Amyloidosis represents a family of diseases characterized by the deposition of proteinaceous material in the extracellular space that, by forming insoluble clusters on various tissues and organs, affects its function.1 Amyloidosis can be classified as systemic or localized, acquired or hereditary and according to their constitutive proteins.3 Systemic subtypes consist of primary AL amyloidosis, secondary amyloid A (AA), familial amyloidosis and β2-microglobulin-related amyloidosis.1,4 AA amyloidosis is classically associated with chronic infections (essentially in developing countries), inflammatory chronic diseases (such as sprondyloarthropathy, inflammatory bowel disease and rheumatoid arthritis), periodic fever syndromes, among other examples.1–4 Clinical and laboratory manifestations depend on the type of amyloidosis and may include, for example, easy bruising, macroglossia, signs and symptoms of heart failure or arrhythmias, hepatomegaly, coagulation disorders or nephrotic proteinuria.1–3
We present a case of an older woman with nephrotic syndrome and a previously non clarified inflammatory peripheral arthropathy, with the latter becoming responsible for AA amyloidosis. This 48-month follow-up proves the need and effectiveness of properly treating the underlying disease.
O. G. D., a 78-year-old caucasian woman with a personal history of seronegative peripheral polyarthritis, poor therapeutic compliance and corticoid-induced diabetes mellitus, was admitted for etiological study of constitutional syndrome, inflammatory arthralgias, diarrhea and anasarca refractory to oral diuretics. Usual medication included methotrexate, deflazacorte, esomeprazole and tapentadol, folic acid, calcium carbonate plus cholecalciferol and insulin. Denied drug allergies.
The complementary study revealed anemia (normocytic, normochromic) of 9.2g/dL, normal leukogram, thrombocytosis (720×10^9/L), pCr of 1.3mg/dL, a 24-h proteinuria (Pu) of 10.4g, severe hypoalbuminemia of 1.4g/dL, mixed dyslipidemia as well as elevated sedimentation rate (117mm/h) and C-reactive protein (77mg/L). Urinary sediment also showed microscopic hematuria (+). Nephrotic syndrome was considered.
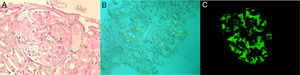
In this context, occult neoplasia was excluded. Viral and bacterial screening was negative, as well as autoimmunity. In relation to articular manifestations, there were mostly peripheral joint complaints. However, as it was a seronegative disease (rheumatoid factor and anti-citrulline protein antibodies) and the patient also described low back pain for a long time, this raised the hypothesis of spondyloarthritis, which was confirmed (bilateral radiographic sacroiliitis; antigen HLA-B27 positive) and a definitive diagnosis of ankylosing spondylitis (AS) was established. Intestinal mucosal and renal biopsies revealed amyloid AA deposits (amyloid A protein). Concerning the latter, light microscopy showed amorphous deposition in mesangium and invasion of the basement membrane (Fig. 1, panel A), and congo red evidenced amyloid deposits in glomeruli and small arteries (Fig. 1, panel B). IMF is also available (Fig. 1, panel C). Diffuse tubular atrophy was also described. Additionally, echocardiogram did not suggest cardiac involvement.
Anticipating the need for institution of biological treatment, all appropriate prophylactic measures were implemented (the whole vaccination plan was updated, influenza and antipneumococcal vaccination administered) and microbiological screening was completed, including screening for latent tuberculosis. Due to positive tuberculin skin test and thoracic CT scan sequels, started therapy for nine months with isoniazid (300mg/day).
At discharge date, reference to pCr of 2mg/dL and albumin of 2.5g/dL, beyond the resolution of anasarca and remaining symptomatology. Follow-up consultations in rheumatology, internal medicine and nephrology were scheduled.
Later, given worsening renal function (maximum pCr of 2.9mg/dL) and aggravated proteinuria, which reached 20g/day (with several hypoalbuminemia of 1.9g/dL), biological therapy with etanercept was instituted (50mg/week).
One-year follow-up showed improvement in renal function, with pCr reduction from 2.9 to 1.6mg/dL, proteinuria up to 5.1g/day, albuminemia from 1.9g/dL to 3.4g/dL and sedimentation rate from 68mm/h to 39mm/h.
At 48 months of follow-up, pCr is stable (1.4mg/dL) and proteinuria improved, under 400mg/day. About AS, also with a good and sustained response, with low disease activity, keeping etanercept as the only immunosuppressive treatment.
Success after administration of etanercept – widely used in the treatment of inflammatory arthropathies – demonstrates once again that one of the key factors in the treatment and control of AA amyloidosis lies in controlling the underlying disease despite the presence of histologically chronic stigmas.3
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.