La calcificación arterial (CA) es una complicación común en la enfermedad renal crónica y la enfermedad renal en etapa terminal, y cuyo alcance es diagnóstico de una posterior mortalidad cardiovascular más allá de los factores de riesgo convencionales establecidos. La CA se desarrolla en dos ubicaciones diferentes: en las capas íntima y media de las paredes arteriales de gran y medio tamaño. Estas dos formas se encuentran frecuentemente asociadas. La CA está estrechamente relacionada con el envejecimiento y el remodelado arterial, que incluye el engrosamiento de la íntima-media y los cambios en la geometría y la función de las válvulas aórticas. Se han recogido evidencias que señalan la naturaleza activa y regulada del proceso de calcificación. Elevados niveles de fosfatos y calcio pueden estimular el cotransporte de fosfato dependiente del sodio que implique cambios osteoblásticos en la expresión genética celular. La CA es responsable del endurecimiento de las arterias, con un aumento de la poscarga ventricular izquierda y perfusión coronaria anormal como principales causas clínicas.
Arterial calcification (AC) is a common complication of CKD and ESRD, and the extents of AC are predictive of subsequent cardiovascular mortality beyond established conventional risk factors. AC develop in two distinct sites: the intima and media layers of the large and medium-sized arterial wall. These two forms are frequently associated. AC is tightly associated with aging and arterial remodeling, including intima-media thickening, but also changes of the geometry and function of aortic valves. Evidence has accumulated pointing to the active and regulated nature of the calcification process. Elevated phosphate and calcium may stimulate sodium–dependent phosphate cotransport involving osteoblast–like changes in cellular gene expression. AC is responsible for stiffening of the arteries with increased left ventricular afterload and abnormal coronary perfusion as the principal clinical consequences.
INTRODUCTION
The cardiovascular complications are leading cause of mortality and morbidity in chronic and end-stage renal diseases, in great part related to arterial diseases, i.e. atherosclerosis and arteriosclerosis1,2. While atherosclerosis and plaque-associated occlusive lesions are the frequent causes of these complications, arteriosclerosis is characterized by outward remodeling and stiffening of large arteries3. These arterial structural and functional changes are, in many aspects, similar to an accelerated age-related process3. One characteristic feature of arterial alterations observed in renal patients is the presence of extensive vascular calcifications4-6 whose extents are predictive of subsequent cardiovascular mortality beyond established conventional risk factors7-9. AC develop in two distinct sites: the intima and media layers of the large and medium-sized arterial wall10. These two forms are frequently associated. Intima calcification occurs within atherosclerotic plaque and is a progressive feature of common atherosclerosis, while media calcifications can occurs independently from atherosclerotic plaques and is frequently observed in medium sized arteries in CKD/ESRD, diabetes. AC is tightly associated with aging and arterial remodeling, including intima-media thickening, but also changes of the geometry and function of aortic valves, e.g., decreased aortic valve surface area and smaller valve opening11.
MECHANISMS OF ARTERIAL CALCIFICATION
The presence of dystrophic calcification in the arterial walls is a response to tissue injury, represents a repair process, and is a form of scar tissue12. Experimental and clinical studies have shown that AC is a process reflecting changes of the vascular smooth-muscle cells (VSMC) and pericytes from contractile to secretory phenotype). VSMC synthesize bone-associated proteins, including alkaline phosphatase, osteocalcin, osteopontin and a coat of collage-rich extracellular matrix, and includes the formation of matrix vesicles, nodules and apoptotic bodies, which serve as initiation sites for apatite crystallization13,14.
In vitro, VSMC differentiation towards osteoblast-like cells, with subsequent mineralization, is regulated by the balance between promoters and inhibitors of calcification, and results from disruption of this balance in favor of promoters. The secretory phenotype is initiated by the activation of Runx2 (Cbfa1) and osterix (Osx), transcrition factors that promote the differentiation of mesenchymal cells into the osteoblastic lineage15,16. The Runx2 and Osx are activated upstream by several factors including Msx2, Wnt and b-catenin signaling17. The stimuli initiating this “osteogenic cascade” include bone morphogenic proteins (BMP 2, 4) and chronic injurious stimuli and metabolic toxicities including generation of reactive oxygen species (ROS)17-19. The result could be either VSMC apoptosis or stimulation of NFk-B and activation of inflammatory mediators TNFa, IL-1, IL-6, and activation of macrophages19-23. Experimental studies using molecular imaging clearly showed that calcifications develops in parallel with inflammation in two phases: early activation of macrophages and inflammation and calcification at later stage22 (figure 1).
Pooled uremic serum with high phophate concentration, induced expression of Runx224 and blocks the expression of genes responsibles for expression of contractile molecules13,14. In vitro, the phosphate-stimulated calcification process can be inhibited by adding pyrophosphates that antagonize the cellular sodium-phosphate cotransport system (PIT-1)25. Recent study has shown tha phosphate induces the calcification process through a common pathway: increasing mitochondrial ROS and activation of NFk-B pathway and transcription of osteogenic program with expression of Msx2-Wnt-Runx226.
In the presence of normal serum, VSMC do not calcified and can inhibit spontaneous calcium and phosphate precipitation in solution, indicating that systemic calcification inhibitors such as fetuin-A are present in the serum27 and also in VSMCs who constitutively express potent local inhibitors of calcification, such as matrix GLA protein28,29, which may limit AC by binding to bone morphogenic proteins (BMP-2)29. Osteopontin and osteoprotegerin are potent inhibitors of AC in vivo, and inactivation of their gene enhances the calcification process30,31.
CLINICAL IMPACT OF ARTERIAL CALCIFICATIONS
Intima calcification occurs in the context of common atherosclerosis, progresses in parallel with the plaque evolution. The arterial dysfunction result from narrowing of the arterial lumen with ischemia affecting the tissues and organs downstream. The acute coronary events and infarction are more related to biomechanical stability of atherosclerotic plaques and the rupture of the plaque’s fibrous cap. This results from mechanical discontinuity between the inclusion of rigid material (calcium crystals) into distensible material (lipid core) resulting in plaque vulnerability and rupture. Although a higher coronary AC score is associated with a poorer cardiovascular prognosis, the influence of calcification on plaque stability is controversial. The results of several studies indicated that AC does not increase plaque vulnerability, which seems more attributable to a large lipid pool, thin fibrous cap and intensity of local inflammation32,33.
Media calcification (Mönckeberg’s sclerosis or media calcinosis) is characterized by diffuse mineral deposits within the arterial tunica media. While media calcification is frequently observed with aging in the general population, it is significantly more pronounced in patients with metabolic disorders, such as metabolic syndrome, diabetes or CKD. Media calcification is concentric, not extending into arterial lumen in its typical pure form and is associated with abnormal cushioning function of blood vessels (arteriosclerosis-arterial hardening) by promoting arterial stiffness34. The principal consequences of arterial stiffening are an abnormal arterial pressure wave (characterized by increased systolic and decreased diastolic pressures, resulting in high pulse pressure) and increased aortic characteristic impedance, a measure of the opposition of the aorta to oscillatory input (i.e., stroke volume)35. Because the two forms of AC are frequently associated the conduit and cushioning abnormalities could be associated.
MANAGEMENT AND PREVENTION
AC rarely regress, therefore, the primary goals are prevention and stabilization of existing calcifications. Because intimal AC are related to atherosclerosis, the general approach is non-specific as advocated for patients with atherosclerosis: control of blood lipids (but no evidence of a benefit with statins), use of aspirin, treatment of obesity and hypertension, physical activity, smoking cessation, and control of diabetes. More specific preventive measures for patients with CKD or ESRD include controlling serum calcium and phosphate levels, thereby avoiding oversuppression of parathyroid activity and ABD36. Disturbances in calcium and phosphate metabolism are associated with uremic bone disease, and the results of several studies indicated that calcium overload is associated with AC development and progression, suggesting that the overuse of high doses of calcium-based phosphate binders, pharmacological doses of vitamin D, and high calcium concentration in the dialysate should be avoided36-39. Those data suggest that the use of calcium-containing phosphate binders, high intradialytic calcium load, and overuse of active vitamin D should be avoided in elderly patients and in those who already have AC.
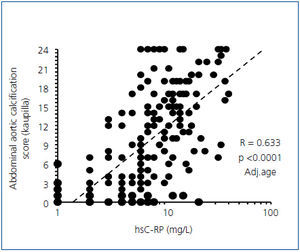
Figure 1. Correlations between the abdominal aortic calcification score and high-sensitive C-RP.