There is scarce clinical experience with etelcalcetide in patients with secondary hyperparathyroidism uncontrolled with cinacalcet. The effect of etelcalcetide on serum sclerostin levels remains to be clarified.
Materials and methodsProspective cohort study in prevalent hemodialysis patients with uncontrolled sHPT under cinacalcet for at least 3 months, mean parathyroid hormone (PTH)>800pg/mL and calcium (Ca)>8.3mg/dL. Etelcalcetide 5mg IV/HD was initiated after cinacalcet washout. Levels of PTH, Ca, and phosphorus (Pi) followed monthly for 6 months. Plasma sclerostin levels measured before etelcalcetide treatment and after 6 months.
ResultsThirty-four patients were enrolled, 19 (55.9%) male gender. Mean age 60.7 (± 12.3) years; median time on HD 82.5 (7–296) months and median cinacalcet dose was 180mg/week (Interquartile Range: 180–270).
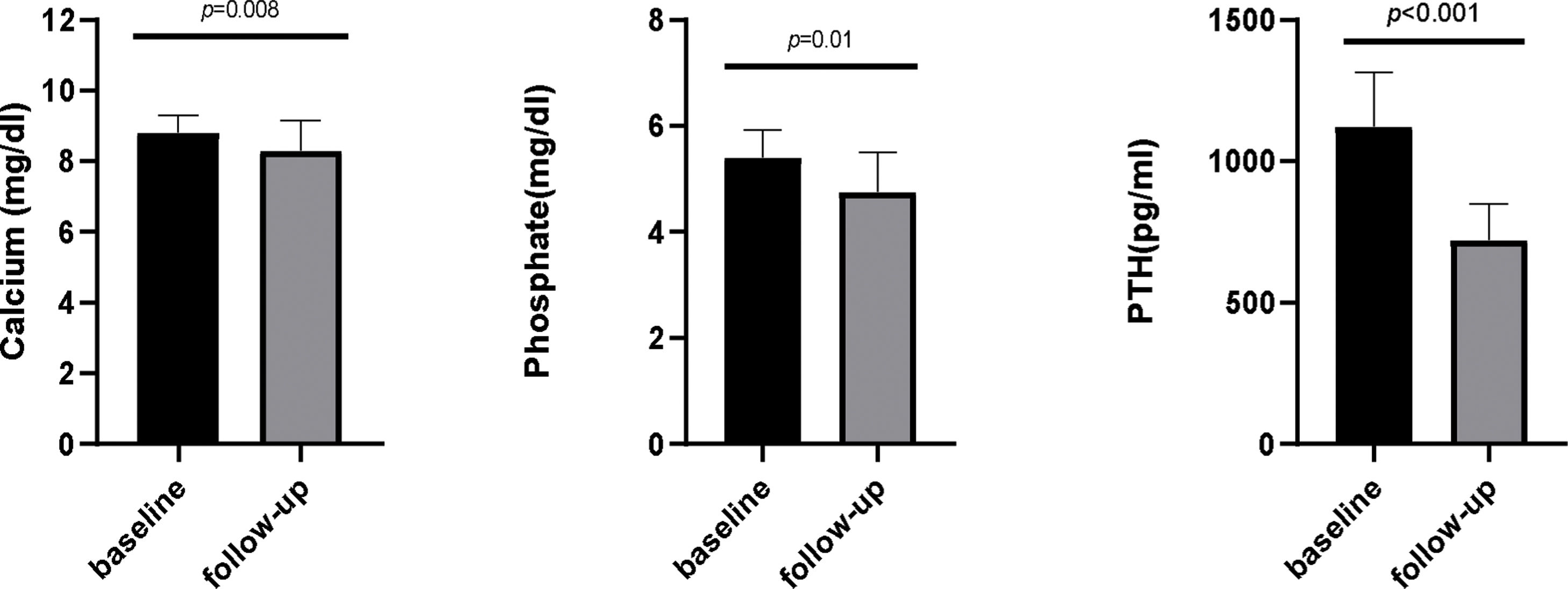
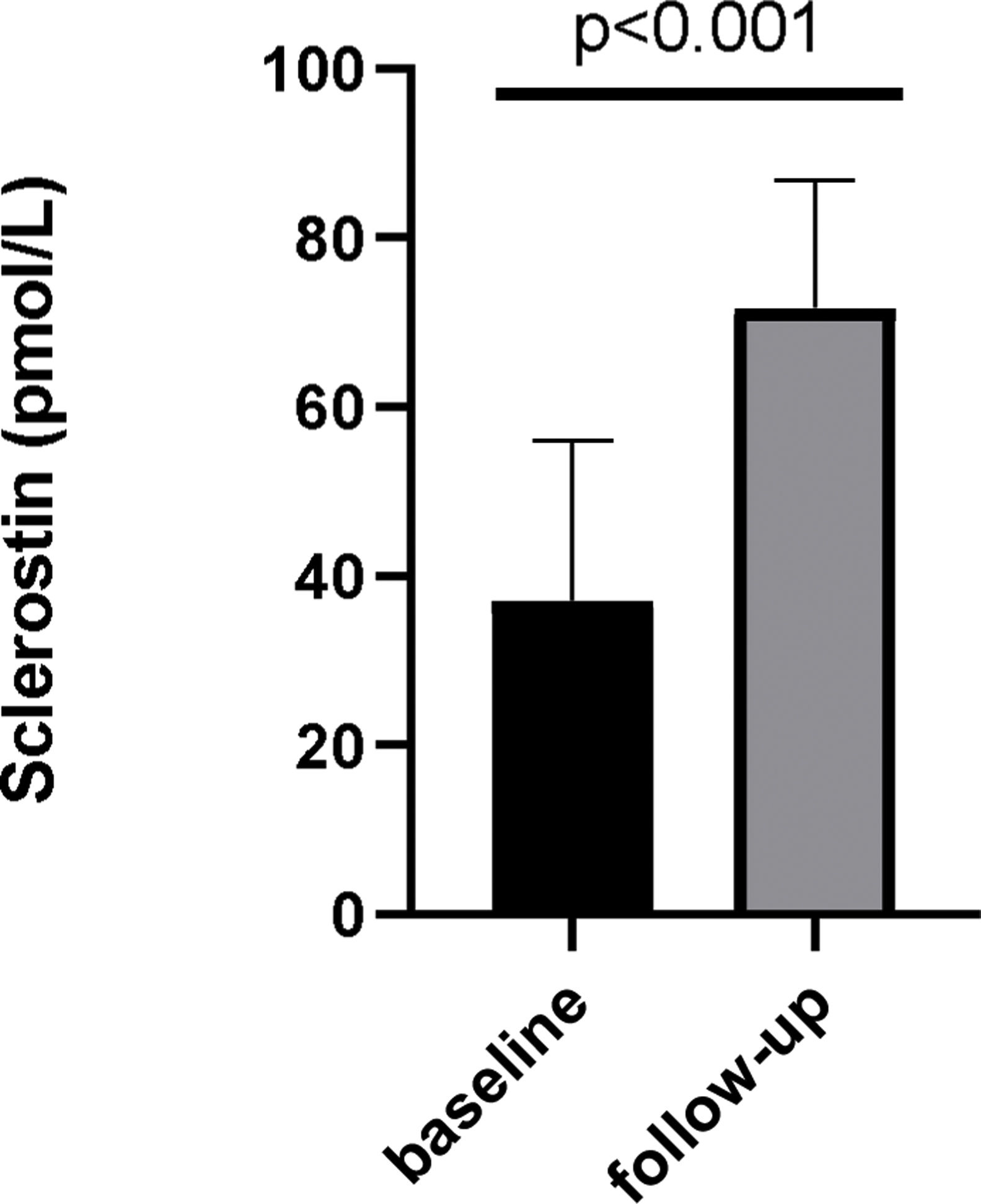
Serum Ca, Pi and PTH levels showed a significant reduction after etelcalcetide treatment from 8.8mg/dL, 5.4mg/dL and 1005pg/mL to 8.1mg/dL (p=0.08), 4.9mg/dL (p=0.01) and 702pg/mL (p<0.001), respectively. Median etelcalcetide dose remained at 5mg/HD. Plasma sclerostin concentration increased from 35.66pmol/L (IQR11.94–54.58) to 71.05pmol/L (IQR54.43–84.91) (p<0.0001).
ConclusionEtelcalcetide improved sHPT control in this group of patients, previously under cinacalcet treatment, and significantly increased plasma sclerostin concentration. The impact of etelcalcetide treatment on sclerostin levels is a novel finding.
Existe escasa experiencia clínica sobre el uso de etelcalcetida en pacientes con hiperparatiroidismo secundario no controlado con cinacalcet. Asimismo, el efecto de la etelcalcetida sobre los niveles de esclerostina aún no ha sido aclarado.
Materiales y métodosRealizamos un estudio de cohorte prospectivo en pacientes en hemodiálisis (HD) con hiperparatiroidismo secundario no controlado con cinacalcet durante al menos 3 meses, hormona paratiroidea media> 800 pg/ml y calcio (Ca)> 8,3mg/dl. Tras un periodo de lavado, se inició administración intravenosa de etelcalcetida 5mg/HD y se realizó un seguimiento mensual de los niveles de hormona paratiroidea, Ca y fósforo (Pi) durante 6 meses. Además, los niveles de esclerostina plasmática fueron medidos antes del tratamiento con etelcalcetida y después de 6 meses.
ResultadosSe incluyeron 34 pacientes, 19 (55,9%) de sexo masculino. Edad media 60,7±12,3 años; la mediana de tiempo en HD fue 82,5 (7-296) meses y la mediana de la dosis de cinacalcet fue de 180mg/semana (rango intercuartílico 180-270).
Los niveles séricos de Ca, Pi y hormona paratiroidea mostraron una reducción significativa después del tratamiento con etelcalcetida desde 8,8mg/dl, 5,4mg/dl y 1005 pg/ml hasta 8,1mg/dl (p=0,08), 4,9mg/dl (p=0,01) y 702 pg/mL (p<0,001) respectivamente. La dosis media de etelcalcetida se mantuvo en 5mg/HD. La concentración de esclerostina plasmática aumentó de 35,66pmol/L (rango intercuartílico 11,94-54,58) a 71,05pmol/L (rango intercuartílico 54,43-84,91; p <0,0001).
ConclusiónEn este grupo de pacientes previamente en tratamiento con cinacalcet, la etelcalcetida mejoró el control de hiperparatiroidismo secundario y resultó en un aumento de la concentración plasmática de esclerostina. El efecto del tratamiento con etelcalcetida sobre los niveles de esclerostina es un hallazgo novedoso.
Secondary hyperparathyroidism (sHPT) is associated with increased bone turnover, risk of fractures, vascular calcifications and cardiovascular and all-cause mortality.1 Recent observational data indicate that parathyroid hormone (PTH)>600pg/mL is associated with higher risk of cardiovascular mortality, all-cause and cardiovascular hospitalization.2
Etelcalcetide is a calcimimetic agent with a pharmacokinetic profile that allows thrice weekly intravenous (IV) dosing after hemodialysis (HD), recently approved for sHPT treatment.3 A randomized, double-bind, double-dummy active clinical trial was conducted comparing etelcalcetide with cinacalcet in 683 HD patients with sHPT.4 In this trial, etelcalcetide was not inferior to cinacalcet in reducing PTH levels over 26 weeks and also met superiority criteria. However, there is scarce clinical experience with etelcalcetide, namely its efficacy in patients with sHPT previously uncontrolled with cinacalcet.
Sclerostin is an inhibitor of the wingless-type mouse mammary tumor virus integration site (Wnt) β-catenin pathway.5 The role of sclerostin in chronic kidney disease-mineral and bone disorder (CKD-MBD) spectrum has been an area of active research.6 Studies evaluating the association between sclerostin serum levels with vascular calcification, cardiovascular and all-cause mortality have been published, with conflicting results. Some authors reported a positive association between sclerostin levels and all-cause mortality,7,8 while others found the opposite.9 The relationship between sclerostin levels and mortality in CKD patients awaits further clarification. The treatment of sHPT with calcimimetics decreases calcium, phosphate and FGF-23 levels in CKD patients10 but the effect of these agents in sclerostin concentration remains to be clarified.
In this work, we studied the use of IV etelcalcetide in the treatment of prevalent HD patients with previously uncontrolled sHPT under cinacalcet. The effect of etelcalcetide treatment on sclerostin levels was also evaluated.
Materials and methodsStudy populationWe conducted a prospective cohort study in adult prevalent HD patients in 3 DaVita Dialysis Centers in Portugal who had uncontrolled sHPT under cinacalcet treatment for at least 3 months.
Inclusion criteria were: patients under cinacalcet treatment for at least 3 months, with mean PTH (intact PTH)>800pg/mL and mean corrected total calcium (Ca)>8.3mg/dL in the previous 3 months. Exclusion criteria included: patients unable to give informed consent; previous or current prescription of corticosteroids, bisphosphonates, anticonvulsants or denosumab; scheduled living-donor kidney transplant.
The study was approved by DaVita Local Ethics Committee. Written consent was obtained for all patients.
Conversion from cinacalcet to etelcalcetideAfter 1 week of cinacalcet washout, etelcalcetide 5mg IV/HD was initiated. Etelcalcetide dose was adjusted monthly, without a pre-specified protocol, under the judgment of the patient's assistant Nephrologist according clinical practice guidelines, considering Ca and PTH levels. Concurrent sHPT treatments like active vitamin D and phosphate binders were also maintained throughout the study.
Blood for biochemistry was obtained before the midweek dialysis. Serum levels of PTH, Ca, and phosphorus (Pi) were followed monthly for 6 months. Plasma sclerostin levels were analyzed before the start of etelcalcetide treatment and after 6 months.
Dialysate calcium concentration was 1.25–1.5mmol/L in all patients and it remained unchanged throughout the 6 months of follow-up.
ELISA for sclerostin measurementsThe assay used for sclerostin levels measurement was performed by ELISA method on the fully automated Gemini Stratec (Diatrom) equipment, with Biomedica reagents and using the manufacturer protocols (Biomedica Medizinprodukte GmbH & Co, Wien, Austria). The assay was performed on plasma EDTA samples collected using BD Vacutainer K2E. Plasma separation was performed immediately by centrifugation (10min at 1500g). Samples were transported to the laboratory at 2–8°C and then storage at −25°C.
Statistical analysisStatistical analysis included mean, median, interquartile range (IQR) and percentage unless otherwise specified. Wilcoxon and McNemar testes were used for paired variables ordinal and dichotomic respectively. Significance level was considered p<0.05. Analysis was done with SPSS IBM (IBM SPSS Statistics for Windows, version 25.0, Armonj, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA).
ResultsBaseline characteristicsThirty-four patients were enrolled in the study, 19 (55.9%) male gender. The mean age was 60.7 (± 12.3) years; median time on HD was 82.5 (7–296) months and median cinacalcet dose was 180mg/week (Interquartile Range (IQR): 180–270). Mean Ca, Pi and PTH levels were 8.8mg/dL, 5.4mg/dL and 1005pg/mL, respectively.
Mineral and bone metabolism control – the impact of conversion from cinacalcet to etelcalcetideSerum Ca, Pi and PTH levels showed a statistically significant reduction after etelcalcetide treatment (Fig. 1). Median etelcalcetide dose remained at 5mg/HD during the follow-up. Concurrent sHPT treatments (active vitamin D, phosphate binders) were not significantly different after conversion to etelcalcetide.
At 6 months of follow-up, 30 patients completed the study. Four patients did not complete the entire follow-up period due to: kidney transplantation (one patient), transfer to another HD Center (one patient), loss of follow-up due to holydays (one patient), suspension of etelcalcetide due to paraesthesia (one patient with Ca 7.6mg/dL).
Etelcalcetide was able to control PTH serum levels even in patients with severe sHPT uncontrolled under cinacalcet treatment. In patients with baseline PTH levels>1000pg/mL, etelcalcetide reduced median PTH from 1231pg/mL (IQR 1138–1612) to 763pg/mL (IQR 441–954) (p=0.012). Considering patients with baseline PTH levels<1000pg/mL, etelcalcetide treatment reduced mean PTH from 924pg/mL (IQR 830–941) to 627pg/mL (IQR 524–752) (p=0.046). There was no significant difference in the proportion of patients who reached target levels of PTH<600pg/mL regardless baseline PTH levels (above or below 1000pg/mL (25% vs 40%, p=NS)).
Hypocalcemia with serum Ca<8.0mg/dL occurred in 12 cases (40% of patients); severe hypocalcemia with serum Ca<7.5mg/dL occurred in 3 cases (10% of patients). All patients remained asymptomatic, except for the patient with paresthesia, without cardiovascular or other adverse events related to hypocalcemia.
Bone alkaline phosphatase (BAP) concentration did not change after 6 months of etelcalcetide treatment (from 18.3μg/L to 17.7μg/L, p=0.81).
Globally, the conversion from cinacalcet to etelcalcetide improved bone and mineral metabolism control as measured by blood biochemical parameters. Reference values used in our hemodialysis units were Ca: 8–10.5mg/dL; Pi: <5.5mg/dL and PTH: 150–600pg/mL. Under cinacalcet treatment, the percentage of patients within the reference values were 76.7%, 63.3% and 0% for Ca, Pi and PTH, respectively; none of the patients had all the 3 referred parameters in the reference target. After conversion to etelcalcetide the percentage of patients within target was 60%, 83.3% and 30% for Ca, Pi and PTH, respectively; furthermore, 6.7% of patients had all the 3 parameters on target under etelcalcetide.
Sclerostin levels – baseline and after etelcalcetide treatmentIn a subset of patients, sclerostin levels (n=29) were available. In those, over 6 months of etelcalcetide treatment, serum sclerostin increased from 35.66pmol/L (IQR 11.94–54.58) to 71.05pmol/L (IQR 54.43–84.91) (p<0.0001) (Fig. 2).
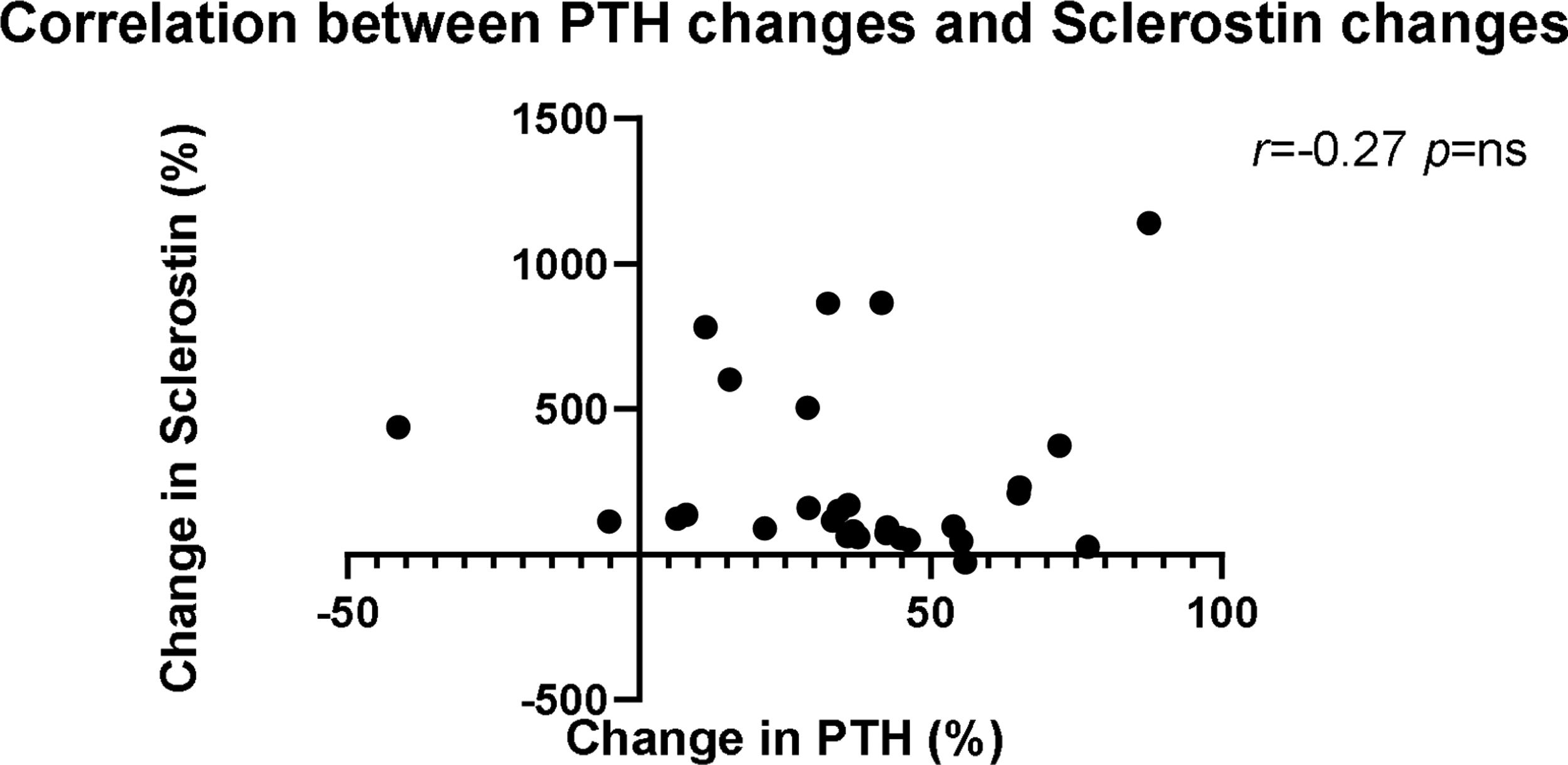
A negative association occurred between changes in sclerostin and PTH concentrations (Fig. 3) (r=−0.27, p=NS). The magnitude of plasma sclerostin concentration increase was not associated with changes in Ca, Pi or PTH after 6 months of etelcalcetide treatment.
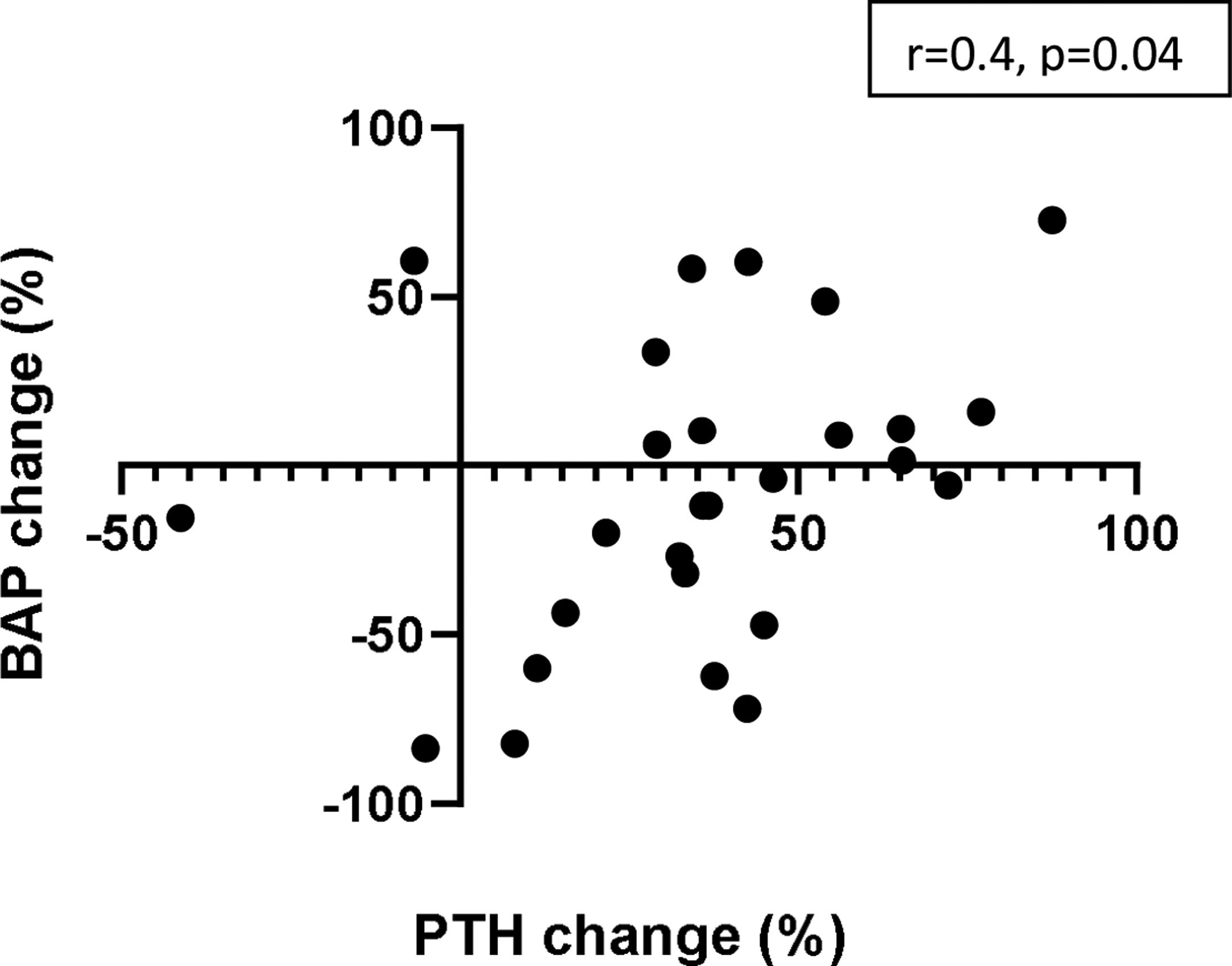
Changes in BAP levels showed a moderate (positive) correlation with changes in PTH concentrations after 6 months of etelcalcetide treatment (Fig. 4) (r=0.431, p=0.019).
DiscussionOur study showed that the conversion from oral cinacalcet to IV etelcalcetide improved the control of severe sHPT in prevalent HD patients. Also etelcalcetide treatment leads to a significant reduction in Ca and Pi levels and an increase in sclerostin concentration from the baseline values.
Etelcalcetide significantly reduced mean PTH levels in this group of patients, previously uncontrolled with cinacalcet. In the head-to-head randomized clinical trial comparing etelcalcetide with cinacalcet in HD patients with sHPT,4 etelcalcetide achieved a reduction of 30% of PTH levels in 68.2% of patients compared with 57.7% patients treated with cinacalcet (p for noninferiority<0.001; p for superiority=0.004) over 26 weeks of treatment. This trial included patients with moderate to severe sHPT with an inclusion criteria of PTH levels>500pg/mL and, more importantly, patients without cinacalcet treatment during the 3 months prior to the first screening laboratory assessment. The present study adds a piece to the puzzle – etelcalcetide was efficacious even in patients with severe sHPT uncontrolled under cinacalcet treatment.
Our results are in line with Arenas et al.11 These authors evaluated the use of etelcalcetide in 25 HD patients with sHPT classified as adherent (10 patients) and non-adherent (15 patients) to cinacalcet according to the Simplified Medication Adherence Questionnaire. They found that etelcalcetide treatment in 8 months reduced PTH levels from 818±395pg/mL to 367±289pg/mL (p<0.001) in non-adherents, and from 496±172 to 228±111pg/mL in adherent patients, respectively. The lack of adherence to cinacalcet was considered a possible cause to the apparent lack of response. Conversely, the use of etelcalcetide ensured compliance and control of sHPT in both non-adherent and adherent patients. Similar results were obtained by Xipell M et al.12 in a prospective observational study including 29 HD patients who were switched from cinacalcet to etelcalcetide with a follow-up of 6 months. Baseline PTH level was not considered as an inclusion criteria. Patients were classified as adherent or non-adherent using a questionnaire survey. The authors reported that etelcalcetide was more effective than cinacalcet in controlling sHPT, with an overall decrease in PTH levels that was significant from the second month. Patients who were non-adherent to cinacalcet (38% of included patients) showed a significant reduction in PTH during follow-up with etelcalcetide (from 530pg/mL at baseline to 201pg/mL at six months of follow-up; p<0.005); the adherent group (62%) tended to have lower PTH levels with etelcalcetide, reaching statistical significance after 5 months of follow-up (from 437pg/mL at baseline to 235pg/mL at six months of follow-up; p<0.001). In our study, lack of adherence to cinacalcet may also explain in some extent the better control of sHPT in patients treated with etelcalcetide but we did not formally address the adherence to cinacalcet.
Treatment with etelcalcetide was well-tolerated in our patients similar to the recent clinical reports11,12 and in line with our group clinical experience. These results contrast with the head-to-head randomized trial4 comparing etelcalcetide with cinacalcet in which self-reported symptoms of nausea and vomiting were not significantly different between the 2 randomized groups. The reasons for this discrepancy between the data from clinical trials and the real world remains speculative.
In the present study, hypocalcemia was a frequent event, as expected – as many as 40% of patients had Ca<8.0mg/dL and 10% of patients with Ca<7.5mg/dL. These results are similar to previous reports.4,11–13 However, all patients remained asymptomatic except for one patient with paresthesia. The clinical implications of hypocalcemia in the patients treated with calcimimetics are uncertain, but it may be less harmful. Floege J et al.14 published an interesting work aiming to investigate incidence, predictors and therapeutic consequences of hypocalcemia in a post hoc analysis of the EVOLVE trial. At least one episode of hypocalcemia occurred within 16 weeks after the first administered dose of cinacalcet in 58.3% of patients compared to 14.9% of patients randomized to placebo. Hypocalcemia was severe (defined as total serum calcium<7.5mg/dL) in 18.4% in cinacalcet group compared with 4.4% in placebo group. In the majority of patients, hypocalcemia was asymptomatic and resolved spontaneously within 14 days with no modification of therapy. Among patients who received an intervention, the most common was an increase in active vitamin D dose. Interestingly, there were no increase in PTH following the hypocalcemia episode. These findings explain the KDIGO group recommendation15 that is not necessary to correct hypocalcemia in all patients but significant or symptomatic hypocalcemia still should be addressed.
We showed an increase in sclerostin levels in patients already under cinacalcet treatment. This is an original observation and deserves further investigation. This effect is not surprising because PTH downregulate sclerostin expression in osteocyte.16 However, an etelcalcetide effect on calcium sensing receptor (CaSR) cannot be excluded. Dvorak-Ewell MM et al. showed reduced sclerostin gene expression in humeral cortices in CaSR knock-out mice.17 We are not aware of another published paper exploring the effect of etelcalcetide in sclerostin levels. Kuczera P et al. reported that in 58 HD patients with PTH>300pg/mL, sclerostin levels increased after 3 and 6 months of cinacalcet treatment from 1.66 (1.35–1.96) ng/mL to 1.77 (1.43–2.12) ng/mL and to 1.87 (1.50–2.25) ng/mL, respectively.18 Plasma sclerostin concentration correlated inversely with PTH at baseline and also after 6 months of treatment.
Sclerostin levels are affected by many clinical and biological factors.6 In our patients, serum sclerostin levels increased after 6 months of etelcalcetide treatment. However, the magnitude of sclerostin concentration increase was not significantly associated with changes in Ca, Pi or PTH. Our results contrast with Pietrzyk B et al.19 These authors evaluated 150 chronic HD patients and showed that sclerostin concentration positively correlated with 25-OH-vitamin D, Pi and inversely with PTH and alkaline phosphatase.
In CKD patients increased sclerostin levels have been associated with vascular calcification and renal osteodystrophy.20 Also it has been suggested that the sclerostin produced in the vessel wall, may not only have protective beneficial paracrine effects, suppressing the transformation of vascular smooth muscle cell in to a osteoblast-like cell (retards the progression of vascular calcification) but also, when spilled over to the circulation decreases osteoblastogenesis and bone formation. Although this is an interesting concept supported by laboratory data and with biological plausibility, this remains to be proved in clinical grounds.
It is possible that this effect of etelcalcetide increasing sclerostin levels will translate into a protective effect on vascular calcification at the same time reducing osteoblast activity and consequently bone remodeling in the setting of high turnover bone disease. This very appealing possible protective cardiovascular effect of etelcalcetide induced rise in sclerostin levels remains speculative and needs to be demonstrated in properly designed prospective clinical trials. Perhaps the best clinical evidence available so far comes from the osteoporosis field. Romosozumab is a monoclonal antibody that binds to and inhibits sclerostin, increases bone formation, and decreases bone resorption. A randomized clinical trial enrolled 4093 postmenopausal women with osteoporosis and a fragility fracture and randomly assigned them in a 1:1 ratio to receive monthly subcutaneous romosozumab (210mg) or weekly oral alendronate (70mg) in a blinded fashion for 12 months.21 A lower risk of new vertebral fractures was observed in the romosozumab group than in the alendronate group. However, this trial raised relevant concerns about romosozumab. During the first year, serious cardiovascular adverse events were observed more often with romosozumab than with alendronate (50 of 2040 patients [2.5%] vs. 38 of 2014 patients [1.9%]). This study supports the potential protective role of sclerostin in vascular heath and the potential cardiovascular risks of sclerostin blockade.
Our work has some strengths. It is the largest “real-world” study published to date evaluating etelcalcetide in patients previously treated with cinacalcet; also, we only included patients with severe sHPT with PTH>800pg/mL and it is multi-center study involving 3 hemodialysis facilities. The limitations of this study should also be noted. We did not perform electrocardiogram in order to exclude QT interval prolongation; other bone-derived and bone turnover markers were not studied (for example FGF-23, collagen degradation products); there was not a strict protocol for adjustment of other concurrent factors such as active vitamin D and phosphate binders or hemodialysis prescription. It is also worth to mentioning that there was no placebo-control arm, although performing a placebo-controlled study in this particular setting would raise ethical concerns.
ConclusionEtelcalcetide improved sHPT control in this group of patients, previously under cinacalcet treatment, and significantly increased plasma sclerostin concentration. This is the first study describing the impact of etelcalcetide treatment on plasma sclerostin levels.
Conflict of interestThe authors declare that they have no conflict of interest.
This work was performed in three DaVita Hemodialysis Clinics in Portugal. The authors want to acknowledge DaVita Portugal for supporting this study.