Dear Editor,
De novo Hemolytic Uremic Syndrome (HUS) occurs in 1-5% of renal transplant recipients, most frequently within the first 3 months after transplantation.1,2
Thrombotic Microangiopathy (TMA) usually sets in the first weeks post-transplant when patients are treated with high dose of immunosuppressant.
De novo TMA has been documented in approximately 1% of patients receiving tacrolimus.2 Calcineurin inhibitor – induced nephrotoxicity primarily results from dose dependent renal arteriolar vasoconstriction,3 owing of the enhanced production of vasoconstrictive factors, particularly endothelin-1 and angiotensin II.4,5 Moreover, calcineurin inhibitors might promote a procoagulant state enhancing platelet aggregation and activating plasminogen activator.6 De novo post-transplant HUS has been reported both with the use of an mTOR inhibitor alone and in combination therapy.7 The viral infections, to which patients on immunosuppression are susceptible, have been implicated in pathogenesis of de novo HUS in organ transplant recipients. A CMV infection8 has been associated with both the novo and recurrent9 forms of post-transplant HUS. Parvovirus B19 and polioma BK virus infection have also been associated with de novo HUS in renal transplant recipients.10-12
The authors describe the clinical case of a Caucasian male, 33 years old with a history of chronic kidney disease of unknown etiology, in hemodialysis for nearly 7 years, with past medical history of severe hypertension, severe hypertriglyceridemia and chronic pancreatitis. Family history was irrelevant.
In May 2010 he was underwent a renal transplantation with deceased kidney allograft (donor: man aged 23, suffered TCE) with CMV (D + / R +), HBV, HCV and HIV negative and with ABO compatibility. In the presence of low immunological risk situation with PRA 0% and with HLA 4:6 matches, he did not submitted to induction and began a immunosuppression regimen with mycophenolic acid (1440 mg/day), Tacrolimus LP (0.15mg/kg), methylprednisolone (8mg/kg) and adopting an initial maintenance scheme comprising mycophenolic acid (1440mg/day), LP tacrolimus (16mg) and prednisone (20mg/day).
Initial graft function was immediate, without surgical complications but with severe hypertension of difficult to control. Doppler renal graft was performed that excluded vascular complications and showed a normal resistance index.
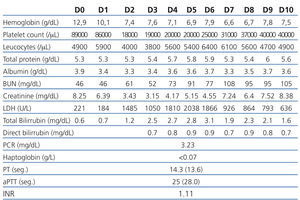
The 2nd day postoperatively for 3rd day there was a deterioration of renal graft function (serum creatinine of 2.92 to 3.15mg/dL) associated with abrupt drop in platelet count (97,000 to 19.000/μL), drop in serum hemoglobin (10.4 to 7.6g/dL) and increase serum levels of lactate dehydrogenase (LDH 1485U/L) with tacrolimus plasma level of 10ng/mL. The Coombs test (direct and indirect) was negative, serum haptoglobin decreased and were found 3% of schistocytes in peripheral blood smear.
The survey of CMV, BK virus and parvovirus B19 in blood by P.C.R. were negative. It was found normal plasma activity of the ADAMTS13 in the absence of inhibitors. The autoimmunity study with anti-nuclear antibodies, anti-dsDNA and anti-cytoplasmic antibodies and antiphospholipid antibody were negative as well as was negative donor-specific antibody. In relation to severity of thrombocytopenia was not possible to perform biopsy of renal allograft.
The patient remained clinically well and without gastrointestinal or neurological changes. Given the evidence of worsening of renal function (acute dysfunction of renal allograft) associated with markers of intravascular hemolysis (nonimmune: Coombs direct and indirect negative) was established the diagnosis of De novo hemolytic uremic syndrome post-transplant, probably associated with tacrolimus.
We suspended tacrolimus and mycophenolic acid (by myelosuppressive effects) and was introduced everolimus on the 5th day. We obtained improved of blood pressure control. Despite the attitudes adopted on the 9th day maintained parameters hemolysis (6.6g Hb/dL, and 31,000/μL platelet LDH 926U/L) and worsening of graft function (7.24mg/dL) with diuresis maintained (Table 1). We decided to initiate daily plasmapheresis (D9 to D23) (1.5 x the plasma volume) with fresh frozen plasma inactivated. The patient remained without neurological disorders. After 14 sessions of plasmapheresis and 3 daily hemodialysis sessions (D9 to D12) we observed recovery of renal graft function accompanied by the disappearance of markers of hemolysis. At discharge date he had a serum creatinine of 2.39 mg/dL and 6 months after the level of seric creatinine was of 1.0mg/dL, with no evidence of recurrent HUS (without analytical signal of intravascular hemolysis).
There are no treatment guidelines for this entity. Among 29 patients with De novo post-transplant TMA, 80% recovered graft function after cyclosporine stop and plasma therapy.13 Our experience demonstrates that switching from tacrolimus to everolimus with plasmapheresis with fresh plasma proved be an adequate strategy for this situation without increased risk of acute rejection.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 1. Laboratorial evolution