Acute kidney injury (AKI) has been associated with cardiovascular disease, but this is sparsely studied in non-selected populations and with little attention to the effect in age and renal function. Using nationwide administrative data, we investigated the hypothesis of increased one-year risk of cardiovascular event or death associated with AKI.
MethodsIn a cohort study, we identified all admissions in Denmark between 2008 and 2018. AKI was defined as ≥1.5 times increase from baseline to peak creatinine during admission, or dialysis. We excluded patients with age <50 years, estimated glomerular filtration rate (eGFR) <15ml/min/1.73m2, renal transplantation, index-admission due to cardiovascular disease or death during index-admission. The primary outcome was cardiovascular risk within one year from discharge, which was a composite of the secondary outcomes ischemic heart disease, heart failure or stroke. To estimate risks, we applied multiple logistic regression fitted by inverse probability of censoring weighting and stratified estimations by eGFR and age. We adjusted for proteinuria in the subcohort with measurements available.
ResultsAmong 565,056 hospital admissions, 39,569 (7.0%) cases of AKI were present. In total, 18,642 patients sustained a cardiovascular outcome. AKI was significantly associated with cardiovascular outcome with an adjusted OR [CI] of 1.33 [1.16–1.53], 1.43 [1.33–1.54], 1.23 [1.14–1.34], 1.38 [1.18–1.62] for eGFR ≥90, 60–89, 30–59 and 15–29ml/min/1.73m2, respectively. When omitting the outcome heart failure, these results were 1.24 [1.06–1.45], 1.22 [1.11–1.33], 1.05 [0.95–1.16], 1.25 [1.02–1.54]. Results did not change substantially in strata of age groups, in AKI stages and in the subcohort adjusted for proteinuria.
ConclusionNon-selected patients aged 50 years or above with AKI during admission had significantly higher one-year risk of cardiovascular event or death, especially, but not only due to heart failure, independent of age and eGFR.
La lesión renal aguda (LRA) se ha asociado a la enfermedad cardiovascular, pero se ha estudiado poco en poblaciones no seleccionadas y se ha prestado escasa atención al efecto en la edad y la función renal. Utilizando datos administrativos a escala nacional, se investigó la hipótesis de un mayor riesgo de acontecimiento cardiovascular o muerte al cabo de un año asociado a la LRA.
MétodosEn un estudio de cohortes se identificaron todos los ingresos que tuvieron lugar en Dinamarca entre 2008 y 2018. La LRA se definió como un aumento mayor o igual a 1,5 veces desde los valores iniciales hasta el pico de creatinina durante el ingreso o la diálisis. Se excluyeron a los pacientes con una edad inferior a 50 años, una tasa de filtración glomerular estimada (TFGe) inferior a 15ml/min/1,73m2, un trasplante renal, un ingreso inicial por enfermedad cardiovascular o la muerte durante el ingreso. El resultado primario fue riesgo cardiovascular en el plazo de un año desde el alta, entendido como una combinación de los criterios de valoración secundarios de cardiopatía isquémica, insuficiencia cardíaca o accidente cerebrovascular. Para estimar los riesgos, se aplicó una regresión logística múltiple ajustada por la ponderación de la probabilidad inversa de censura y las estimaciones estratificadas por la TFGe y la edad. Se ajustó por proteinuria en la subcohorte para la que se disponía de mediciones.
ResultadosDe entre 565.056 ingresos hospitalarios, en 39.569 (7,0%) de los casos había LRA presente. En total, 18.642 pacientes mantuvieron un desenlace cardiovascular. La LRA estuvo asociada de forma significativa con los criterios de valoración cardiovasculares, con una tasa global (índice de confianza) de 1,33 (1,16-1,53); 1,43 (1,33-1,54); 1,23 (1,14-1,34); 1,38 (1,18-1,62) para una TFGe≥90, 60-89, 30-59 y 15-29ml/min/1,73m2, respectivamente. Cuando se omitió el criterio de valoración de insuficiencia cardíaca, los resultados fueron 1,24 (1,06-1,45); 1,22 (1,11-1,33); 1,05 (0,95-1,16); 1,25 (1,02-1,54). Los resultados no cambiaron sustancialmente en los estratos de los grupos de edad, en los estadios de LRA ni en la subcohorte ajustada por proteinuria.
ConclusiónLos pacientes no seleccionados de 50 años o más con LRA durante el ingreso tenían un riesgo significativamente mayor de sufrir un acontecimiento cardiovascular o muerte al cabo de un año, sobre todo, aunque no solamente, debido a insuficiencia cardíaca, con independencia de la edad y de la TFGe.
Acute kidney injury (AKI) is a serious condition that complicates one in five hospital admissions and one in two admissions to intensive care units.1 AKI is associated with early mortality2 and is a risk factor for chronic kidney disease (CKD) and vice versa.3 Since the main complication to CKD is cardiovascular disease,4 it is plausible AKI is a risk factor for cardiovascular disease.
The pathophysiological processes underlying this association are still uncertain, but transition to or worsening of CKD could partly mediate it. Nevertheless, AKI has been shown to elicit systemic inflammation with distant but direct organ changes including the heart.5
Several studies have found an association between AKI and cardiovascular outcomes. However, these studies are primarily limited to specific patient populations at high risk, e.g., admission due to cardiac events or cardiac surgery.6 As such, studies on non-selected hospital populations remain sparse.7–9 Those studies available have only focused on heart failure or only found an association with heart failure, but not atherosclerotic events. Furthermore, the effect of, as well as the potential interaction from, different age groups and prior renal function groups on the attributable risk of AKI have received little attention. In Denmark, the automatically recorded national patient registers facilitate large-scale studies of populations with equal access to health care, which is limited in previous studies. On this basis, we investigated one-year risk of cardiovascular event or death after admission with AKI in a cohort of non-selected patients.
Materials and methodsData sourcesThe Danish health care system administrates tax-funded health services for all 5.8 million Danish inhabitants. Through this, Statistics Denmark collects comprehensive health data in many nationwide registers. The Central Person Register number that all Danish citizens are issued makes individual cross-reference of data between these registers possible, and it holds information on any migration.10
We extracted results from blood and urine samples from laboratory databases from four of five administrative regions, which is gathered in the Register of Laboratory Results for Research.11 The Danish National Prescription Registry provided prescription data via Anatomical Therapeutic Chemical Classification System (ATC) codes. As medication costs are partly reimbursed by the healthcare authorities, every pharmacy is obligated to deliver complete data.12 Comorbidities were based on diagnosis codes (10th edition of the International Classification of Diseases (ICD-10)), from both hospital discharges and outpatient clinics. Surgical interventions were based on the Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP). ICD-10 and NCSP-codes were extracted from the validated Danish National Patient Register.13 Causes of death were gathered from the National Causes of Death Registry.
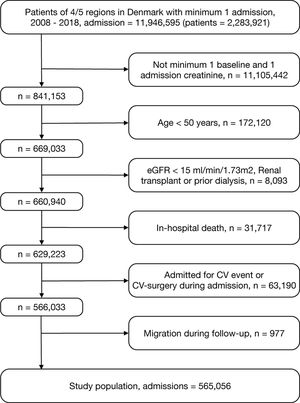
Study designIn this retrospective cohort study, we identified patient admissions from a hospital in Denmark between January 1st, 2008 and December 31st, 2018. Patients were included if they had at least one baseline creatinine measurement between one week and one year before admission, which is a validated baseline period.14 They were also required to have a second measurement during admission. Start of follow-up (index-date) was date of discharge. Multiple admissions of the same patient were included if they were at least three months apart to ensure a new admission with AKI was independent. Patients were excluded if age was less than 50 years, they had an estimated glomerular filtration rate (eGFR) <15ml/min/1.73m2, end-stage renal disease or had prior renal transplantation. The age criterium was set to gain more homogeneity in causes and pathologies of AKI and comorbidities. Further exclusion criteria were index-admission due to cardiovascular disease, death during index-admission or migration during follow-up.
Study exposureThe baseline creatinine was calculated as the mean of creatinine measurements in the baseline period. AKI was defined as 1.5 times increase or more from baseline plasma creatinine to peak creatinine during admission. This was an operational approximation of the international guidelines defined by Kidney Disease: Improving Global Outcomes (KDIGO).15
Severity of AKI was divided into stages, also following KDIGO guidelines. Stage 1 was defined as 1.5–1.9-fold increase from baseline creatinine, stage 2 as 2.0–2.9-fold increase, and stage 3 as threefold or more increase or the initiation of acute dialysis, identified by ICD-10-code.
Prior comorbidities and medicationMedication was determined by redeemed prescriptions up to six months before admission date by the use of ATC-codes (listed in Table S3 in supplementary). Medication comprised antidiabetics, beta-blockers, renin-angiotensin-system inhibitors (RASi), calcium channel blockers, non-loop diuretics, loop diuretics, antihyperlipidemic agents, non-steroidal anti-inflammatory drugs and acetylsalicylic acid.
Comorbidities were based on ICD10-codes registered within five years prior to admission (Table S4 in supplementary) and included ischemic heart disease, heart failure, stroke, atrial fibrillation/flutter (AFF), diabetes, hypertension, chronic obstructive pulmonary disease, liver disease, and cancer. In addition to diagnostic codes, diabetes was identified by a redeemed prescription of any antidiabetic medication according to ATC-codes. Hypertension was also determined by two or more antihypertensive agents, with the exception of loop diuretics. We computed eGFR from mean baseline creatinine with the CKD-EPI formula.16 Proteinuria was defined as urine dipstick ≥+1 or albumin creatinine ratio ≥30mg/g. Hyperlipidaemia was defined by a total cholesterol ≥5mmol/L.
OutcomesThe primary outcome was first occurrence of a cardiovascular event, defined as a composite of ischemic heart disease (ICD-10: DI20-25), heart failure (ICD-10: DI42, DI50, DJ81) and stroke (ICD-10: DI60-69, DG458, DG459, NCSP: KAAL10, KAAL11) or the death due to one of these. Secondary outcomes were the individual diagnoses that composed a cardiovascular event, along with AFF (ICD-10: DI48) and all-cause death.
Statistical analysesContinuous variables were described with median and interquartile range (IQR) and categorical variables with counts and percentages. Cumulative incidence curves for AKI-stages and mortality were estimated with the Aalen-Johansen estimator. We used multiple logistic regression to model the association between AKI and cardiovascular outcome within one year to report adjusted odds ratios (OR). Models were fitted by inverse probability of censoring weighted equations to account for censoring and competing risk of death and other causes than cardiovascular events.17 Models were adjusted for gender, age groups (50–65, 66–80, >80 years), prior ischemic heart disease, heart failure, stroke, AFF, hypertension, diabetes, total cholesterol, antihyperlipidemic drugs and non-steroidal anti-inflammatory drugs. Estimations of ORs were performed separately for different eGFR groups using an interaction term between AKI and eGFR groups. We also fitted the model with adjustment by proteinuria18 in the subcohort with proteinuria measurements available. In further stratification analyses we estimated the association separately within age groups by further adding an interaction term in the model between AKI and age. In similar models, we estimated the association between stages of AKI and cardiovascular outcome (stage 1 vs. no AKI and stage 2–3 vs. no AKI). In a sensitivity analysis we evaluated estimates of patients aged 18–49 years, and first-time admissions in another analysis.
Data management was carried out with SAS software (version 9.4, SAS Institute), and statistical analyses with R (version 3.6.3).19
ResultsThe total cohort included of 565,056 patient admissions (Fig. 1). Overall, median [IQR] age was 72 [63–81] years and median eGFR [IQR] was 78ml/min/1.73m2 [61–90]. AKI was identified in 39,569 admissions (7.0%). The distribution of AKI-severity was stage 1, n=24,519 (4.3% of total cohort and 62% of AKIs), stage 2, 9448 (1.7%/24%) and stage 3, 5602 (1.0%/14%). Baseline characteristics, stratified by AKI, for the total cohort is presented in Table 1 and for the subcohort with proteinuria measurements in Table S1 in supplementary. Admissions involving AKI were characterized by greater preponderance for male gender, increased age, and greater burden of comorbidity. In those patients excluded from the study due to death during admission, 40% had AKI (Fig. 1).
Baseline characteristics of hospitalized adult patients stratified by AKI (AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; RASi, renin–angiotensin–aldosterone-receptor-system inhibitor; SD, standard deviation).
| Characteristics | No AKI | AKI |
|---|---|---|
| Patient admissions | 525,487 | 39,569 |
| Males (%) | 242,481 (46.1) | 19,976 (50.5) |
| Age (mean(SD)) | 71.8 (11.5) | 74.7 (11.0) |
| eGFR [ml/min/1.73m2] (%) | ||
| 90+ | 135,212 (25.7) | 8800 (22.2) |
| 60–89 | 273,455 (52.0) | 17,368 (43.9) |
| 30–59 | 105,120 (20.0) | 11,345 (28.7) |
| 15–29 | 11,700 (2.2) | 2056 (5.2) |
| Hypertension (%) | 188,704 (35.9) | 19,112 (48.3) |
| Diabetes (%) | 85,308 (16.2) | 9811 (24.8) |
| Ischemic heart disease (%) | 38,691 (7.4) | 2934 (7.4) |
| Heart failure (%) | 20,726 (3.9) | 2923 (7.4) |
| Stroke (%) | 29,644 (5.6) | 2330 (5.9) |
| Atrial fibrillation/flutter (%) | 50,388 (9.6) | 4907 (12.4) |
| COPD (%) | 34,351 (6.5) | 3007 (7.6) |
| Cancer (%) | 10,730 (2.0) | 1223 (3.1) |
| Liver disease (%) | 75,171 (14.3) | 6278 (15.9) |
| Proteinuria (%) | ||
| No | 163,104 (31.0) | 10,598 (26.8) |
| Yes | 57,964 (11.0) | 8152 (20.6) |
| Unknown | 304,419 (57.9) | 20,819 (52.6) |
| Cholesterol (%) | ||
| <5mmol/L | 257,162 (48.9) | 22,102 (55.9) |
| ≥5mmol/L | 197,210 (37.5) | 12,006 (30.3) |
| Unknown | 71,115 (13.5) | 5461 (13.8) |
| Anti-lipids | 184,890 (35.2) | 15,593 (39.4) |
| Loop diuretics (%) | 79,890 (15.2) | 11,690 (29.5) |
| Non-loop diuretics (%) | 143,004 (27.2) | 14,424 (36.5) |
| Calcium channel blockers (%) | 113,552 (21.6) | 10,318 (26.1) |
| Betablockers (%) | 112,694 (21.4) | 11,110 (28.1) |
| RASi (%) | 200,459 (38.1) | 20,416 (51.6) |
| Acetylsalicylic acid (%) | 2330 (0.4) | 170 (0.4) |
| NSAID (%) | 87,859 (16.7) | 6754 (17.1) |
| Admission number (%) | ||
| 1 | 368,737 (70.2) | 26,446 (66.8) |
| 2 | 110,146 (21.0) | 9123 (23.1) |
| 3–5 | 46,604 (8.9) | 4000 (10.1) |
| Speciality (%) | ||
| Medical | 305,118 (58.1) | 23,502 (59.4) |
| Surgical | 183,132 (34.8) | 12,419 (31.4) |
| Other | 37,237 (7.1) | 3648 (9.2) |
| Length of stay [days] (median [IQR]) | 2.0 [1.0, 5.0] | 7.0 [3.0, 13.0] |
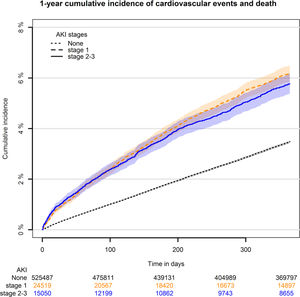
Within the one-year follow-up period from discharge 18,642 cardiovascular events or deaths were found, hereof heart failure, n=5092, ischemic heart disease, n=7348 and stroke n=6202. Of AFF events/deaths 16,330 were found and all-cause deaths were 58,788. One-year cumulative incidence of cardiovascular events showed higher risk in patients with AKI compared to no AKI, which was 3.5% for no AKI (Fig. 2). There was with no difference between AKI stage 1 (=6.1%) and stage 2–3 (=5.6%).
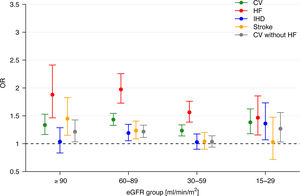
AKI was significantly associated with a cardiovascular event with an adjusted OR of 1.33 [1.16–1.53], 1.43 [1.33–1.54], 1.23 [1.14–1.34], 1.38 [1.18–1.62] for eGFR ≥90, 60–89, 30–59 and 15–29ml/min/1.73m2, respectively (Fig. 3). Corresponding results for cardiovascular events without heart failure were 1.24 [1.06–1.45], 1.22 [1.11–1.33], 1.05 [0.95–1.16], 1.25 [1.02–1.54]. In subgroup analyses of patients with data on proteinuria overall results remained principally unchanged (supplementary, Fig. S1).
One-year adjusted odds ratios of cardiovascular event/death, individual diagnosis of CV and CV without HF with 95% CI for AKI vs. no AKI in different intervals of eGFR (AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; CV, cardiovascular; HF, heart failure; IHD, ischemic heart disease).
Results for AFF were 1.36 [1.10–1.69], 1.22 [1.07–1.38], 0.86 [0.74–1.00], 1.19 [0.85–1.66], in the respective eGFR levels.
When estimating the associations within age groups, the main results were largely unaffected (Fig. S2). Percentual distribution of age groups were 24%, 43%, 38% in 50–65, 66–80 and >80 years, respectively. In age 50–65 years ORs were higher than the remainder of the age groups, but also with more uncertainty, as less data was available, especially in eGFR 15–29ml/min/1.73m2 (n=291). In the largest age group, age 66–80 years, OR [CI] was 1.19 [0.98–1.44], 1.38 [1.24–1.54], 1.17 [1.02–1.35], 1.51 [1.14–2.00] in the respective eGFR levels. For age >80 years ORs were all significant, except in eGFR ≥90ml/min/1.73m2 (1.68 [1.00–2.84], n=771).
In the analysis of AKI severity, no substantial differences between stage 1 vs. no AKI and stage 2–3 vs. no AKI were found (supplementary, Fig. S3). For stage 1 vs. no AKI in the age group 66–80 years ORs [CI] for the respective eGFR levels were 1.20 [1.00–1.46], 1.38 [1.24–1.54], 1.21 [1.05–1.38], 1.56 [1.18–2.07]. Correspondingly, for stage 2–3 vs. no AKI, ORs [CI] were 1.10 [0.80–1.51], 1.37 [1.16–1.61], 1.19 [0.96–1.46], 1.65 [1.10–2.49]. In contrast, cumulative mortality was graded by AKI stages: no AKI, 10,5%; AKI stage 1, 23%; AKI stage 2–3, 26% (supplementary, Fig. S6).
No interaction was found between AKI and proteinuria, prior cardiovascular disease, (supplementary, Fig. S4), hypertension or diabetes (not shown). The sensitivity analysis of patients aged 18–49 showed overall somewhat higher ORs in the various eGFR intervals, as compared to the age intervals of the main results, but with wide CIs (supplementary Table S2). No major differences were found for first-time admission only, compared to multiple admissions, apart from slightly wider CIs (Fig. S5).
DiscussionIn this nationwide study of non-selected, hospitalized patients, we found AKI to be associated with increased one-year risk of cardiovascular event or death in all eGFR groups. Though this increase was mainly driven by heart failure the risk was remained significantly increased when heart failure was omitted from the composite endpoint, except in eGFR 30–59ml/min/1.73m2. Results were similar in strata of age groups and stages of AKI, though significance in some eGFR groups was lost.
Prior studiesIn the last decade, focus on the association between AKI and cardiovascular disease has increased, with nearly all studies reporting a clear association. However, most of these studies are limited to specific high-risk populations, particularly those admitted due to cardiovascular disease,20,21 cardiac surgery,22–24 general surgery25,26 or contrast induced nephropathy.27 While this probably includes a more uniform and comparable cohort, which strengthen the internal validity, it also limits the external validity/extrapolation to larger populations. A meta-analysis from 2017 that included the majority of these studies found a relative risk of 1.38 [1.23–1.55] for cardiovascular events (median [IQR] follow-up 1.4 years [1.2–1.9]), compared to no AKI, which was especially due to heart failure (relative risk 1.58 [1.46–1.72]). Relative risk of cardiovascular death was 1.86 [1.72–2.01] (median follow-up 2.6 years [2.0–3.4]).6 These results resembled ours.
A few studies on patients hospitalized for non-specific reasons have been published. In a large study of 430,159 patients from the Kaiser Permanente database in the US, cases of AKI were matched with no-AKI controls.7 It was possible for enrolled patients to be admitted with prevalent as well as due to cardiovascular disease. Rate of cardiovascular events, including heart failure, was significantly increased within the first year (hazard ratio (HR) 1.18 [1.13–1.25] and 1.44 [1.33–1.56], respectively), regardless of AKI severity. These results resembled ours, except that atherosclerotic events in isolation were not significant, which was possibly due to lack of power and/or more complete adjustment for proteinuria. The strengths of the study were the large dataset with almost full follow-up, adjustment of many important confounders, including proteinuria, and a proxy for severity of illness. A limitation was the potential selection bias from the use of the Kaiser Permanente database. This database only holds insured patients, which precludes equal access to health care.28 Another large study, comprising 1,120,145 patients from US Veterans Affairs health care records, focused solely on incident heart failure.8 This study also matched AKIs with no-AKIs and found a one-year HR of 1.28 [1.23–1.34]. Results were unchanged in subgroups of age, ischemic heart disease, CKD and diabetes. In contrast to our study they accounted for renal recovery, i.e. restoration of renal function up to certain points, which revealed that HR was dampened in a graded way with increasing levels of recovery. The study was limited by the use of a cohort restricted to primarily males who had a heightened heart failure risk. A study of 210,895 patients from the primary care setting of Baylor Scott & White Healthcare found that after 90, 180 and 365 days adjusted ORs for de novo heart failure were all more than twofold. Adjustment did not include prior medication or proteinuria.9 A Danish study of 21,556 unspecific patients admitted to intensive care unit reported increasing HR of incident heart failure with increasing severity of AKI, through three years of follow-up. HR of incident myocardial infarction was only increased with moderate to severe AKI.29 In two post hoc studies of prospective cohorts there was also increased risk of cardiovascular events. One was a French study of patients with diabetes,30 the other a study of the high-risk cohort of the SPRINT study.31
The most severe degree of the exposure, i.e. dialysis requiring AKI, has been compared with non-dialysis AKI in a couple of studies. Two studies found no evidence of increased risk of cardiovascular events with dialysis requiring AKI per se,32,33 but in studies also accounting for renal recovery, risk of cardiovascular events was increased (though results were divergent for stroke).34–36
Renal recovery seems an important interacting factor in several of the mentioned studies. A recent meta-analysis on the duration/recovery of AKI reported that renal recovery provided additional prognostic value, particularly for heart failure.37 This aligns with the augmented increase in cardiovascular risk observed in patients with CKD with a steep annual decline in renal function.38 Acute kidney disease describes a suspected continuum from non-recovery AKI toward CKD. In this phase, renal pathophysiologic processes are ongoing that could be responsible for the association with cardiovascular events.39 Another risk factor could be the development or worsening of proteinuria subsequent to AKI, which followed a dose–response pattern, as recently reported.40
Several pathophysiological processes have been suggested. These include “organ crosstalk” with cytokine mediated inflammation and subsequent fibrosis of cardiac cells, activation of the renin-angiotensin system, mitochondrial dysfunction, fluid overload/hypertension and electrolyte disturbances.41 Both pathophysiological processes and renal recovery are probably strongly influenced by the phenotype of the renal insult, i.e. whether structural damage or hemodynamic alteration arises,42 together with the renal functional reserve, i.e. the capacity that can be recruited in periods of increased demands.43 The phenotype can be adjudged by biomarkers, though still not validated to predict short- and long-term adverse outcomes, including cardiovascular disease.44 In a study of patients undergoing cardiac surgery, none of five urinary biomarkers were significantly associated with cardiovascular events, whereas four of five cardiac biomarkers were.23 In mediation analyses, pooled cardiac biomarkers accounted for half of the association, although CI was broad (1–97%). The authors concluded that the association was more likely due to hemodynamic changes or cardiac dysfunction rather than intrinsic kidney damage.23 In this perspective, the kidneys act as a barometer of systemic hemodynamic changes. They have a stronger impact on renal and cardiac function than “organ crosstalk”45 that could explain the strong association of heart failure across studies. This is supported by the reported risk of increased blood pressure after AKI, graded by severity of AKI, which is a plausible mechanism for development of heart failure.46 Existing studies, including our, lack complete information on whether heart failure outcomes were primary in origin or secondary to worsening renal function, since results from echocardiographies were not available. A contributing aspect in this regard is the withdrawal of RASi after an episode of AKI, as a preventative measure, whereby the protective effect on heart failure, as well as any proteinuria, is lost.47
Whether AKI contributes to cardiovascular disease or merely is a marker of subclinical cardiovascular risk/injury remains unknown. Plausibly, some degree of continuous bidirectional influence exists as described in the cardio-renal syndrome, where mutual insults to the two organs creates a vicious circle of deterioration of their respective functions.41 Compromised microcirculation in both vascular beds, e.g. due to systemic atherosclerosis, secondary to shared risk factors such as diabetes and hypertension, could play a role. This has been visualized with ultrasound in unselected patients by the independent association between increased renal resistance index and both central pulse pressure and intracardiac Doppler blood flow indices.48,49
Strengths and limitationsThis study has several strengths. A large number of diverse patients from a general population with equal access to health care from the recent ten years was included. Due to the comprehensive follow-up and validation, demographic sampling bias was limited. Furthermore, we analyzed data in subgroups of age and baseline eGFR, with important covariates adjusted for and plausible interactions accounted for. The exposure was defined by the more sensitive creatinine rather than diagnostic coding.
There were also limitations. We did not have access to the clinical motivations to perform creatinine measurements, although creatinine is part of the standard set of blood samples. We did not know the specific causes of AKI or if any renal recovery occurred, which could have indicated the AKI phenotype and transition to/worsening of CKD. The lengthy time-period for obtaining baseline creatinine measurements made risk of misclassification of rapid deterioration of CKD as AKI possible. Accurate calculation of eGFR with body surface and race was not possible. Despite adjustment for central, validated covariates, residual confounding in exposed versus non-exposed patients could not be ruled out. In addition, we could not account for all cardiovascular risk factors, such as smoking, exercise or degree of hypertension. As comorbidities rely on correct diagnosis coding, their true prevalence may be underestimated, especially in conditions not only diagnosed in relation to admissions or outpatient clinics.
ConclusionIn conclusion, non-selected patients with AKI during admission had significantly higher one-year risk of cardiovascular event or death after discharge, especially but not only due to heart failure, compared to patients without AKI, independent of age and baseline renal function.
Experiencing AKI during a general admission should result in intensified focus on cardiovascular protection after discharge, regardless of any age above 50 years or prior renal function. As survival after hospitalization with AKI is fortunately increasing,50 the risk time of sustaining a cardiovascular event has expanded, underlining the importance of this focus.
EthicsRetrospective, register-based studies do not need prior ethical approval in Denmark. The Danish Data Protection Agency has approved use of data (ref. P-2019-191).
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestMS reports lecture grants from Astra Zeneca, Novo, Bohringer and Novartis. The authors declare no potential conflicts of interest with respect to the research, authorship or publication of this article.