The advent of direct-acting antiviral agents promises to change the management of hepatitis C virus infection (HCV) in patients with chronic kidney disease (CKD), a patient group in which the treatment of hepatitis C was historically challenging. We investigated the safety and efficacy of all-oral, interferon-free direct-acting antiviral agents for the treatment of hepatitis C in a ‘real-world’ cohort of patients with CKD.
MethodsWe performed an observational single-arm multi-centre study in a large (n=198) cohort of patients with stage 1–3 CKD who underwent antiviral therapy with DAAs for the treatment of HCV. The primary end-point was sustained virologic response (serum HCV RNA <15IU/mL, 12 weeks after treatment ended) (SVR12). We collected data on on-treatment adverse events (AEs), severe AEs, and laboratory abnormalities.
ResultsThe average baseline eGFR (CKD-EPI equation) was 70.06±20.1mL/min/1.72m2; the most common genotype was HCV 1b (n=93, 51%). Advanced liver scarring was found in 58 (46%) patients by transient elastography. Five regimens were adopted: elbasvir/grazoprevir (n=5), glecaprevir/pibrentasvir (n=4), ritonavir-boosted paritaprevir/ombitasvir/dasabuvir (PrOD) regimen (n=40), simeprevir±daclatasvir (n=2), and sofosbuvir-based combinations (n=147). The SVR12 rate was 95.4% (95% CI, 93.8%; 96.8%). There were nine virological failures – eight being relapsers. Adverse events occurred in 30% (51/168) of patients, and were managed clinically without discontinuation of therapy or hospitalization. One of the most common AEs was anaemia (n=12), which required discontinuation or dose reduction of ribavirin in some cases (n=6); deterioration of kidney function occurred in three (1.7%).
ConclusionsAll-oral, interferon-free therapy with DAAs for chronic HCV in mild-to-moderate CKD was effective and well-tolerated in a ‘real–world’ clinical setting. Studies are in progress to address whether sustained viral response translates into better survival in this population.
La aparición de los antivíricos de acción directa (AAD) promete cambiar el tratamiento de la infección por el virus de la hepatitis C (VHC) en los pacientes con nefropatía crónica (NC), un grupo de pacientes en el que el tratamiento de la hepatitis C siempre supuso una dificultad. Se investiga la seguridad y la eficacia de los antivíricos de acción directa, sin interferones orales, en todos los casos para el tratamiento de la hepatitis C en una cohorte en condiciones reales de pacientes con NC.
MétodosSe llevó a cabo un estudio multicéntrico, de un solo grupo y observacional en una cohorte amplia (n=198) de pacientes con NC en estadio 1-3 a los que se administró tratamiento antivírico con AAD para el VHC. El criterio principal de valoración fue la respuesta virológica sostenida (ARN sérico del VHC<15UI/ml, 12 semanas después de la finalización del tratamiento) (RVS12). Se recogieron los datos sobre acontecimientos adversos (AA) surgidos durante el tratamiento, AA graves y anomalías analíticas.
ResultadosLa FGe inicial media (ecuación de CKD-EPI) fue de 70,06±20,1ml/min/1,72m2; el genotipo más frecuente fue VHC 1b (n=93; 51%). Se observó cicatrización hepática avanzada en 58 (46%) pacientes mediante elastografía transitoria. Se adoptaron 5 pautas: elbasvir/grazoprevir (n=5), glecaprevir/pibrentasvir (n=4), pauta de paritaprevir/ombitasvir/dasabuvir (PrOD) potenciada con ritonavir (n=40), simeprevir ± daclatasvir (n=2) y combinaciones basadas en sofosbuvir (n=147). La tasa de RVS12 fue del 95,4% (IC del 95%: 93,8; 96,8%). Hubo 9 fracasos virológicos, 8 de ellos recidivantes. Se produjeron acontecimientos adversos en el 30% (51/168) de los pacientes, que se trataron clínicamente sin suspensión del tratamiento ni hospitalización. Uno de los AA más frecuentes fue la anemia (n=12), que precisó la suspensión o la reducción de la dosis de ribavirina en algunos casos (n=6); se produjo deterioro de la función renal en 3 casos (1,7%).
ConclusionesEl tratamiento sin interferón oral en todos los casos con AAD para el VHC crónico en la NC de leve a moderada fue eficaz y bien tolerado en un contexto de la práctica clínica real. Hay estudios en curso para abordar si la respuesta viral sostenida se traduce en una mejor supervivencia en esta población.
Hepatitis C virus (HCV) infection is a common infectious disease with a prevalence of 71 million HCV-infected individuals all over the world.1 Evidence has accumulated over the last two decades reporting a variety of extra-hepatic diseases induced by HCV; HCV-negative individuals have lower non-liver-related mortality compared to those who are chronically infected with HCV.2 The extra-hepatic activity of HCV is added to its action on the liver that manifest itself with cirrhosis and hepatocellular carcinoma, the main complications of chronic liver disease.
The kidneys are an important target of chronic HCV, the relationship between HCV and the kidneys has bidirectional nature and is complex. On one side, kidney failure supports the diffusion of HCV (mostly via dialysis environment) particularly in the developing world where the compliance to the infection control procedures against HCV spread within dialysis units is frequently missing. On the other, HCV increases the risk of renal impairment. In addition to conventional risk factors for chronic kidney disease (ageing, metabolic syndrome, arterial hypertension, and diabetes) HCV infection may be an additional risk factor.
The detrimental role of chronic HCV on the incidence and progression of chronic kidney disease in the general population has been recently emphasized. A meta-analysis of observational studies (n=40 studies, n=4,072,867 unique patients) demonstrated an association between positive anti-HCV serologic status and increased incidence of CKD.3 We found a significant association between positive anti-HCV serologic status and increased frequency of proteinuria, adjusted risk of proteinuria associated with HCV across the surveys, 1.633 (95% CI, 1.29; 2.05). Test for homogeneity of the adjusted risk of proteinuria across the ten studies gave a Q value of 37.47 (I2=75.9%) (P=0.0001). That is, the homogeneity assumption was rejected.3
The advent of the direct-acting antiviral agents (DAAs) is dramatically changing the management of HCV in the general populations including the ‘difficult-to-treat’ groups such as CKD patients. According to the guidelines provided by the AASLD/IDSA, two regimens based on DAAs have been suggested for HCV in advanced CKD.4 On the contrary, various combinations have been recommended in patients with mild-to-moderate renal impairment.4,5 However, the data in the medical literature regarding the use of DAAs in patients with stage 1–3 chronic kidney disease are extremely limited.6–9
The aim of this study is to evaluate efficacy and safety of therapy with DAAs for HCV in patients with mild-to-moderate chronic kidney disease in a ‘real-life’ clinical practice. Various regimens based on DAAs have been retrospectively reviewed including the most recent combinations.
Material and methodsStudy design and eligibility of patientsThis was a retrospective analysis of patients with stage 1–3 CKD (followed at some units of Europe and America) and chronic HCV who received antiviral therapy with DAAs. We included adult patients aged 18 years and older diagnosed with chronic HCV infection, who took at least one dose of a DAA therapy between April 2013 and August 2018. Patients were included irrespective of their liver fibrosis stage, genotype, or prior HCV treatment status.
Pre-treatment stage of liver fibrosis was evaluated by transient elastography (Fibroscan®; Echosens, Paris, France). Liver fibrosis stage (F1-F4) was derived from the liver stiffness values in kPa obtained by transient elastography on the grounds of the indications provided by the manufacturer.
Antiviral regimenDAA regimens were prescribed at the discretion of the treating physician depending on the genotype, baseline HCV viraemia, presence of liver fibrosis/cirrhosis, and prior treatment experience. Various antiviral regimens were administered: EBR/GZR (elbasvir was prescribed at 50mg administered orally once daily in co-formulation with grazoprevir 100mg administered orally once daily fixed dose); PrOD regimen (ritonavir-boosted paritaprevir/ombitasvir/dasabuvir)±ribavirin, administered at standard doses (ombitasvir, 25mg once daily/paritaprevir, 150mg once daily/ritonavir, 100mg once daily/dasabuvir, 250mg twice daily); GP (glecaprevir was prescribed at 100mg administered orally in co-formulation with pibrentasvir, 40mg, three times daily for 12 weeks). Sofosbuvir-based regimens were: LDV/SOF±ribavirin (ledipasvir was prescribed at 90mg administered orally once daily in co-formulation with sofosbuvir 400mg [fixed dose]); sofosbuvir and ribavirin (sofosbuvir was prescribed at 400mg administered orally once daily and weight-adjusted ribavirin doses orally once daily); SOF/DCV±ribavirin (daclatasvir was prescribed at 60mg administered orally once daily in co-formulation with sofosbuvir 400mg); SOF/VEL±ribavirin (velpatasvir was prescribed at 100mg administered orally in co-formulation with sofosbuvir at 400mg orally once daily); sofosbuvir (400mg) and simeprevir (150mg) (SOF/SIM) were administered in a single tablet fixed-dose combination once daily. A minority of patients received sofosbuvir (400mg once daily orally) plus daclatasvir (60mg once daily orally)±ribavirin.
Sustained virological response was defined according to AASLD/IDSA recommendations as an undetectable HCV RNA 12 weeks after the end of antiviral therapy.4 Duration of treatment ranged from 12 to 24 weeks depending on the treating physician, in accordance with product label. RBV was never administered in a syrup and the minimum prescribed dose was 200mg a day; the dose of RBV dose was prescribed from the treating physician based on body weight and renal function.
Laboratory assessmentsSerum HCV RNA was measured using the quantitative COBAS AMPLICOR HCV (Roche) Monitor Assay (limit of detection, 15logIU/mL). HCV genotyping was determined at baseline by the SIEMENS Versant HCV Genotype 2.0 Assay (LiPA) (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). The eGFR was assessed by CKD-EPI Equation in all patients. All measurements of AST, ALT and gamma-GT were made by spectrophotometric method. The upper limits in the serum AST and ALT assays were 40 and 40IU/L respectively; the upper limits in the serum gamma-GT were 55IU/L. eGFR was estimated using the CKD Epidemiology Collaboration equation.10
Safety evaluationThe patients were monitored on a regular basis for treatment efficacy and side-effects. The patients’ visits were scheduled as follows: treatment initiation, treatment weeks 4,8, 12, and 12 weeks post-treatment. Each visit consisted of a query on medical history and side effects, check of concomitant medication, physical examination, laboratory analyses, and drug delivery. Laboratory analyses included blood chemistry, blood count, prothrombin time, and HCV RNA. The structure of recordings of adverse events (AEs) was as follows: any AE or serious adverse event (SAE), which included any event requiring hospitalization, life-threatening event, or death; the relationship with the administered medication was also assessed. The study was reported according to the STROBE initiative.11 The 22 items regarding the current manuscript are reported in the Supplemental File n. 1.
Statistical analysisData are presented as means and standard deviations or medians with respective ranges, as appropriate. Serum aminotransferase and gamma-glutamyl transpeptidase were logarithmically transformed (natural logarithm) to obtain normal distribution and then were subjected to statistical tests. For all comparisons, a two-sided P value <0.05 was considered to indicate statistical significance throughout the study. All analyses were made with Stata, version 9.0 (StataCorp, College Station, TX).
Ethical standardAll procedures during the study were conducted in accordance with the International Conference on Harmonization guidelines, and ethical principles that have their origin in the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective design of the study and use of data from which the patients’ identification information had been removed.
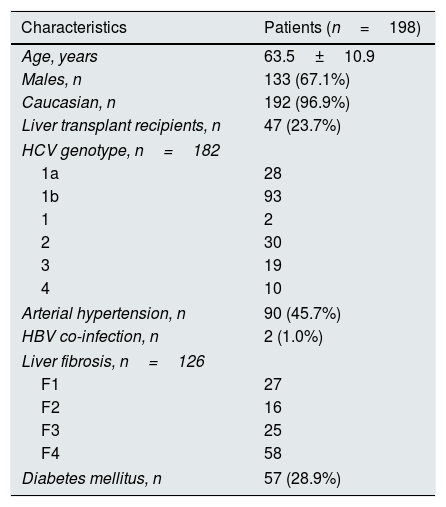
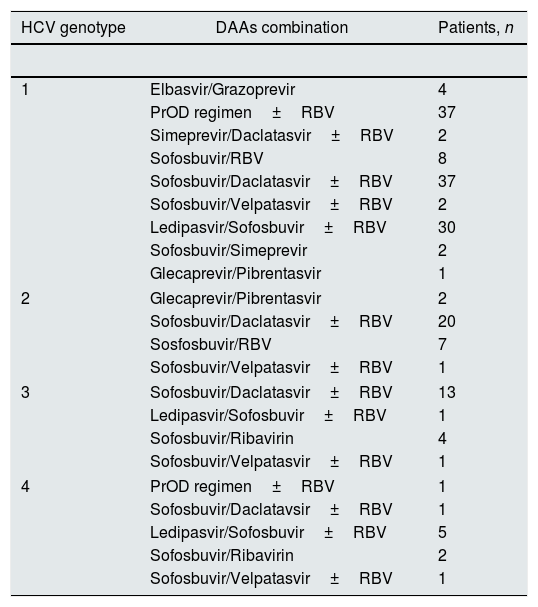
ResultsBaseline patient demographicsThe baseline demographic and clinical characteristics of patients included in the study group are shown in Tables 1 and 2. The majority of patients were Caucasian (96.5%) and male (70%). As listed in Table 1 the study group included patients with functioning liver transplant and no significant differences occurred between LT and non-LT recipients regarding baseline parameters (data shown). Liver fibrosis was assessed by transient elastography in a subset (n=126) of patients. Stage 3 CKD was found in 66 (33.3%) patients. The most common genotype was HCV 1b (50.0%), and 55 (30%) patients were treatment-experienced. Antiviral therapy was conducted with various (n=5) regimens of DAAs: EBR/GZR (n=5), GP (n=4), PrOD regimen±RBV (n=40), simeprevir±daclatasvir±RBV (n=2), and sofosbuvir-based combinations (n=147). Sofosbuvir-based combinations included sofosbuvir±RBV, SOF/DCV±RBV, SOF/VEL±RBV, LDV/SOF±RBV, and SOF/SIM (Table 3).
Demographic and clinical patients’ characteristics at baseline: patients with stage 1–3 chronic kidney disease.
| Characteristics | Patients (n=198) |
|---|---|
| Age, years | 63.5±10.9 |
| Males, n | 133 (67.1%) |
| Caucasian, n | 192 (96.9%) |
| Liver transplant recipients, n | 47 (23.7%) |
| HCV genotype, n=182 | |
| 1a | 28 |
| 1b | 93 |
| 1 | 2 |
| 2 | 30 |
| 3 | 19 |
| 4 | 10 |
| Arterial hypertension, n | 90 (45.7%) |
| HBV co-infection, n | 2 (1.0%) |
| Liver fibrosis, n=126 | |
| F1 | 27 |
| F2 | 16 |
| F3 | 25 |
| F4 | 58 |
| Diabetes mellitus, n | 57 (28.9%) |
HBV, Hepatitis B virus infection; HCV, hepatitis C virus infection.
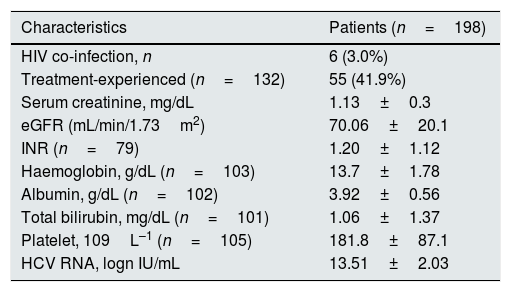
Clinical and biochemical patients’ characteristics at baseline: patients with stage 1–3 chronic kidney disease.
| Characteristics | Patients (n=198) |
|---|---|
| HIV co-infection, n | 6 (3.0%) |
| Treatment-experienced (n=132) | 55 (41.9%) |
| Serum creatinine, mg/dL | 1.13±0.3 |
| eGFR (mL/min/1.73m2) | 70.06±20.1 |
| INR (n=79) | 1.20±1.12 |
| Haemoglobin, g/dL (n=103) | 13.7±1.78 |
| Albumin, g/dL (n=102) | 3.92±0.56 |
| Total bilirubin, mg/dL (n=101) | 1.06±1.37 |
| Platelet, 109L–1 (n=105) | 181.8±87.1 |
| HCV RNA, logn IU/mL | 13.51±2.03 |
eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; HCV RNA, hepatitis C virus ribonucleic acid; INR, international normalized ratio.
Combinations of direct-acting antiviral agents and treatments in patients with stage 1–3 chronic kidney disease (n=182 patients).
| HCV genotype | DAAs combination | Patients, n |
|---|---|---|
| 1 | Elbasvir/Grazoprevir | 4 |
| PrOD regimen±RBV | 37 | |
| Simeprevir/Daclatasvir±RBV | 2 | |
| Sofosbuvir/RBV | 8 | |
| Sofosbuvir/Daclatasvir±RBV | 37 | |
| Sofosbuvir/Velpatasvir±RBV | 2 | |
| Ledipasvir/Sofosbuvir±RBV | 30 | |
| Sofosbuvir/Simeprevir | 2 | |
| Glecaprevir/Pibrentasvir | 1 | |
| 2 | Glecaprevir/Pibrentasvir | 2 |
| Sofosbuvir/Daclatasvir±RBV | 20 | |
| Sosfosbuvir/RBV | 7 | |
| Sofosbuvir/Velpatasvir±RBV | 1 | |
| 3 | Sofosbuvir/Daclatasvir±RBV | 13 |
| Ledipasvir/Sofosbuvir±RBV | 1 | |
| Sofosbuvir/Ribavirin | 4 | |
| Sofosbuvir/Velpatasvir±RBV | 1 | |
| 4 | PrOD regimen±RBV | 1 |
| Sofosbuvir/Daclatavsir±RBV | 1 | |
| Ledipasvir/Sofosbuvir±RBV | 5 | |
| Sofosbuvir/Ribavirin | 2 | |
| Sofosbuvir/Velpatasvir±RBV | 1 | |
DAAs, direct-acting antiviral agents; PrOD, ritonavir-boosted paritaprevir/ombitasvir/dasabuvir; RBV, ribavirin.
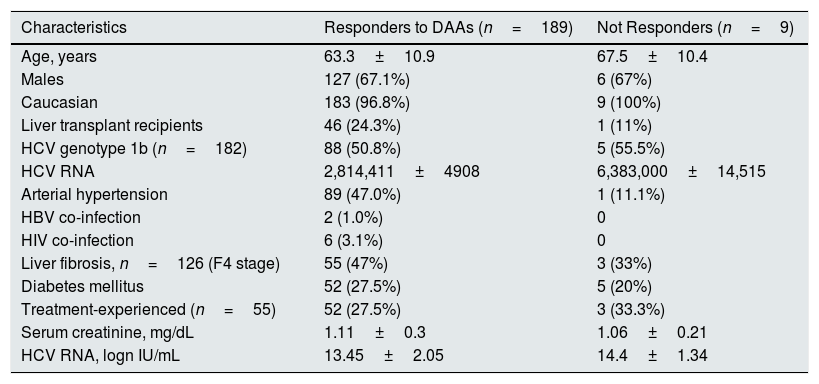
All patients completed treatment course and subsequent follow-up. The SVR12 rate was 95.4% (189/198) (95% CI, 93.8%; 96.8%), according to an ITT analysis. There were nine virological failures; one and eight were non-responders and relapsers, respectively. The genotypes of virological failures were: 1a (n=1), 1b (n=5) and 2 (n=3), respectively. The combinations of DAAs in virological failures were: elbasvir/grazoprevir (n=1), PrOD (n=1), simeprevir/daclatasvir (n=1), sofosbusvir±RBV (n=2), sofosbuvir/daclatasvir±RBV (n=3), and sofosbuvir/simeprevir (n=1). No difference occurred regarding clinical and biochemical characteristics between responder and non-responder patients at baseline – all the comparisons reported in Table 4 were not significant. There was no significant difference between LT recipients and those without liver grafts with regard to the SVR12 rate, 24.3% (46/189) vs. 75% (143/189), P=0.69.
Demographic and clinical patients’ characteristics: responders vs. non-responders.
| Characteristics | Responders to DAAs (n=189) | Not Responders (n=9) |
|---|---|---|
| Age, years | 63.3±10.9 | 67.5±10.4 |
| Males | 127 (67.1%) | 6 (67%) |
| Caucasian | 183 (96.8%) | 9 (100%) |
| Liver transplant recipients | 46 (24.3%) | 1 (11%) |
| HCV genotype 1b (n=182) | 88 (50.8%) | 5 (55.5%) |
| HCV RNA | 2,814,411±4908 | 6,383,000±14,515 |
| Arterial hypertension | 89 (47.0%) | 1 (11.1%) |
| HBV co-infection | 2 (1.0%) | 0 |
| HIV co-infection | 6 (3.1%) | 0 |
| Liver fibrosis, n=126 (F4 stage) | 55 (47%) | 3 (33%) |
| Diabetes mellitus | 52 (27.5%) | 5 (20%) |
| Treatment-experienced (n=55) | 52 (27.5%) | 3 (33.3%) |
| Serum creatinine, mg/dL | 1.11±0.3 | 1.06±0.21 |
| HCV RNA, logn IU/mL | 13.45±2.05 | 14.4±1.34 |
DAAs, direct-acting antiviral agents; HBV, hepatitis B virus infection; HCV RNA, hepatitis C virus ribonucleic acid; HIV, human immunodeficiency virus; INR, international normalized ratio.
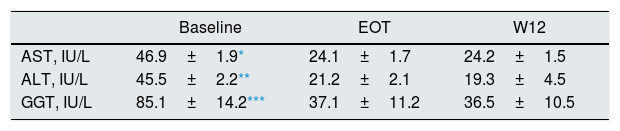
The dynamics of liver enzymes (aminotransferase and gamma-glutamyl transpeptidase) during and after antiviral therapy with DAAs were observed in a subgroup of patients (n=94 patients). AST, ALT and GGT lowered significantly after therapy with DAAs and over the follow-up (Table 5).
Liver biochemical tests at baseline, after antiviral therapy and over the follow-up with DAAs: patients with stage 1–3 chronic kidney disease (n=94 patients).
| Baseline | EOT | W12 | |
|---|---|---|---|
| AST, IU/L | 46.9±1.9* | 24.1±1.7 | 24.2±1.5 |
| ALT, IU/L | 45.5±2.2** | 21.2±2.1 | 19.3±4.5 |
| GGT, IU/L | 85.1±14.2*** | 37.1±11.2 | 36.5±10.5 |
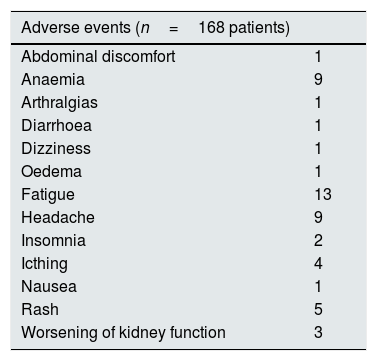
All patients completed antiviral therapy and subsequent follow-up (drop-out rate, 0%). Adverse events were recorded in a subset of patients (n=158); 58 individuals had at least one AE. Most AEs events were mild, and were managed clinically without discontinuation of therapy. The most common AEs were fatigue (n=13), anaemia (n=9), and headache (n=9), respectively. Severe anaemia requiring reduction or discontinuation of ribavirin occurred in some cases (n=6) (Table 6).
AEs experienced during treatment with DAAs (information on AEs was not available in 30 patients).
| Adverse events (n=168 patients) | |
|---|---|
| Abdominal discomfort | 1 |
| Anaemia | 9 |
| Arthralgias | 1 |
| Diarrhoea | 1 |
| Dizziness | 1 |
| Oedema | 1 |
| Fatigue | 13 |
| Headache | 9 |
| Insomnia | 2 |
| Icthing | 4 |
| Nausea | 1 |
| Rash | 5 |
| Worsening of kidney function | 3 |
AEs, adverse events.
Three (1.7%) patients showed irreversible worsening of kidney function during antiviral therapy with DAAs, the increase in serum creatinine was less than 30% of baseline levels (stage 3 CKD in all at baseline). These patients received SOF-based treatments, SOF/DCV (n=2) and LDV/SOF (n=1).
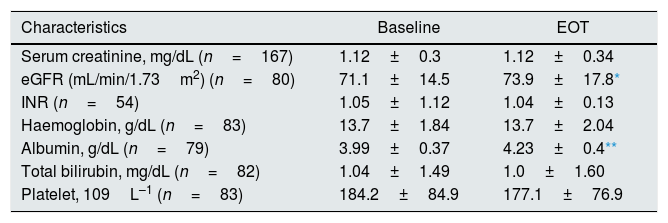
Hospitalizations were not necessary during antiviral therapy and 12-week follow-up. No documented episodes of graft rejection occurred among liver transplant recipients on DAAs (n=47). We found no significant differences regarding several biochemical parameters between baseline and EOT values (Table 6). The average values of serum albumin and eGFR increased significantly after completing antiviral therapy with DAAs (Table 7), this occurred regardless of whether the patient was a LT recipient or not (data not shown).
Clinical and biochemical patients’ characteristics (patients with stage 1–3 chronic kidney disease): baseline vs. end-of-treatment.
| Characteristics | Baseline | EOT |
|---|---|---|
| Serum creatinine, mg/dL (n=167) | 1.12±0.3 | 1.12±0.34 |
| eGFR (mL/min/1.73m2) (n=80) | 71.1±14.5 | 73.9±17.8* |
| INR (n=54) | 1.05±1.12 | 1.04±0.13 |
| Haemoglobin, g/dL (n=83) | 13.7±1.84 | 13.7±2.04 |
| Albumin, g/dL (n=79) | 3.99±0.37 | 4.23±0.4** |
| Total bilirubin, mg/dL (n=82) | 1.04±1.49 | 1.0±1.60 |
| Platelet, 109L–1 (n=83) | 184.2±84.9 | 177.1±76.9 |
Most RCTs of DAAs in the general population included patients with intact kidneys and the majority of studies with CKD patients (such as C-SURFER, RUBY-1, and EXPEDITION-4) involved individuals with advanced CKD.12–14 The aim of this study is to evaluate the efficacy and safety of DAAs for treatment of HCV among patients with mild-to-moderate CKD in a ‘real-world’ setting. We have evaluated the activity of various combinations of DAAs (such as PrOD or sofosbuvir-based regimens) in a large cohort of patients with stage 1–3 CKD followed at some outpatient clinics all over the world. We found a high viral response (>95%) and this occurred irrespective of viral features, demographic and clinical characteristics. The viral response was great despite our cohort was a difficult group to cure- many patients had advanced liver fibrosis, treatment-experience, and a high rate of co-morbidities (such as arterial hypertension and diabetes); genotypes other than 1 and 4 were frequently found.
Another important point was tolerance to DAAs. This was satisfactory as no drop-outs or hospitalizations occurred; dose reduction or discontinuation of RBV was made in a minority of patients only. Worsening of kidney function was experienced in a few individuals (all having stage 3 CKD at baseline). This fact appears to confirm what has been already observed in the HCV-TARGET database, where patients with eGFR <45mL/min more frequently experienced worsening of kidney function compared with a control group with eGFR >45mL/min per 1.73m2.7 No difference in serum creatinine and eGFR levels at the beginning vs. EOT was observed in the whole population.
From a historical point of view, the purpose of antiviral therapy towards HCV has been to treat and prevent liver-related complications such as cirrhosis, HCV and liver-related death. The World Health Organization 1 has recently proposed the universal treatment of HCV, regardless the liver disease stage, and this recommendation is in keeping with the recent evidence showing that antiviral treatment of HCV prevents the development or deterioration of diabetes, cardiovascular disease, and chronic kidney disease. Survival studies performed in patients with intact kidneys or dialysis population have shown the association between positive anti-HCV serologic status and higher cardiovascular mortality.15,16 The detrimental role of chronic HCV on the incidence and progression of CKD in the adult general population has been repeatedly emphasized. In addition to the role of HCV in the development of glomerular disease, several biological mechanisms have been advocated to explain the kidney injury in HCV-positive patients; it can be given by endothelial dysfunction with is promoted by enhanced oxidative stress, pro-inflammatory cytokines, peripheral and hepatic insulin resistance, or non-alcoholic steatohepatitis (NASH).17–20
The findings from the current study have some limitations. First, some regimens (such as EBR/GZR or GP) have been administered in a few cases only as these have been recently introduced in the market. The retrospective nature of the current study may have led to incorrect reporting of AEs but we reviewed with accuracy the clinical records of our series and felt that many AEs were associated with the typical comorbidities of these patients. A subgroup of patients was excluded from the safety analysis because the information on AEs was incomplete. The aetiology of CKD was not addressed in our cohort and a few patients had undergone kidney biopsy; this commonly occurs in the ‘real-life’ clinical practice. Finally, we included patients with CKD identified by calculating eGFR, proteinuria measurements have not been considered for the diagnosis of CKD in our population. This is in analogy with what reported approach in prior studies8 as quantitative proteinuria assessment is still infrequent in HCV-infected individuals in the ‘real-world’ activity. Another limitation of the current study is that the post-treatment data are available only over a short follow-up (12 weeks). Studies are in progress in order to evaluate the effects of HCV eradication on the incidence and progression of CKD over longer periods of time.
In summary, various interferon-free combinations of DAAs are currently available for patients with early stage chronic kidney disease. These have given excellent safety and efficacy. What we need now is to accumulate data on the new treatments for HCV able to provide shorter treatment durations, lower pill burden, and no ribavirin use. In addition, studies are under way to assess the link between the eradication of HCV and better renal and cardiovascular survival among patients with CKD.
AbbreviationsAASLD, American Association for the Study of Liver Diseases; AEs, adverse events; AH, arterial hypertension; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD-EPI, chronic kidney disease epidemiology collaboration; DAAs, direct-acting antiviral agents; DDIs, drug-drug interactions; DM, diabetes mellitus; EOT, end-of-treatment; eGFR, estimated glomerular filtration rate; gamma-GT, gamma glutamyltranspeptidase; GI, gastrointestinal; HCV, hepatitis C virus; IDSA, Infectious Disease Society of America; INR, international normalized ratio; ITT, intention-to-treat; MDRD, Modification of Diet in Renal Disease; NA, not available; OLT, orthotopic liver transplant; PKD, polycystic kidney disease; SAEs, serious adverse events; SVR, sustained virological response; W12, week 12 (12 weeks after the end of treatment).
Abbreviations (antiviral agents)DCV, daclatasvir; DSV, dasabuvir; EBR, elbasvir; GP glecaprevir/pibrentasvir; GZR, grazoprevir; SOF, sofosbuvir; IFN, interferon; LDV, ledipasvir; OBV, ombitasvir; pegIFN, pegylated interferon; PrOD, ritonavir-boosted paritaprevir, ombitasvir and dasabuvir; PTV, paritaprevir; r, ritonavir; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir; VEL, velpatasvir.
FundingNo sources of funding were used for the preparation of this manuscript.
Conflict of interestsEzequiel Ridruejo: advisor/lecturer for Bristol-Myer Squibb, Gilead, MSD. Manuel Mendizabal: advisor/speaker for BMS, AbbVie; grants from AbbVie and MSD. Marcelo Silva: advisor/speaker for MSD, AbbVie, BMS, Gilead; grants from MSD, AbbVie, BMS. Fabrizio Fabrizi: consultant/advisor to AbbVie, Merck & Co.