Finerenone is a selective nonsteroidal mineralocorticoid receptor antagonist (MRA) with anti-inflammatory and anti-fibrotic effects, which may translate into a prognostic benefit with fewer adverse effects than other MRAs.1 In patients with type 2 diabetes (T2D) and chronic kidney disease (CKD), finerenone has been shown to reduce fatal and nonfatal cardiovascular (CV) events, hospitalisations for heart failure (HF), and CKD progression.2,3
The FIDELITY pooled analysis, which included 13,026 patients followed for three years, demonstrated that composite CV and kidney outcomes were significantly reduced in the finerenone group compared to placebo (HR 0.86; 95% CI 0.78–0.95 and HR 0.77; 95% CI 0.67–0.88, respectively), with similar safety outcomes between both groups.1 Based on this evidence, finerenone was approved in Spain in 2024 for treating T2D patients with CKD and albuminuria.
Patients with T2D and CKD are at high risk for CV events and kidney progression, with a worse prognosis associated with the severity and stage of CKD.4 Early treatment with renin-angiotensin-aldosterone inhibitors (RAASi), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and finerenone can reduce CV outcomes and kidney disease progression.4 However, the current clinical practice of treating these patients and the potential population who could benefit from new therapies is poorly understood.
We conducted a retrospective analysis to identify potential candidates for finerenone treatment according to current local recommendations: CKD (estimated glomerular filtration rate (eGFR) ≥25mL/min/1.73m2), T2D, and urine albumin-creatinine ratio (uACR) ≥30mg/g despite treatment with optimised stable doses of RAASi and/or SGLT2i, or patients who are intolerant to these drugs. According to 2024 KDIGO guidelines, CKD was defined as eGFR <60mL/min/1.73m2 and/or the presence of uACR ≥30mg/g (a marker of kidney damage) for a minimum of 3 months.4 We analysed various variables: (a) clinical characteristics (sex, age, CV risk factors, previous HF and ischemic disease, CKD stage4); (b) chronic pharmacological treatment (RAASi, MRA, SGLT2i, diuretics, statins); and (c) analytical parameters (creatinine, eGFR, Na, K and uACR). The analysis was based on all uACR determinations performed at the laboratory of a two-level hospital during 2023 (194,731 population), including those referred from primary care. The ethics committee approved the study. The analysis was performed using STATA 17.0.
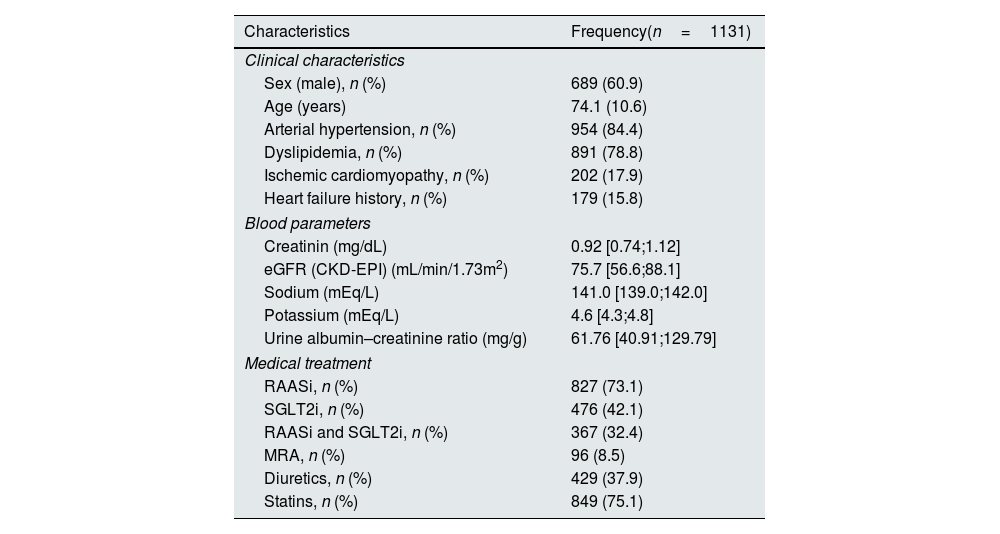
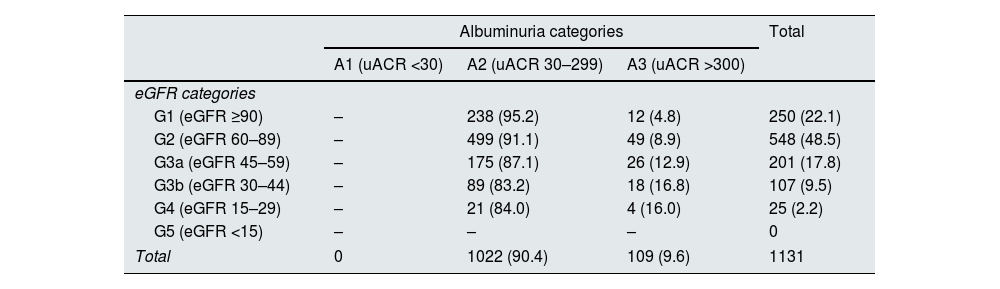
We identified 29,574 single uACR determinations, 8,734 of which were from DT2 patients (29.5%). Of these, 1,131 had an eGFR ≥25mL/min/1.73m2 and uACR ≥30mg/g (12.9%). These 1,131 patients met the criteria for initiation of treatment with finerenone. The cohort was predominantly male, with a mean age of 74 years and a high prevalence of CV risk factors (Table 1). The mean eGFR was 75.7mL/min, and the uACR was 61.76mg/g. According to the KDIGO classification,4 most patients were in the A2 category (90.4%) and the G2 (48.5%) and G3a (17.8%) categories (Table 2). Regarding pharmacological treatment, most patients were treated with RAASi (73.1%), while SGLT2i were less frequently prescribed (42.1%). Only 32.4% of patients were treated with both therapies.
Clinical characteristics of patients suitable for finerenone treatment.
| Characteristics | Frequency(n=1131) |
|---|---|
| Clinical characteristics | |
| Sex (male), n (%) | 689 (60.9) |
| Age (years) | 74.1 (10.6) |
| Arterial hypertension, n (%) | 954 (84.4) |
| Dyslipidemia, n (%) | 891 (78.8) |
| Ischemic cardiomyopathy, n (%) | 202 (17.9) |
| Heart failure history, n (%) | 179 (15.8) |
| Blood parameters | |
| Creatinin (mg/dL) | 0.92 [0.74;1.12] |
| eGFR (CKD-EPI) (mL/min/1.73m2) | 75.7 [56.6;88.1] |
| Sodium (mEq/L) | 141.0 [139.0;142.0] |
| Potassium (mEq/L) | 4.6 [4.3;4.8] |
| Urine albumin–creatinine ratio (mg/g) | 61.76 [40.91;129.79] |
| Medical treatment | |
| RAASi, n (%) | 827 (73.1) |
| SGLT2i, n (%) | 476 (42.1) |
| RAASi and SGLT2i, n (%) | 367 (32.4) |
| MRA, n (%) | 96 (8.5) |
| Diuretics, n (%) | 429 (37.9) |
| Statins, n (%) | 849 (75.1) |
eGFR: estimated glomerular filtration rate; MRA: mineralocorticoid receptor antagonist; RAASi: renin–angiotensin–aldosterone inhibitors; SGLT2i: sodium-glucose cotransporter-2 inhibitors (SGLT2i).
Distribution of patients according to chronic kidney disease severity.
| Albuminuria categories | Total | |||
|---|---|---|---|---|
| A1 (uACR <30) | A2 (uACR 30–299) | A3 (uACR >300) | ||
| eGFR categories | ||||
| G1 (eGFR ≥90) | – | 238 (95.2) | 12 (4.8) | 250 (22.1) |
| G2 (eGFR 60–89) | – | 499 (91.1) | 49 (8.9) | 548 (48.5) |
| G3a (eGFR 45–59) | – | 175 (87.1) | 26 (12.9) | 201 (17.8) |
| G3b (eGFR 30–44) | – | 89 (83.2) | 18 (16.8) | 107 (9.5) |
| G4 (eGFR 15–29) | – | 21 (84.0) | 4 (16.0) | 25 (2.2) |
| G5 (eGFR <15) | – | – | – | 0 |
| Total | 0 | 1022 (90.4) | 109 (9.6) | 1131 |
The classification was based on KDIGO 2024 guidelines4. eGFR is expressed in mL/min/1.73m2 and uACR in mg/g.
eGFR: estimated glomerular filtration rate; uACR: urine albumin–creatinine ratio.
Finerenone has demonstrated a reduction in CV events and CKD progression in patients with T2D and CKD with albuminuria. Our single-centre study identified 1,131 patients over one year who could benefit from finerenone to improve prognosis. Our cohort was older than the FIDELITY pooled population (74.1 vs 64.8 years), with a higher proportion of patients with a history of HF (15.8 vs 7.7%). Our patients also had better renal function, although the percentage of patients with advanced CKD (eGFR <25) was slightly higher in our cohort (2.2 vs 1.2%). Regarding albuminuria, most of our patients had a uACR between 30 and 300mg/g and in FIDELITY pooled, the majority had proteinuria ≥300mg/g. Potassium levels were slightly higher in our population (4.6 vs 4.4mEq/L). Finally, the treatment patterns differed: our patients had lower rates of RAASi (73.1% vs 99.8%) and diuretics (37.9% vs 51.5%) but higher rates of SGLT2i (42.1% vs 6.7%).
Our study highlights the significant number of T2D patients with CKD who, based solely on albuminuria, remain at residual risk for CV events, renal disease progression and HF onset.1,5 Current evidence supports the use of RAASi and SGLT2i in these patients, but these therapies are still underused. Finerenone can potentially optimise treatment in these patients further, offering an additional benefit. The only attention that needs to be paid is to potassium levels, which should be ≤4.8mEq/L at baseline (most of the patients in our cohort), although the use of potassium binders should be considered to facilitate the initiation and titration of MRA.6 The starting dose is 10mg, which can be increased to 20mg depending on eGFR.
CRediT authorship contribution statementStudy design: AEF, GRM. Data collection: CBR, MFU, EGP, BLG. Data review and statistical analysis: AEF. Manuscript drafting: AEF. Review, editing, and acceptance of the manuscript: all authors.
FundingThere are no sources of funding for this work.
Conflicts of interestAEF has received payments from Bayer for his participation as a speaker at scientific meetings. The rest of the participants declare that they have no conflict of interest.
Data availabilityThe datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.