Patients with proteinuria especially nephrotic syndrome are more prone to infections both due to immunosuppressive drugs used in treatment and low Ig G levels because of loss through urine.1 In addition, certain infections such as hepatitis B and C, human immunodefficiency virus (HIV) might be responsible for the glomerulonephritis.2 So detailed medical history and certain screening tests are mandatory for discrimination of primary glomerulonephritis from secondary post-infectious forms and for protection of the patients from reactivation of silent micro-organisms with induction of immunosuppressive therapy.3
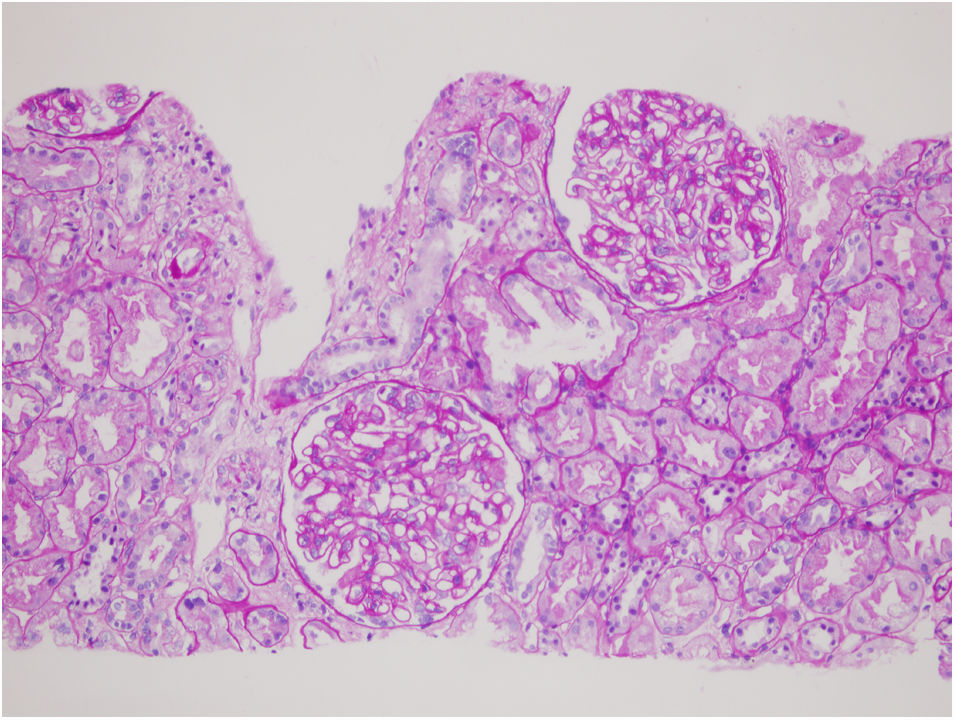
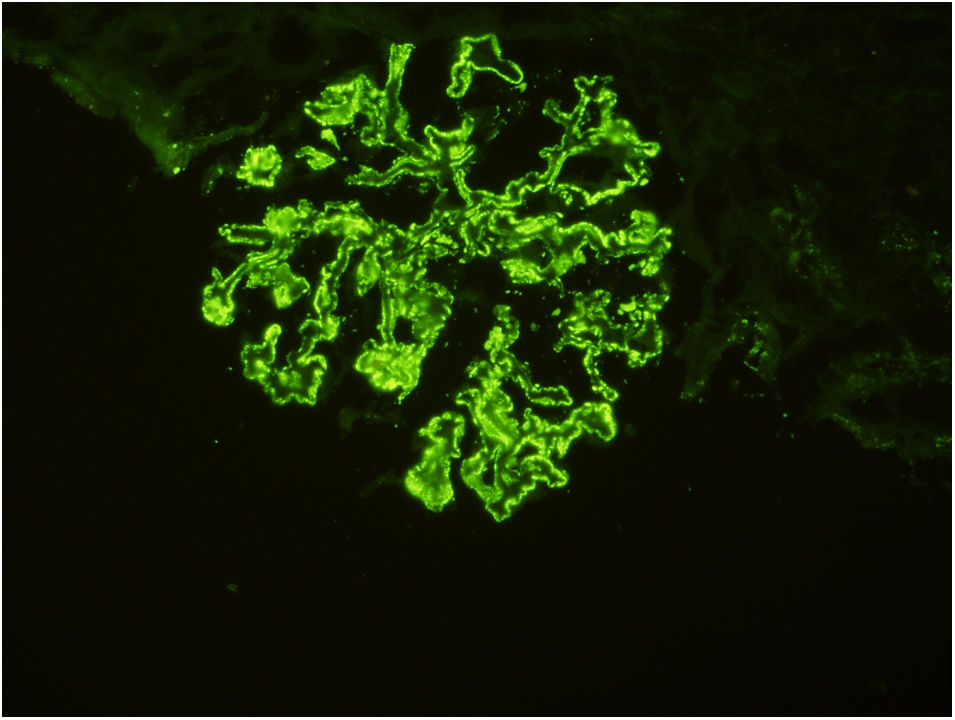
37 years old male married patient with a history of panic attack and hypertension was presented with dispnea due to pleural effusion. Biochemically: serum creatinine: 0.64mg/dL, haemoglobin: 13.2g/dL, albumin: 2.1g/dL, creatinine clearance: 126mL/min, LDL-C: 315mg/dL, 24-h urine protein excretion: 19g/day, 24-hour urine albumin excretion: 10.7g/day. Ultrasonographically kidneys were normal. Renal biopsy revealed membranous nephropathy stage 2 (Figs. 1 and 2). Our routine infection screening revealed positive results for syphilis antibodies with ELISA test (1/250 cut off index). Validation tests (Treponema palladium hemagglutination assay – TPHA – for both Ig M and Ig G) confirmed the diagnosis of asymptomatic (latent) syphilis. As there was no expressed recent history of high risk sexual behaviour, late latent syphilis was thought and three weekly doses of penicillin G benzathine (2.4 million units i.m.) were given to the patient. Serum complement levels of the patient were normal and proteinuria level of patient was not decreased with this therapy. As anti-phospholipase A2 receptor antibody (PLA2Rab) was positive (N≤20 RU/mL) with ELISA test (result: 164RU/mL), diagnosis of primary membranous glomerulopathy was ascertained. Under treatment of steroid and cyclosporine, his serum albumin levels increased to 3.1g/dL, and proteinuria decreased to 1g/day within one month.
Most of the membranous glomerulopathy (nearly 75%) cases are known as idiopathic (primary) without any identifiable causes such as infections, drugs, auto-immune diseases, neoplasms.4 Serological tests (PLA2Rab, hepatitis B, C, HIV and syphilis) are important for both differential diagnosis of membranous glomerulopathy and minimize side effects of immunosuppressive treatment.5 KDIGO guidelines do not advice to screen for syphilis routinely in patients with primary membranous glomerulopathy.6 In addition, literature review showed many cases of syphilis as a cause of membranous glomerulopathy but as far as we know no report with latent syphilis along with primary membranous glomerulopathy was published. Also, one should remember false positive syphilis results (due to cross reactions with other antibodies) if one of nontreponemal tests (VDRL, RPR) is reactive and other one of treponemal tests (ELISA, TPHA, FTA-ABS) is nonreactive. In order to diagnose the organism directly PCR or direct fluorescent antibody testing for treponema palladium are used.7 Presence of positive serology for syphilis (two different types of treponemal tests for antibodies against syphilis including Ig M and Ig G), absence of any sign of infection and treatment history is defined as -presumed- latent syphilis as in our case.
Anti-PLA2Rab is very specific for diagnosis and monitoring immunological activity of primary membranous glomerulopathy.8 In our patient, with the presence of anti-PLA2Rab and absence of remission in proteinuria after 3 weeks of optimum treatment against syphilis, primary membranous glomerulopathy related nephrotic syndrome was diagnosed. In this particular patient, if the screening for syphilis had not been done, immunosuppressive treatment for primary membranous glomerulopathy might have led to progression through advanced (tertiary) syphilis infection.9 This case makes us think it should be better to screen for syphilis before onset of immunosuppressive treatment even among patients with primary membranous glomerulopathy.
Authorship contributionsConcept: Kubra Kaynar; Design: Kubra Kaynar, Beyhan Guvercin; Control: Sukru Ulusoy; Data Collection: Sevdegul Mungan; Literature review: Kubra Kaynar; Writing the manuscript: Kubra Kaynar, Beyhan Guvercin.
Compliance with ethical standardsNo conflict of interests was declared. No fund was taken. Informed consent was obtained from the patient.
Conflict of interestNone.