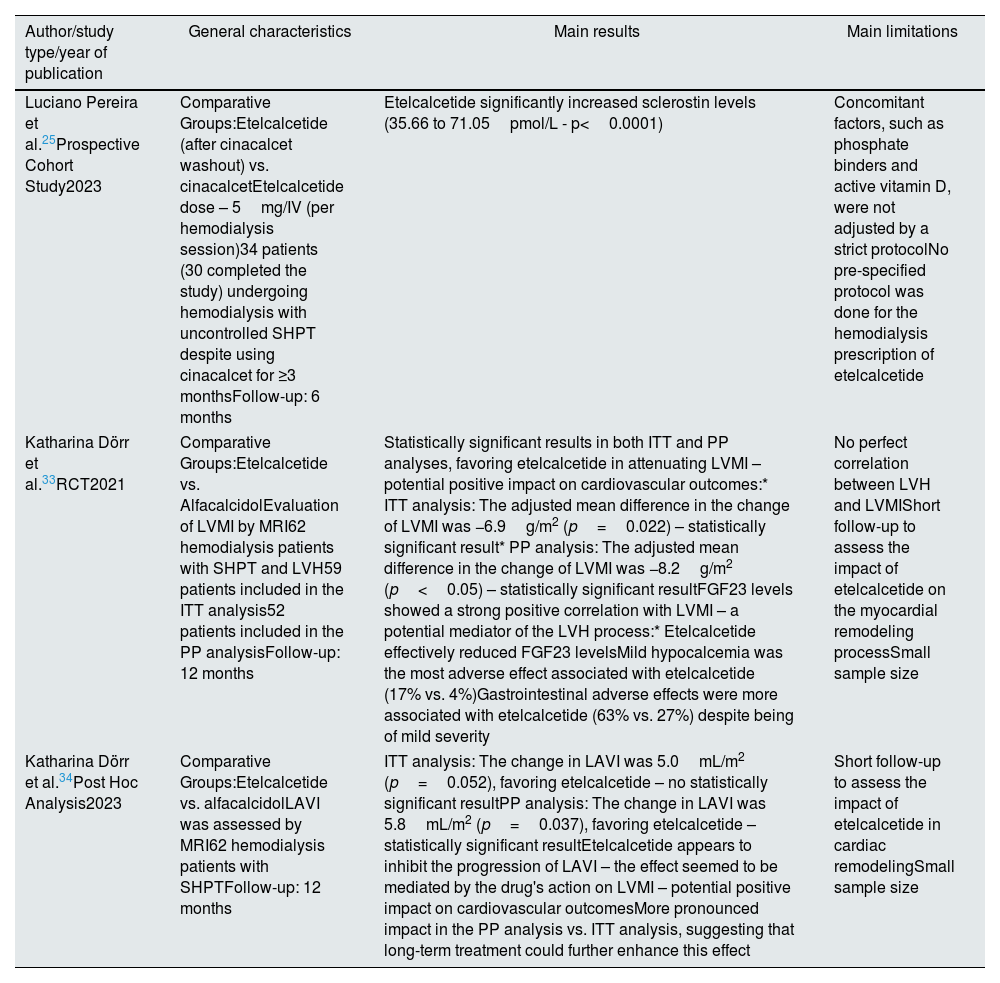
LVH and left atrial volume (LAV) increase the risk of cardiac events and associated mortality, particularly in patients with CKD.33,34 The association between CKD and CV disease, especially with LVH, is depicted in Fig. 3.
Pathophysiological mechanisms and corresponding association between chronic kidney disease and cardiovascular disease. Description: Demonstration of the correlation between chronic kidney disease and cardiovascular disease, highlighting the complications associated with these diseases, as well as the different mechanisms that contribute to both pathologies. Abbreviations: P04(3-), phosphate; FGF23, fibroblast growth factor 23; ↑, increase.
According to Park et al., an increase >10% in left ventricular mass index (LVMI – good correlation with LVH) is an independent predictor for adverse CV events.35 Conversely, a 10% reduction in hemodialysis patients was associated with reductions in CV and all-cause mortality.36 According to Dörr et al.’s post hoc analysis, left atrial volume index (LAVI – good correlation with LAV) correlates with CV events and all-cause mortality. Delaying the progression of LAVI may imply better outcomes at this level.34
Dörr et al.’s RCT evaluated the progression of LVH (measured through LVMI, assessed by magnetic resonance imaging) in patients undergoing etelcalcetide versus alfacalcidol therapy for 12 months. Both intent-to-treat (ITT) and per-protocol (PP) analyses showed statistically significant differences favoring etelcalcetide in stabilizing the progression of LVMI and corresponding LVH. The adjusted mean difference in LVMI estimate was −6.9g/m2 (p=0.022) and −8.2g/m2 (p=0.008), respectively.33 This study also assessed the impact of therapies on FGF23 levels, with significant reductions in the etelcalcetide-treated group (factor 0.13; 95% CI, 0.06–0.26; etelcalcetide versus alfacalcidol, ITT analysis).33 There was a positive association between FGF23 levels and LVMI (LVMI increased by 2.0g/m2 by doubling the ratio of FGF23 values; 95% CI, 1.0–3.1g/m2),33 consistent with another study indicating that FGF23 was independently correlated with LVMI (p=0.01) and with the risk of LVH (odds ratio 2.1; 95% CI, 1.03–4.2), with both results statistically signifcant.37 Therefore, Dörr et al.’s study concluded that FGF23 suppression by etelcalcetide helped to stabilize the progression of LVH compared to alfacalcidol (which increased that progression), particularly in hemodialysis patients.33 The post hoc analysis by Dörr et al. evaluated the relationship between etelcalcetide and alfacalcidol in LAVI progression. Both ITT and PP analyses showed a reduction in LAVI of 5.0mL/m2 (95% CI: −0.04, 10; p=0.052) and 5.8mL/m2 (95% CI: 0.36, 11; p=0.037), respectively, in favor of etelcalcetide.34 In a sub-analysis, a close relationship between LAVI and LVMI was demonstrated, with the effect of etelcalcetide on inhibiting LAVI progression partly mediated by its effect on LVMI.34 Thus, both studies suggest that since LAV and LVH are CV risk factors, etelcalcetide, by stabilizing their progression, may lead to a reduction of CV mortality, particularly in hemodialysis patients.33,34 Studies directly addressing the impact of etelcalcetide on CV and all-cause mortality are needed for more informed conclusions.
FGF23 is a hormone synthesized in the bone and its levels are increased in CKD.38 FGF23 levels are independently associated with CV outcomes and mortality in individuals who have not started dialysis, those who recently initiated it, and even in chronically dialyzed patients.38–40 According to the post hoc analysis of the EVOLVE trial, reductions in FGF23 levels were associated with lower rates of major CV events and CV mortality.41 In line with this evidence, sub-analysis results from this trial showed that patients treated with cinacalcet had higher survival rates in the group with low FGF23 levels compared to those with high levels.41 Etelcalcetide, as mentioned earlier, significantly reduces FGF23 levels, suggesting a potential beneficial impact on CV outcomes.33
Concerning CV outcomes, vascular calcification is another important aspect as it correlates with CV mortality.42 Vascular calcification is a common complication in CKD patients, resulting from mineral homeostasis dysregulation.43 High levels of calcium and phosphate as well as extreme levels of PTH are essential for the initiation and progression of this issue.43
Studies assessing the impact of etelcalcetide on vascular calcification are scarce, with no human studies to date. A preclinical study in rats with CKD and SHPT compared etelcalcetide and paricalcitol, with vascular calcification being measured by aortic calcium content (ACC).44 63% of the 24 rats treated with paricalcitol had ACC>400μg/g, while none of the 24 rats treated with etelcalcetide had it. This result suggests a positive impact of etelcalcetide on the attenuation of vascular calcification, consistent with previous findings on cinacalcet.44
The pathophysiology explaining the attenuation of vascular calcification by etelcalcetide remains unclear. Other calcimimetics had shown a direct effect on vascular cells due to CaSR activation in endothelial and vascular smooth muscle cells, increasing matrix Gla protein (calcification inhibitor) in the arterial wall. Thus, since etelcalcetide acts on CaSR in a similar way as other calcimimetics, the mechanism that explain the attenuation of vascular calcification process is probably identical.12,17,44
In a more recent prospective cohort study, sclerostin, a protein apparently relevant in the vascular calcification process, was evaluated.25 Sclerostin is expressed in osteocytes and it is elevated in the early stages of CKD.45 Elevated levels of sclerostin were associated with better outcomes in terms of vascular calcification.46 Physiologically, it is believed to have beneficial protective paracrine effects, suppressing the transformation of vascular smooth muscle cells into osteoblast-like cells, slowing down the vascular calcification process.25 Also, in this Pereira et al's study, a negative correlation between sclerostin and PTH levels was found, both at the beginning and at the end of the study,25 a predictable correlation since PTH negatively regulates sclerostin in osteocytes.47 In individuals already taking cinacalcet, sclerostin concentration significantly increased after etelcalcetide use.25 Thus, this seems to be another mechanism that can explain the possible role of etelcalcetide in attenuating the vascular calcification process beyond its effect on vascular CaSR, as mentioned earlier. In severe cases of SHPT, PTH levels are higher and, consequently, sclerostin levels are lower.25 In these cases, drugs aiming to increase sclerostin levels may be advantageous, particularly in attenuating the progression of vascular calcification.
Table 4 summarizes the main results of the aforementioned studies regarding this topic (CV outcomes).
Summary of key studies addressing the impact of etelcalcetide on surrogate cardiovascular outcomes.
| Author/study type/year of publication | General characteristics | Main results | Main limitations |
|---|---|---|---|
| Luciano Pereira et al.25Prospective Cohort Study2023 | Comparative Groups:Etelcalcetide (after cinacalcet washout) vs. cinacalcetEtelcalcetide dose – 5mg/IV (per hemodialysis session)34 patients (30 completed the study) undergoing hemodialysis with uncontrolled SHPT despite using cinacalcet for ≥3 monthsFollow-up: 6 months | Etelcalcetide significantly increased sclerostin levels (35.66 to 71.05pmol/L - p<0.0001) | Concomitant factors, such as phosphate binders and active vitamin D, were not adjusted by a strict protocolNo pre-specified protocol was done for the hemodialysis prescription of etelcalcetide |
| Katharina Dörr et al.33RCT2021 | Comparative Groups:Etelcalcetide vs. AlfacalcidolEvaluation of LVMI by MRI62 hemodialysis patients with SHPT and LVH59 patients included in the ITT analysis52 patients included in the PP analysisFollow-up: 12 months | Statistically significant results in both ITT and PP analyses, favoring etelcalcetide in attenuating LVMI – potential positive impact on cardiovascular outcomes:* ITT analysis: The adjusted mean difference in the change of LVMI was −6.9g/m2 (p=0.022) – statistically significant result* PP analysis: The adjusted mean difference in the change of LVMI was −8.2g/m2 (p<0.05) – statistically significant resultFGF23 levels showed a strong positive correlation with LVMI – a potential mediator of the LVH process:* Etelcalcetide effectively reduced FGF23 levelsMild hypocalcemia was the most adverse effect associated with etelcalcetide (17% vs. 4%)Gastrointestinal adverse effects were more associated with etelcalcetide (63% vs. 27%) despite being of mild severity | No perfect correlation between LVH and LVMIShort follow-up to assess the impact of etelcalcetide on the myocardial remodeling processSmall sample size |
| Katharina Dörr et al.34Post Hoc Analysis2023 | Comparative Groups:Etelcalcetide vs. alfacalcidolLAVI was assessed by MRI62 hemodialysis patients with SHPTFollow-up: 12 months | ITT analysis: The change in LAVI was 5.0mL/m2 (p=0.052), favoring etelcalcetide – no statistically significant resultPP analysis: The change in LAVI was 5.8mL/m2 (p=0.037), favoring etelcalcetide – statistically significant resultEtelcalcetide appears to inhibit the progression of LAVI – the effect seemed to be mediated by the drug's action on LVMI – potential positive impact on cardiovascular outcomesMore pronounced impact in the PP analysis vs. ITT analysis, suggesting that long-term treatment could further enhance this effect | Short follow-up to assess the impact of etelcalcetide in cardiac remodelingSmall sample size |
Abbreviations: IV, intravenous; SHPT, secondary hyperparathyroidism; RCT, randomized clinical trial; LVMI, left ventricular mass index; MRI, magnetic resonance imaging; LVH, left ventricular hypertrophy; ITT, intention-to-treat; PP, per-protocol; FGF23, fibroblast growth factor 23; LAVI, left atrial volume index; vs., versus.
Some studies have addressed the impact of etelcalcetide on certain complications of the bone metabolism in SHPT, especially renal osteodystrophy.24,29,48,49
In Dudar et al.’s prospective cohort study, with a 12-month follow-up, 203 hemodialyzed patients with SHPT were evaluated, divided into two groups: one under etelcalcetide treatment and another without calcimimetic treatment (this control group was evaluated retrospectively).24 Regarding primary endpoints, the assessment of bone fracture frequency stood out. At the end of the 12-month follow-up, the bone fracture frequency was about three times higher in the control group compared to the etelcalcetide group, although these results were not statistically significant (p>0.1). The small sample size (203 patients) may explain the lack of power to find statistically significant differences.24
In a very recent study with a 36-week follow-up, the impact of etelcalcetide on bone quality and strength was assessed, in hemodialyzed patients, with the advantage of being the first bone biopsy trial using nanoscale measures of bone quality to assess the impact of calcimimetics on bone.48 In addition, this was the first trial specifically designed to evaluate the effect of etelcalcetide on bone tissue. At the end of the study, PTH levels decreased significantly (67±9%; p<0.001), areal bone mineral density of the spine, femoral neck and hip increased (3±1%, 7±2% and 3±1%, respectively; p<0.05), there was an improvement in trabecular microarchitecture of the spine (with an increase in spine trabecular bone score of 10±2%; p<0.001) and etelcalcetide was associated with a decrease in bone formation rate (p<0.01) without negatively affecting intrinsic bone properties.48 Despite these positive results, the number of participants ultimately limited the power of the study, with only 13 of the initial 22 participants completing the follow-up and only 5 of them undergoing bone biopsy. Despite this limitation, in this studied population, treatment with etelcalcetide was associated with suppression of bone turnover markers, improvement in bone mineral density (with an increase in Z scores adjusted for age and sex at the spine and femoral neck) and improvement in trabecular properties of the central skeleton.48
A preclinical study on rats with SHPT showed that etelcalcetide treatment significantly reduced PTH, FGF23 and osteocalcin (marker of bone turnover).49 The study also found reduction in bone turnover, attenuation of mineralization defects and bone marrow fibrosis, and preservation of cortical bone structure and strength.49 Additionally, etelcalcetide reduced levels of a bone resorption marker, tartrate-resistant acid phosphatase 5b (TRACP-5b).29,49 These findings suggest that etelcalcetide has a possible beneficial impact on renal osteodystrophy.24,29,48,49
In summary, these data suggest that etelcalcetide appears to have a positive impact on bone structure with a possible reduction in the risk of fractures.24,29,48,49 However, to date, there is a lack of studies specifically designed to evaluate the impact of etelcalcetide on reducing the risk of fractures in patients with CKD and SHPT.
Table 5 summarizes the main results of the aforementioned studies regarding this topic (bone metabolism).
Summary of key studies addressing the impact of etelcalcetide on bone metabolism.
| Author/study type/year of publication | General characteristics | Main results | Main limitations |
|---|---|---|---|
| Iryna Dudar et al.24Cohort Study2022 | Comparative Groups:Etelcalcetide vs. control203 patients (71 in the etelcalcetide group and 132 in the control group) undergoing hemodialysis with SHPTFollow-up: 12 months | Approximately 3× reduction in the incidence of bone fractures in the etelcalcetide group | The control group (“historical”) was evaluated retrospectively vs. the “main” group was evaluated prospectively |
| Masafumi Fukagawa et al.29RCT2017 | Comparative Groups:Etelcalcetide vs. placeboEtelcalcetide – initial dose of 5mg, with adjustments between 2.5 and 15mg155 hemodialyzed patients with SHPT (with PTH levels>300pg/mL)Follow-up: 12 weeks | The proportion of patients achieving target PTH levels (60–240pg/mL) was 59% vs. 1.3%The proportion of patients achieving ≥30% reductions in PTH levels was 76.9% vs. 5.9%Significant reductions in serum FGF23, albumin-corrected calcium and phosphate levels in the etelcalcetide group vs. placebo groupEtelcalcetide reduced TRACP-5b levels (a marker of bone resorption) – potential impact on renal osteodystrophy | Short follow-up duration limits some interpretations |
| Pascale Khairallahet al.48Prospective Trial (single arm)2023 | The impact of etelcalcetide on bone quality and structure was assessed22 hemodialysis patients with SHPT were analyzed13 patients completed the study5 patients underwent bone biopsyFollow-up: 36 weeks | Etelcalcetide was associated with an improvement in bone mineral density of the axial skeleton and trabecular quality:* Bone mineral density of the spine, femoral neck and hip increased by 3%, 7% and 3%, respectively, with the use of etelcalcetide (p<0.05)* Improvement in trabecular microarchitecture of the spine (p<0.001)Etelcalcetide was associated with a reduction in bone turnover/bone formation rate without negatively affecting intrinsic bone properties (p<0.01)Conclusion:The drug appeared to be useful in patients with severe SHPT already with significant renal osteodystrophyEtelcalcetide may have an impact on reducing the number of bone fractures by improving bone quality and structure | The small sample size limited the generalizability of the results and the population in this study was not representative of the general populationAbsence of a control groupSignificant losses in terms of follow-up could introduce selection biasThe method used (“quadruple label approach”) to assess bone-tissue quality had some problems: was not validated against the turnover, mineralization and volume system in CKD; only bone volume after treatment was assessed; changes in static measures of histomorphometry were not quantified |
Abbreviations: SHPT, secondary hyperparathyroidism; PTH, parathormone; RCT, randomized clinical trial; FGF23, fibroblast growth factor 23; TRACP-5b, tartrate-resistant acid phosphatase 5b; CKD, chronic kidney disease; vs., versus.
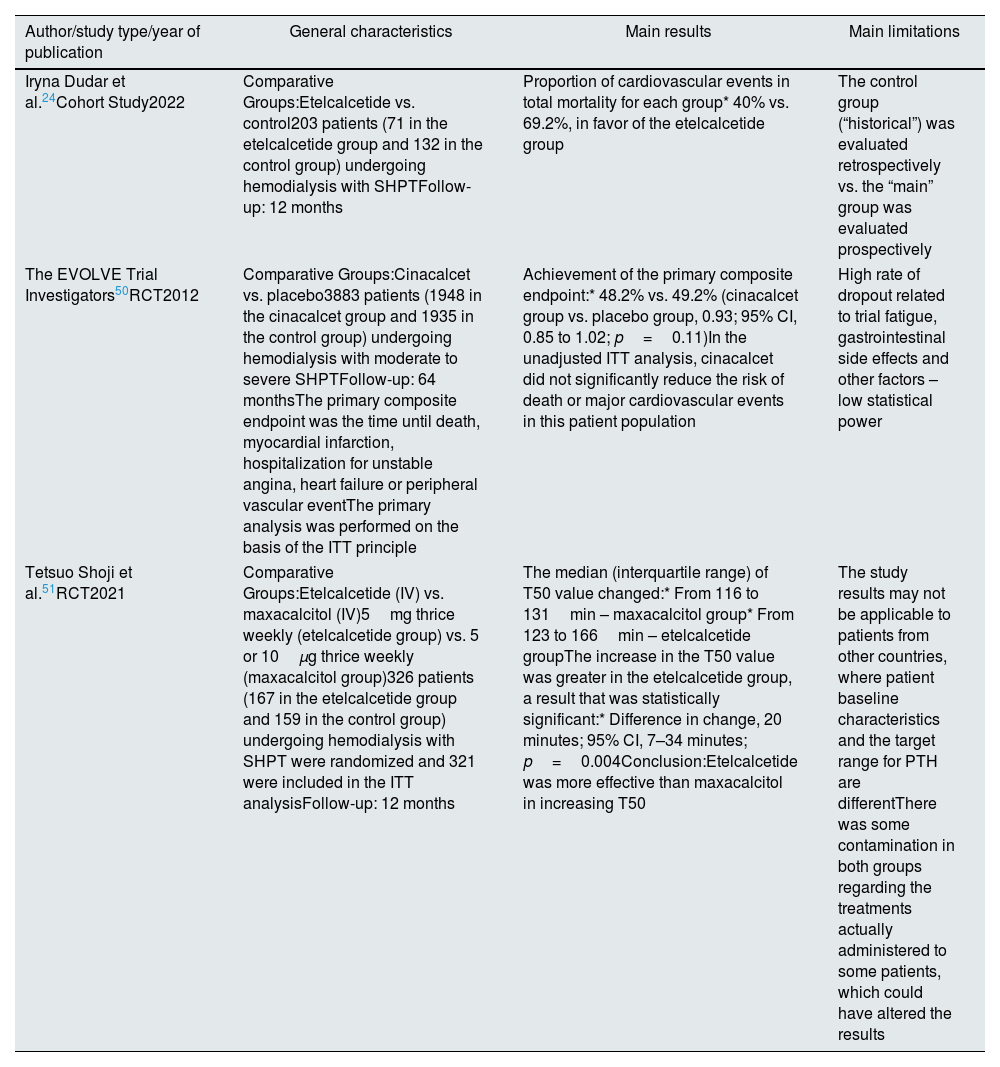
To date, there is a lack of studies that have directly addressed the primary outcome of the impact of calcimimetics on reducing CV and all-cause mortality. However, some studies have assessed their impact on surrogate markers of mortality in patients with CKD and SHPT.24,33,34,50,51
Before the introduction of etelcalcetide, some authors studied the relationship between cinacalcet and CV mortality.50 In the EVOLVE trial (RCT), 3883 patients with moderate to severe SHPT undergoing hemodialysis were evaluated over 64 months.50 The primary composite endpoint included time until death, myocardial infarction, hospitalization for unstable angina, heart failure and peripheral vascular events. 48.2% of patients on cinacalcet versus 49.2% of patients on placebo reached the primary composite endpoint, with results not statistically significant in favor of cinacalcet (hazard ratio 0.93, CI 0.85–1.02, p=0.11). Thus, in this studied population, cinacalcet did not appear to significantly reduce the risk of death and major CV events.50
Regarding the study by Dudar et al., previously mentioned, one of the evaluated primary endpoints was mortality due to CV events. At the end of the 12-month follow-up, all-cause mortality was not statistically lower in the etelcalcetide group versus in the non-calcimimetic group (p>0.2).24 The small sample size (203 patients) may explain the lack of power to find statistically significant differences. However, considering overall mortality in each group, the proportion of cases due to CV mortality was much lower in the etelcalcetide group (40% versus 69.2%) (both groups presented identical characteristics), with authors noting a greater than 2.5-fold reduction in CV mortality rate in this group compared to the control group.24
A RCT performed by Shoji et al. assessed the impact of etelcalcetide versus maxacalcitol on the reducing of a surrogate marker of mortality (serum calcification propensity – measured through T50 value) in patients with SHPT.51 A total of 425 patients from 23 dialysis centers were evaluated, with 321 included in the ITT analysis. At the end of the study, the authors observed an increase in T50 value for both drugs, but its increase (which means a decrease in the calcification propensity) was greater in the etelcalcetide group versus maxacalcitol group, with a statistically significant result (p=0.004).51 Low T50 levels correlate with a higher risk of all-cause mortality in patients with CKD (dialyzed or not),51 suggesting that etelcalcetide may have a positive impact on this outcome. It is important to notice that there was no difference in cognition and handgrip strength between both drugs.51
In conclusion, as previously described, etelcalcetide has demonstrated an impact on reducing LVH and LAV, suggesting it may have a beneficial effect on reducing CV mortality.33,34 Since the EVOLVE trial and the study by Dudar et al. did not confirm the possible effect of calcimimetics on mortality,24,50 more studies specifically designed, such as RCTs, are needed to evaluate this association.
Table 6 summarizes the main results of the aforementioned studies regarding this topic (CV and all-cause mortality).
Summary of key studies addressing the possible impact of etelcalcetide on cardiovascular and all-cause mortality.
| Author/study type/year of publication | General characteristics | Main results | Main limitations |
|---|---|---|---|
| Iryna Dudar et al.24Cohort Study2022 | Comparative Groups:Etelcalcetide vs. control203 patients (71 in the etelcalcetide group and 132 in the control group) undergoing hemodialysis with SHPTFollow-up: 12 months | Proportion of cardiovascular events in total mortality for each group* 40% vs. 69.2%, in favor of the etelcalcetide group | The control group (“historical”) was evaluated retrospectively vs. the “main” group was evaluated prospectively |
| The EVOLVE Trial Investigators50RCT2012 | Comparative Groups:Cinacalcet vs. placebo3883 patients (1948 in the cinacalcet group and 1935 in the control group) undergoing hemodialysis with moderate to severe SHPTFollow-up: 64 monthsThe primary composite endpoint was the time until death, myocardial infarction, hospitalization for unstable angina, heart failure or peripheral vascular eventThe primary analysis was performed on the basis of the ITT principle | Achievement of the primary composite endpoint:* 48.2% vs. 49.2% (cinacalcet group vs. placebo group, 0.93; 95% CI, 0.85 to 1.02; p=0.11)In the unadjusted ITT analysis, cinacalcet did not significantly reduce the risk of death or major cardiovascular events in this patient population | High rate of dropout related to trial fatigue, gastrointestinal side effects and other factors – low statistical power |
| Tetsuo Shoji et al.51RCT2021 | Comparative Groups:Etelcalcetide (IV) vs. maxacalcitol (IV)5mg thrice weekly (etelcalcetide group) vs. 5 or 10μg thrice weekly (maxacalcitol group)326 patients (167 in the etelcalcetide group and 159 in the control group) undergoing hemodialysis with SHPT were randomized and 321 were included in the ITT analysisFollow-up: 12 months | The median (interquartile range) of T50 value changed:* From 116 to 131min – maxacalcitol group* From 123 to 166min – etelcalcetide groupThe increase in the T50 value was greater in the etelcalcetide group, a result that was statistically significant:* Difference in change, 20 minutes; 95% CI, 7–34 minutes; p=0.004Conclusion:Etelcalcetide was more effective than maxacalcitol in increasing T50 | The study results may not be applicable to patients from other countries, where patient baseline characteristics and the target range for PTH are differentThere was some contamination in both groups regarding the treatments actually administered to some patients, which could have altered the results |
Abbreviations: SHPT, secondary hyperparathyroidism; RCT, randomized clinical trial; EVOLVE, evaluation of cinacalcet hydrochloride therapy to lower cardiovascular events; ITT, intention-to-treat; IV, intravenous; CI, confidence interval; vs., versus.
About etelcalcetide, there are some areas that need further exploration.
Regarding tolerability, it is necessary to address the discrepancy between the results of RCTs and observational studies (in a real-world setting).
The relationship between etelcalcetide and CV and all-cause mortality still needs to be more thoroughly studied, with studies specifically designed to assess this association.
The topic of bone fractures is scarcely mentioned in some articles about etelcalcetide, and it is also necessary to have studies specifically designed to evaluate this association.
Finally, bone biopsy is an excellent method for assessing bone quality and structure. More studies that preferentially use this method are needed.
ConclusionsThe post-approval evidence of etelcalcetide has supported the initial trials regarding its significant efficacy in reducing serum PTH levels. It seems to be more effective when compared to cinacalcet in reducing PTH, calcium, phosphate and FGF23 levels. Also, etelcalcetide has demonstrated a rapid and sustained effect over time.
Controversy persists about adverse effects. Hypocalcemia seems to be more pronounced with the use of etelcalcetide. Gastrointestinal tolerability appears to be higher with etelcalcetide in real-world setting studies, possibly due to the supposed lower severity of these adverse effects compared to cinacalcet.
CV outcomes are promising, showing a potential positive impact of etelcalcetide on LVH and vascular calcification. The association between FGF23 suppression and stabilized LVH progression suggests potential CV benefits. The recent link between etelcalcetide, sclerostin and vascular calcification opens new avenues for discovering another pathophysiological mechanism associated with the drug.
Etelcalcetide appears to improve bone quality and structure in patients with CKD and SHPT, with an apparent positive impact on renal osteodystrophy.
Finally, it is necessary to develop studies specifically designed to assess the impact of etelcalcetide on reducing bone fractures, CV and all-cause mortality.
FundingThis narrative review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.