One of the primary functions of dialysis in the treatment of stage five chronic kidney disease (CKD) is the elimination of uremic toxins (TU). The model to follow is the kidney, capable of purifying all types of TU continuously without discarding albumin. The elements of this renal function are the glomerular filtration, the tubular functions and their urinary elimination to the outside. Dialysis is far from emulating these functions, although its purification capacity has improved over the years.
Knowledge of TUs has advanced in the last decades.1,2 They have been molecularly typified, the concentrations they reach in CKD have been measured, and their individual toxicity has been described.1–3 TU retention is related to cardiovascular risk,3–5 the main cause of mortality in dialysis patients. TUs are usually classified by their molecular weight (MW) and their binding or not to proteins, mainly albumin (Table 1).1,2 Recently, some authors propose the division of medium TU into two groups: molecules of medium and high MW,6 placing the limit of these at 15,000 Da, according to different authors. This classification is of particular interest, since these high PM TUs have been associated with some of the main comorbidities derived from CKD and dialysis, 3 specifically, with inflammation and cardiovascular disease. New techniques and hemodialysis (HD) membranes allow the elimination of a greater number of medium-sized molecules than conventional HD.
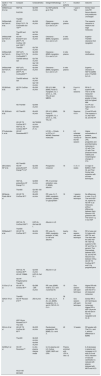
Classification of uremic toxins and clearance by the different techniques.
| Small water soluble molecules | Middle molecules | Protein-bound molecules | ||
|---|---|---|---|---|
| Middle molecules | Large molecules | |||
| PM Daltons | <500 | 500–15,000 | 15,000–60,000 | |
| Urea | Atrial natriuretic peptide | Leptin | P-cresol sulfate | |
| Creatinine | Endothelin | Myoglobin | Indoleacetic | |
| Match | PTH | Light chains κ | Indoxylsulfate | |
| Oxalate | β2 microglobulin | Interleukin 6 | Pentosidine | |
| Purines/ac. uric | Cystatin C | Hepcidin | Neuropeptide | |
| Guanidine (ADMA) | Light chains λ | |||
| TNFα | ||||
| TDE/debugging mechanism | HD-LF, HD-HF/broadcast | HD-HF/broadcast | HDF-OL, HDx/diffusion and convection | Adsorption, HFR/diffusion, convection and adsorption |
| HDF-OL, HDx/diffusion and convection | HDF-OL, HDx/diffusion and convection | |||
OL-HDF: online hemodiafiltration; HD-HF: high flow hemodialysis; HD-LF: low-flow hemodialysis; HDx: extended hemodialysis; HFR: hemofiltration with reinfusion of the ultrafiltrate; MW: molecular weight; TDE: extrarenal purification technique.
At the beginning of dialysis, the dialyzers-membranes available only allowed the removal of low-MW TUs. Low-flux (LF) dialyzers only significantly purify low-PM TU and have a coefficient of hydraulic permeability (Kf) of less than 20 mL/h/mmHg. Later, high flux or high flux (HF) dialyzers appeared with a Kf greater than 20 mL/h/mmHg, capable of eliminating the so-called medium molecules, with a MW of up to 20,000 Da, including β2 -microglobulin (β2 -mG) and leptin. Thus, depending on the permeability of the dialyzer, two types of HD are distinguished, high (HD-HF) and low flow (HD-LF). Currently, we have numerous dialyzers with Kf greater than 50 mL/h/mmHg, called very high hydraulic permeability. The Membrane Permeability Outcome (MPO) study is the greatest exponent of the best clinical results of HF-HD compared to LF-HD.7,8 Techniques with high convective transport and especially on-line hemodiafiltration (OL-HDF) with HF dialyzers, were the next step, managing to purify some of the high-MW TU, avoiding the elimination of significant amounts of albumin. The Online Hemodiafiltration Study (ESHOL) and other randomized trials have demonstrated the superiority in terms of survival of OL-HDF patients over HF-HD and LF-HD.9,10 We could conclude that eliminating the TU of PM larger and in a greater quantity is related to a better prognosis of dialysis patients, independently of other morbidity and mortality cofactors.
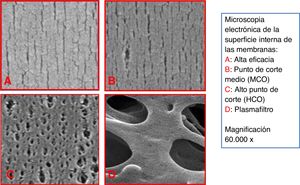
In the last five years, a new type of membrane has been developed, with a higher cut-off point (CO), called mid-cut point (MCO), with the ability to remove high PM molecules, as well as This is done by high CO (HCO) membranes, used in myeloma, but capable of retaining albumin.11,12 These new membranes have a high retention point or “retention onset”. This concept is determined by the PM from which more than 10% of the solutes will be retained, that is, the PM for which the coefficient of screening (Cc) will be 0.9. The size of the pores of the OLS membrane is intermediate between those of the HF and HCO membranes (Fig. 1). In addition, and as we will explain below, these membranes have a dialyzer design that enhances internal convective transport.13 Therefore, with these devices it is possible to obtain a clearance of medium and large molecules superior to that of HF dialyzers (Table 2). HD performed with OLS dialyzers has been called “extended” HD (HDx) because it is capable of increasing the PM range of removed TUs.
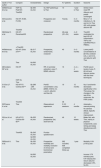
Elimination of uremic toxins measured by RR and clarifications in HDx compared to HD-HF and HDF-OL.
| Quote n. o/1st author | Compare | Characteristics | Design/methodology | n. o patients | Duration | Outcome |
|---|---|---|---|---|---|---|
| 23/Belmouaz M. et al. | HDF-OL | Qb 300 mL/min | OL-HDF patients go to Ther | 10 | 1 year in Ther | Similar mean mole RR |
| Pol210H | Serum albumin and prealbumin unchanged | |||||
| Ther500 | ||||||
| 24/Boschetti-de-Fierro A. et al. | HD-HF: Elisio17H™, FX CorDiax80 and 100™ | Qb 300 mL/min | Clearance : β2MG, myoglobin, CL κ and λ, IL-6 | In vitro plasma | Ther superior clearance in medium and large molecules | |
| Ther400 and 500 | Qd 700 mL/min | |||||
| 24/Boschetti-de-Fierro A. et al. | HD-HF: Elisio21H and 25H™, FX CorDiax 120 and 1000™ | Qb 400 mL/min | Clearance : β2MG, myoglobin, CL κ and λ, IL-6 | In vitro plasma | Superior Ther500 clearance from myoglobin | |
| Ther500 | Qd 700 mL/min | |||||
| 24/Boschetti-de-Fierro A. et al. | HDF (UF): Elisio17H™, FX CorDiax800™ | Qb 300 mL/min | Clearance : β2MG, myoglobin, CL κ and λ, IL-6 | In vitro plasma | Similar clarifications | |
| Ther500 without Quf | Qd 700 mL/min | |||||
| Quf 100 mL/min | ||||||
| 24/Boschetti-de-Fierro A. et al. | HDF (UF): Elisio21H and 25H™, FX CorDiax800™ | Qb 300 mL/min | Clearance : β2MG, myoglobin, CL κ and λ, IL-6 | In vitro plasma | Superior clearance in HDF for β2MG and in Ther500 for IL-6. | |
| Ther500 without Quf | Qd 700 mL/min | |||||
| Quf 100 mL/min | ||||||
| 25.26/Kirsh. et al. | HD FX CorDiax 80™ | Qb 300 mL/min | RR & Cl: MM: β2MG, Factor D, myoglobin, CL κ and λ, α1 -mG, YKL-40 and MBPM. | 39 | Four 4-h HD sessions | RR & Cl superior with Ther in all the measured molecules, greater in those with the highest MW. |
| HD Ther400 | Qd 500 mL/min | Cl of P and higher urea in Ther | ||||
| 25, 26/Kirsch et al. | HD Ther400 | Qb 400 mL/min | RR & Cl: β2MG, Factor D, myoglobin, CL κ and λ, α1 -mG, YKL-40 | 39 | Sessions 4–5 h | Cl and RR with Ther superior in CL than HDF-OL and HD-HF |
| HD HF FX CorDiax 80™ | Qd 700/600 mL/min | |||||
| HDF-OL FX CorDiax 800™ | Quf total 21.4 L | |||||
| 27/Latosinska A. et al. | HD-HF Revaclear400™ | LD 30 L × 16 tests in CETs; LC-MS/MS; Dissolved molecules | 8 | LD collected during the 1st hour of the HD session | Higher concentration of proteins, albumin, B2MG, LPS, proapototic and proinflammatory molecules in LD with Ther. LD with Ther produces worse viability and morphological changes in CTs | |
| HD Ther400 | ||||||
| 28/Cordeiro ISF et al. | HD-HF polysulfoneHF Diacap™ 2 m2 | Qb 350 mL/min | Prospective-crossover | 16 | 4 + 4 + 4 weeks | Cl major of B2MG in HDF-OL and Ther; Similar total extraction with all three techniques. |
| HDF-OL polysulfone HF Diacap™ 2 | Qd 800 mL/min | RR, Cl and total extraction: urea, P, B2MG, albumin | ||||
| Ther400 | Qinf 90–100 mL/min | |||||
| 29/García-Prieto AM et al. | HD-HF FX CorDiax 80™ | Qb 450 mL/min | RR: urea, Cr, P, (2MG, myoglobin, prolactin, cystatin, α1-glycoprotein | 18 | 1 session with each technique | No differences in urea, Cr or P. Ther and HDF-OL superior to HD-HF in all molecules, without differences between them. Albumin 0.03 g/session with Ther and 3.1 g in OL-HDF |
| HDF-OL FX CorDiax1000™ | HDF-OL: Quf total 28 L | Albumin in LD | ||||
| Ther500 | ||||||
| 30/Maduell F. et al. | HD-HF FX CorDiax 80™ | Qb 434 mL/min | RR: urea, Cr, (2MG, myoglobin, prolactin, α1-MG, α1-GP and albumin. | twenty-one | One session with each technique | RR of urea and Cr higher with HDF-OL and Ther than with the other HD-HF; HDF-OL superior to HD-HF and Ther. In general, Ther intermediate between HDF-OL and HD-HF. Albumin loss between 0.54 g HD-HF FX CorDiax 80™ and 3.3 g polyphenylene |
| HDF-OL FX CorDiax 80™ | Qd 400 mL/min | Albumin in LD | ||||
| HD-HF: PMMA | HDF-OL Quf 31 L | |||||
| HD-HF: Polyphenylene | ||||||
| Ther400 | ||||||
| 31/Cho LF. et al. | HD-HF FX CorDiax 80™ | Qb 289 −294 mL/min | RR: urea, β2MG, Vit.B12, CL κ and λ and free Hb | 19 | One session with each technique | Higher RR with Ther400 than with HD-HF in all measured molecules |
| Ther400 | 38 | |||||
| 32/Kim TH et al. | HD-HF Rexeed-21A™ | 250 mL/min | RR: urea, Cr, P, Ac. uric, β2MG, myoglobin, CL κ and λ, FGF-23. Cl: CL λ | 6 | One session with each technique | Similar RR in all 3 techniques for small molecules; Higher RR in HDF-OLpre for B2-mG and in Ther for myoglobin and CL ⌊€. |
| HDF-OLpre Rexeed-21A™ | ||||||
| Ther 400 | ||||||
| 33/Lim JH et al. | HD-HF FX CorDiax 80-60™ | Qb 235 mL/min | Randomized prospective. RR: β2MG, CL κ and λ, | 49 | 12 weeks | RR greater with Ther CL κ and λ, without differences in B2MG |
| Ther400 | Qb 245 mL/min | |||||
| Qd 500 mL/min | ||||||
| 34/Willy K et al. | HD-HF: Revaclear™ | In vitro plasma dialysis model | IL-6 in plasma and LD; Culture VSMC, MGP and OPN | Plasma enriched with LPS in vitro x 2 | IL-6 decreases in plasma and increases in LD with OLS and HCO; induction of vascular calcification is reduced with plasma dialysed with MCO and HCO | |
| HCO1100 | ||||||
| MCO400 |
α1-GP: α1-acid glycoprotein; TSCs: tubular epithelial cells; Cl: clearance; CL: free κ or λ light chains ; Cr: creatinine; Hb: hemoglobin; HCO: high cut-off dialyzer; HD: hemodialysis; HDF: hemodiafiltration; OL-HDF: post-dilution line HDF; OLpre-HDF: predilution line HDF; LC-MS/MS: liquid chromatography/mass spectrometry; LD: dialysis fluid; LPS: lipopolysaccharides; MBPM: low molecular weight molecules; OLS, mid-cut-point dialyzer; MGP: Gla protein; MM: medium and high MW molecules; OPN: osteopontin; P: phosphates; Pol 210H: Polyflux 210 H™; Qb: blood flow; Qd: Dialysis liquid flow; Quf: Ultrafiltration flow; Ther: theranova™; RR: reduction percentage; VSMC: Vascular Smooth Muscle Cells.
The discrepancy regarding the elimination of uremic toxins is explained by the different methodology of the studies.
The objective of this review is to describe the characteristics of OLS membrane dialyzers, their performance in terms of TU elimination, preliminary clinical results, and to position HDx among HD techniques today.
Technical development of dialyzers and dialysisThe evolution of dialyzers has been a key piece in the development of HD. In this evolution, they have highlighted a series of markers of its effectiveness in eliminating TU. Classically, to assess the elimination of low PM TU, the Kt/V or better the Kt corrected by body surface (Ktsc) is used.14 The transition from HD-LF to HD-HF was marked by the Kf titration of the dialyzers, greater than 20 mL/h/mmHg in those of HF.
Convective transport is assessed by the total ultrafiltered volume (VTU) and the Cc coefficient for the different molecules,15 being the Cc of β2 -mG the most used. In this sense, the EUDIAL16 group marked among the requirements to define an effective OL-HDF, to use a dialyzer with a Cc for β2-mG greater than 0.6 and a VTU per session greater than 23 L. The importance of the amount of convective volume administered is due to the fact that it has been directly related to the clearance of medium molecules,17 and as previously commented, with the mortality of HD patients.
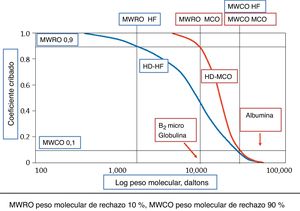
To typify dialyzers with MCO membranes, two markers11 have been used, the retention point or “retention onset” (MWRO) and the cut-off point (MWCO): the first is determined by the MW of the molecule whose Cc is of 0.9 or expressed in another way, the MW of the molecules that begin to be retained by 10%, and the second, the MW that corresponds to a Cc of 0.1, close to the CO of the evaluated membrane (Fig. 2). These two markers have been used in the Cc curve for a membrane/dialyzer, because determining the MW with which a molecule begins to be retained and the MW of CO is very difficult. OLS dialyzers would have a Cc for β2-mG close to 1 and 0.9 for myoglobulin.
Cc in dialyzers depend on the conditions under which they have been measured. The type of plasma, blood flow (Qb), ultrafiltration flow (Quf) and the time it took to obtain the sample are some of the factors that determine the result.15 The comparison of dialyzers, using their Cc is only reliable if the measurement conditions are similar. In the technical data sheet of the devices, it must be specified under which conditions the Cc has been measured.
The MCO membrane has been used in Theranova® (Ther) dialyzers, available in Spain for three years. Their design favors internal filtration/retrofiltration, as a form of purification by convective transport,18 which has been called internal hemodiafiltration (HDFi).19 One of the ways to increase HDFi is to decrease the internal diameter of the capillaries, up to 180 µ in diameter in these dialyzers, to increase internal resistances and achieve greater filtration/back-filtration. The internal radius of the capillary is listed to the fourth power in the Hagen-Poiseuille equation that calculates the resistance of the blood to the passage through the capillaries. A long, narrow dialyzer with a capillary diameter of 180 µ will cause a very large pressure drop within the capillary, of around 170 mmHg for 400 mL/min of Qb. Another way to increase internal filtration is to increase the hydraulic resistance in the dialysis fluid (LD) compartment, increasing the density of the capillaries inside the dialyzer, between 55 and 60%,20 creating a pressure drop of 30 mmHg to 500 mL/min of LD flow (Qd). In internal filtration, Qb and Qd directly influence.
Although internal convective transport has been enhanced in the new MCO dialyzers, recent studies suggest that diffusive transport is the main mechanism for elimination of medium molecules in HDx.21 Always remember the interference of these two types of transport on the same membrane.
A requirement in the development of MCO membrane dialyzers was that they did not lose a significant amount of albumin. The high permeability must be compatible with a minimal elimination of this protein, with a Cc less than 0.01. The loss of albumin is dependent on the size of the pores, its interaction with plasma and the transmembrane pressure (PTM). In OL-HDF, the prefilter pressure can reach 700 mmHg, being the main determinant of the loss of albumin. Dialyzers with OLS membranes should not be used at these pressures, so their use is contraindicated in OL-HDF and isolated ultrafiltration. With the HDF technique, OLS membranes can cause significant albumin losses. In any hemodialysis technique, the loss of this should be less than 4 g per session.6
To assess the effectiveness of OLS dialyzers, we must continue to use Kt/V or Ktsc. In the clinic we cannot measure HDFi, although it has been estimated that for an MCO dialyzer, Ther400, with a Qb of 400 mL/min it would be 41.6 mL/min and 53.1 mL/min with the Ther500,6,22 which would mean a four-hour HD session with an internal filtration of 9,984 mL and 12,744 mL, respectively, to which the programmed ultrafiltration would have to be added. Taking into account that the Cc of this dialyser for β2-mG is >0.9, a clearance of β2-mG similar to that of OL-HDF can be calculated. The Qb, therefore, would also be an important determinant of the purifying efficiency with these dialyzers. The preHD concentration of β2-mG could be useful, and should be kept between 20 and 30 mg/L, except in situations of hyperproduction of β2-mG. With OLS dialyzers, Kf no longer has the importance of HF dialyzers; the Kf and Cc relationship is not good.
The use of HF-HD and OL-HDF has necessitated other advances in dialysis. Among them, the machines with precise ultrafiltration (UF) control, the LD with bicarbonate and ultrapure quality stand out. HDx also requires a dialysis monitor with these advances, although, unlike OL-HDF, it does not require a LD infusion system tied to UF monitoring. The HDx can be performed with any modern HD machine with endotoxin filters.
Uremic toxin clearance and clinical evidence of HDxThe two dialyzers available in Spain with an MCO membrane, composed of polyarylether sulfone and polyvinylpyrrolidone, free of bisphenol A (BPA), are the Ther400 and the Ther500. Both have an internal diameter of the capillaries 180 µm 35 µm thick wall capillary. The Ther400 has a surface area of 1.7 m2 and a Kf of 48 mL/h/mmHg and the Ther500 2.0 m2 and a Kf of 59 mL/h/mmHg (in vitro with bovine blood, Ht. 32 % Pt 6 g/dL and 37 °C). The Cc is 1 for β2 -mG, 0.9 for myoglobin and 0.008 for albumin (measured with human plasma and Qb 300 mL/min and UF 60 mL/min).
The clearance of TU with these dialyzers has been compared with that of the HF-dialyzers and with OL-HDF (Table 2).23–34 HD-HF has similar or slightly lower results regarding the elimination of molecules of low PM in contrast to HDF-OL and HDx, measured by the proportion of the decrease in the concentration of the molecules before and after HD (RR) or by clearing it. Regarding the medium molecules, post-dilution OL-HDF and HDx are superior to HF-HD and if OL-HDF is performed with high convective transport, OL-HDF is superior to HDx. Regarding large molecules, as the MW increases, HDx surpasses OL-HDF, as for example with λ light chains.
The characteristics of dialysis that make OL-HDF superior to HDx have been evaluated.34 With a Qb of 350 mL/min, OL-HDF exceeds HDx in terms of the percentage reduction (RR) of the molecules, evaluated by means of a « Global Removal Score » (GRS), from 80.6 mL/min of Quf, with a Qb of 400 mL/min it would be from 74.1 mL/min. The main problem with this comparison is in the composition of the GRS, which includes, among other molecules, urea. If the RR of large molecules, such as λ light chains and interleukin-6 (IL-6), is analyzed, HDx would be superior to OL-HDF.24–26 The influence of the different large TUs on morbidity and mortality remains to be weighed.
With OLS dialyzers, albumin loss in LD is usually greater than in HF-HD and OL-HDF, in any case less than 3.5 g/session, between 0.03 and 3.15 g/session.25,29,30,32,35,36 Albumin loss is dependent on the type of membrane and transmembrane pressures used and can exceed HDF-OL 10 g/4 h with some dialyzers.37 In HDx patients, serum albumin is maintained after an initial drop.24,38 At 12 weeks and 12 months with OLS membranes, no significant changes in albuminemia have been detected.31,33 In the work of Bunch et al.39 performed in 638 patients, after one year, found a decrease of 3.5%.
One question that arises with MCO membranes is whether, while they cleanse more TU of medium and high MW than HD-HF, they remove more clotting factors, nutrients, drugs, and other molecules that are beneficial to the body. An in vitro study suggests that the change from HD-HF to Ther500 does not require variations in anticoagulation or in the adjustment of a drug such as vancomycin.40 The changes in insulin and erythropoietin concentrations would be similar with Ther500, HD-HF with Polyflux210H™ and HDF with Fx CorDiax 800™.40
Although the ability of MCO dialyzers to retain endotoxins and other pyrogenic substances has been reported, 41,42 given the significant back-filtration of these dialyzers, it is reasonable to use ultra-pure LD.
Clinical results with HDxThere is little clinical evidence in the medium and long term with HDx (Table 3).23,28,31,33,38,43–45 The study that includes the most patients is the Colombian COREXH Registry,39 there they recruit 992 patients dialysed with HDx and 638 complete one year of follow-up (Table 3). They have a mortality of 8.54 per 100 patient-years, low compared to other similar studies with other HD techniques.46,47 The admission rate would be 0.79/patient-year and 6.91 days/patient-year.39 No adverse effects have been described with HDx,25,39,43 nor hypersensitivity reactions to synthetic membranes/polysulfones.48 However, because it is a synthetic membrane, these hypersensitivity reactions may occur.
Clinical aspects with HDx.
| Quote n.o/1st author | Compare | Characteristics | Design | N.o patients | Duration | Outcome |
|---|---|---|---|---|---|---|
| 23/Belmouaz M. et al. | HDF-OL Pol210H | Qb 300 mL/min | OL-HDF patients go to Ther | 10 | 1 year in Ther | Similar mean mole RR |
| Ther500 | Albumin and prealb unchanged | |||||
| 43/Cozzolino et al. | HD-HF: (Fx80, Fx100) | Prospective and crossover | Twenty | 3 + 3 months | Minor n.o of infections, IL-1β and IL-6 in Ther 400, with greater albumin fall | |
| Ther400 | ||||||
| 38/Zickler D. et al. | HD-HF: Revaclear400 | Randomized prospective | 48 ends (23 + 24) | 4 + 8 weeks | Ther400 modulates the best HD-HF inflammation: TNFα and IL6mRNA-RQ in PBMCs. | |
| ≈Ther400 | ||||||
| 44/Belmouaz M. et al. | HD-HF: Elisio 21H™ | Qb 317 mL/min | Prospective, randomized, crossover | 40 | 3 + 3 months | Lower preHD concentrations of β2-mG, oxidized LDL, κ and λ CL and albumin with Ther |
| Ther | Qd 500 mL/min | |||||
| 28/Cordeiro ISF et al. | HD-HF | RR, Cl and total extraction: urea, P, B2MG, albumin | 16 | 4 + 4 + 4 weeks | PreHD serum levels of urea, P, β2-mG and albumin similar with the three techniques. | |
| HDF-OL | ||||||
| Ther | ||||||
| 31/Cho LF. et al. | HD-HF FX CorDiax 80™ | Qb 289 −294 mL/min | PreHD concentration: urea, β2MG, Vit.B12, CL κ and λ, albumin and free Hb | 19 | 12 months | Na, K and ferritin decreased significantly in the Ther group. No changes in the rest of the molecules. No FRR control. |
| Ther400 | 38 | |||||
| 53/Florens N. et al. | Ther | Observational experience | 5,191 sessions | 4 months | Automatic + manual priming | |
| No adverse effects. Specific clinical improvement. | ||||||
| 33/Lim JH. et al. | HD-HF FX CorDiax 80-60™ | Qb 235 mL/min | Randomized prospective: QoL short form 36. | 49 | 12 weeks | Better QoL with Ther, mainly in physical components. Less itching and sleep disturbances with Ther |
| Ther400 | Qb 245 mL/min | Pruritus questionnaire | ||||
| Qd 500 mL/min | ||||||
| 39/Bunch A. et al. | Ther | Qb 350 mL/min | Prospective morbidity and mortality. Evolution of clinical and biochemical parameters | 992 included, 638 completed follow-up | 1 year | Mortality: 8.54 p/100 p-year |
| Qd 500 mL/min | Income: 0.79 events/p-year; 6.91 days/p-year. Serum albumin decreases 3.5%. No EA |
β2-microglobulin: β2-mG ; CL: free light chains; CQ: cytokines; AE: adverse effects; RRF: residual renal function; Hb: hemoglobin; HDx: extended hemodialysis; IL6mRNA-RQ: IL-1β; IL-6: interleukin; 6 Oxidized LDL: oxidized low-density lipoprotein; P: patients; PBMCs: peripheral blood mononuclear cells; Pol 210H: Polyflux 210 H™; Qb: blood flow; Qd: dialysis fluid flow; QoL: quality of life short questionnaire 36 items; Quf: ultrafiltration flow; RR: reduction percentage; Ther: Theranova; TNF-α: Tumor-necrosis factor alpha; β2-mG: β-2-microglobulin; IL-6: interleukin-6; mRNA: messenger RNA; PBMC: peripheral blood mononuclear cells.
The perceived quality of life has been assessed in a prospective study33 in which 49 patients were randomized, 24 were dialyzed with Ther400 and 25 with HD-HF with the FX CorDiax 80-60™. Basically and at 12 weeks, the Kidney Disease Quality of Life Short Form-36 (KQDOL-36) test with 36 items was performed. Ther400 patients expressed a better quality of life, mainly in the physical components/domains, as well as less itching and sleep disturbances.
Cho et al.31 did not find significant differences in the preHD concentration of TUs between patients dialysed with Ther400 and those with FX CorDiax 80 after 12 months. The low serum concentrations of β2-mG, 25.6 mg/L, which rise to 28.4 mg/L per year are noteworthy; these concentrations are common in OL-HDF or when there is significant residual renal function (RRF). The work does not provide this last data, which could mask the effect of the Ther400 through its decrease over time.
With HDx, an improvement in pro-inflammatory parameters has been reported.38 One of the factors that explain the loss of RRF in dialysis is inflammation.49 Some UT with high PM would be harmful to the renal tubules and to RRF2,3,27; their greater clearance by the MCO dialyzers27 could better preserve RRF, which will have to be investigated.
The induction of vascular calcification is reduced with plasma dialyzed with MCO and HCO,34 so a possible beneficial role of HDx to avoid vascular calcifications should be studied.
At the close of this review, there are 16 ongoing HDx studies, registered in the ClinicalTrials.50 Six of these are complete and eight are in recruitment. In six HDx was compared with HD-HF, in four with HDF and in one with both techniques. Some focus on specific aspects such as: anticoagulation, preservation of RRF, calcifications and mineral metabolism or symptoms. Among them is the Spanish multicenter, open, prospective, randomized study MoTHER to explore morbidity and mortality in dialysis patients with HDx compared with OL-HDF.
Key features of the HDxWhat makes HDx different from other forms of HD? Its ability to remove large molecules, high PM TU (Table 1). The HDx can be defined as a high Cc HD, tuning further, with a MWRO in the range of high PM TUs. Therefore, to conceptualize it and differentiate it from conventional HD-HF, the Cc of myoglobin, ≥0.9, should be used. The HDx is therefore a high-screening HD. Myoglobin is a 17,000 Da molecule that is easy to measure and belongs to the low range of high MW molecules.
Where to position HDx as a dialysis techniqueThere is little clinical evidence to determine what types of patients may benefit the most from HDx. Based on previous experience with other high-level TU debugging techniques, such as OL-HDF, we could propose some aspects to check in future studies. 1. Patients without a significant RRF; 2. Patients with the prospect of staying on hemodialysis for years, for example, not candidates for kidney transplantation; 3. Patients with a sufficient intake of nutrients; 4. Patients with a lot of comorbidity; 5. As an alternative to OL-HDF in cases where high convective transport cannot be guaranteed (elevated Hb, suboptimal Qb).51 The cases with greater comorbidity that could benefit from HDx would be those that have a clear relationship with the retention of high-PM TU: chronic inflammation; resistance to EEE; Restless leg syndrome, secondary immunodeficiency, and cardiovascular disease.52
Regarding the competition with OL-HDF, HDx would be useful in patients in whom it is not possible to achieve an adequate convective volume per session (23 L) or when OL-HDF is suspended for safety reasons.
Good results have been reported in people with certain pathologies 43,53: pruritus,33 post-HD asthenia, anorexia, restless legs syndrome, light chain myeloma,54 rhabdomyolysis, severe inflammation. Some of these indications coincide with our own experience.
The superiority of HDx over HD-HF in eliminating high PM TU, its easy implementation and its safety suggest creating a new section in the classification of HD techniques. The HDx is not a “conventional” HD in the sense of being within established standards. With the existing data, the HDx should be in a new category of HD. Morbidity and mortality clinical studies are needed to demonstrate the non-inferiority of HDx over OL-HDF.50
FinancingThis work has not received any type of funding.
Please cite this article as: Perez-Garcia R, Alcazar-Arroyo R, de Sequera-Ortiz P. ¿Cuál es el papel de la hemodiálisis extendida en el tratamiento renal sustitutivo en 2020? Nefrologia. 2021. https://doi.org/10.1016/j.nefro.2020.11.007