La insuficiencia cardíaca (IC) y el fracaso renal agudo (FRA) son dos entidades muy prevalentes en nuestro medio, e inciden de manera directa y sinérgicamente en la morbimortalidad de nuestros pacientes. Cuando es oligoanúrico, el FRA suele conducir a la sobrecarga hídrica, representando esta el núcleo precipitante del mecanismo de descompensación aguda de la IC, y está asociada con el agravamiento de los síntomas, la hospitalización y la muerte. Determinar el balance hídrico en la IC puede ser complejo y depende, en gran medida, de la fisiopatología subyacente. Los nuevos biomarcadores y las nuevas tecnologías están demostrando ser útiles para la detección e identificación de riesgo de IC descompensada aguda que puede permitir una pronta intervención y reversión del FRA que se traduzca en mejores resultados clínicos.
Heart failure (HF) and acute renal failure (ARF) are two very prevalent entities in our environment which impact directly and synergistically in the morbidity and mortality of our patients. ARF, when oligoanuric, often leads to water overload. It represents the precipitating core of the mechanism of acute decompensation of the HF and is associated with the worsening of symptoms, hospitalisation and death. Determining the water balance in HF can be complex and depends, largely, on the underlying pathophysiology. New biomarkers and new technologies are proving to be useful for the detection and identification of risk of acutely decompensated HF that may allow early intervention and reversal of the ARF that translates into better clinical outcomes.
Introduction
Heart failure (HF) and acute renal failure (ARF) are common and they are associated with great consumption of health resources as well as a considerable morbidity and mortality.1-5 In this review, we attempt to provide a general vision of the pathophysiology of fluids accumulation focused on HF and ARF, as well as the importance of the evaluation of water balance on these syndromes and its correlation with the results.
In HF, fluid overload defined as a positive cumulative balance or acute fluid redistribution, represents the precipitating core of the mechanism of acute decompensation and is associated with a worsening of symptoms, hospitalisation and death. Determining the water balance in the HF can be complex and depends largely on the underlying pathophysiology. However, besides the simple balance (inflows minus outflows), new biomarkers (B natriuretic peptide) and new technologies (impedance) are proving to be useful for the detection and identification of acute decompensated HF risk. This advantage may allow early intervention which can account for better clinical outcomes. Recent data have showed the importance of water balance in adults and paediatric ARF patients. In general, a positive balance predicts increased morbidity and a higher risk of poor clinical outcome. Thus, water balance should be recognized as a potentially modifiable biomarker that is determinant of clinical outcome in these patients.
To date, the impact of water balance in both syndromes (even more in ARF) has been underused despite it being reproducible, inexpensive and not requiring equipment. There are few data specifically on water balance in cardiorenal syndrome, in which acute/chronic heart disease can contribute directly to the acute/chronic worsening of renal function and is likely to exacerbate water homeostasis. 6-8
HEART FAILURE
Epidemiology
Acute HF is a growing public health problem. More than 5 million adults in the United States and another 10 million in Europe are diagnosed with HF.2,4 The incidence of HF increases markedly in older age, exceeding 8-15 cases per 1000 inhabitants in people ≥ 65 years. HF is commonly associated with pre-existing coronary disease, hypertension and diabetes mellitus. It currently represents the most frequent reason for hospitalisation (approximately 20% of all admittances) among patients ≥ 65 years.2 Moreover, the hospitalisation and readmission rates due to HF continue to increase, contributing to a projected economic burden of nearly $35 million just in the United States.2,9 Hospital mortality due to acute HF ranges between 4% and 8%10-13, but in survivors mortality rates are 8%-15% at 3 months.10,14,15 At 3 months after initial hospitalisation, readmission estimated rates range between 30% and 38%.12-14,16 In other words, while the prognosis of HF has improved with therapeutic advances, the attributable mortality remains high, and the absolute number of deaths due to HF is increasing.17
Pathophysiology
Numerous factors interact and contribute to the pathophysiology of acute HF. The accumulation of fluid is perhaps one of the most important mechanisms in acute decompensated heart failure (ADHF), directly contributing to worsening ADHF clinical symptoms that end up in hospitalisations.18 HF is a progressive disorder that occurs in response to an acute or chronic trigger (myocardial infarction, left ventricle [LV] pressure/volume overload, family history of cardiomyopathy) that damages the heart muscle. This results in loss of functioning cardiac myocytes and/or interruption of normal myocardial contractility.19 This decrease in pump function represents the common denominator in the HF pathophysiology. In response, a series of compensatory mechanisms that modulate and/or temporarily restore left ventricular function are activated, though eventually become maladaptive. These maladaptive changes contribute to fluids accumulation and to clinical symptoms of the disease.
The increase in diastolic pressure, LV dilation and relative terminal-organ hypoperfusion activate the sympathetic nervous system (SNS), the renin-angiotensin-aldosterone system (RAAS) and stimulate non-osmotic release of arginine vasopressin. These contribute directly and/or worsen the existing fluid imbalance by avid sodium retention and decrease of free water excretion to maintain cardiac output.
Diastolic pressure/volume overload contributes to coronary hypoperfusion and subendocardial ischaemia. In addition, increased expression of inflammatory cytokines (tumour necrosis factor) has been observed in patients with HF. Together, these compensatory mechanisms contribute to LV remodelling, myocardial dilation, valvular regurgitation and eventually deterioration of LV function. These mechanisms also predispose to ARF and can lead to chronic renal failure.
The decrease in glomerular filtration rate (GFR) is an important factor in worsening HF, further reducing the patient's ability to manage fluid homeostasis, decreasing responsiveness to key therapies (loop diuretics) and contributing to fluids accumulation. The HF mechanisms mentioned above describe most commonly patients with chronic HF who have acute decompensation in which fluid accumulation may have occurred more gradually. It is likely that these patients already have a positive fluid balance. Recently an additional subtype of acute HF, called acute vascular insufficiency, has been identified.20,21 The pathophysiology underlying acute vascular insufficiency is characterised by acute arterial hypertension, increased systemic vascular resistance and aortic impedance. The fluid accumulation in this situation is more acute and may be more the result of fluid redistribution from peripheral circulation to pulmonary circulation, manifesting as acute pulmonary oedema.20,21 These patients may or may not present a positive balance. The fluid accumulation, defined as both a positive water balance and acute fluid redistribution, represents a fundamental mechanism in HF decompensation leading to hospitalisation. The management of HF patients requires recognition of the importance of water balance as a biomarker of the disease severity and progression.
Fluid accumulation in heart failure
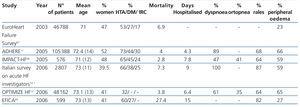
It can be detected and monitored in heart failure patients by different methods, including physical examination, biomarkers and new technologies, many of which correlate with clinical presentation of decompensation. Table 1 shows a summary of the clinical signs and symptoms of fluid accumulation in patients with HF. These may include history of fatigue, dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea, and weight gain. However, changes in body weight are a relatively insensitive predictor of fluid accumulation and subsequent acute decompensation. Physical examination, any evidence of pulmonary rales, jugular venous distension, third heart sound (S3), pleural effusion, ascites, peripheral oedema and pulmonary venous congestion on chest x-ray all suggest clinically significant fluid accumulation or redistribution. In a systematic review of patients who are treated in hospital emergency departments due to dyspnoea, Wang. et. al.22 find five clinical factors that increased the likelihood of HDAF: previous history of HF, symptoms of paroxysmal nocturnal dyspnoea, third heart sound, venous congestion in the chest x-ray and evidence of atrial fibrillation on the electrocardiogram. While these clinical features involve fluid accumulation, the water balance calculations in ADHF patients at the time of presentation can be difficult, and in outpatients such information is not available. Thus, the water balance can be accepted as a biomarker of HF severity and as treatment response in hospitalised patients.
Several biomarkers, specifically atrial natriuretic peptide and B-type (BNP, NT-proBNP)23 peptide have proved useful for diagnosis, early risk stratification, detection of acute decompensation and to guide or adjust HF treatment through serial measurements.24 Plasma levels of natriuretic peptides are positively correlated with LV telediastolic volume and pressure (which is inversely proportional to LV systolic function) and clinical outcomes. In a systematic review of 19 studies of patients with HF using BNP to estimate the risk of heart failure events or death, Doust et al.25 found that each BNP increase of 100pg/ml was associated with a corresponding increase of 35% in relative risk of death. Such biomarkers are a significant promise for improving clinical outcomes compared with adjustment of therapy based on clinical findings only.26 In a small randomized trial of 69 patients with impaired LV systolic function, the HF treatment guided by serial measurements of BNP, compared with a clinical algorithm applied rigorously, was associated with a significantly lower rate of HF events and death.26 Some clinical trials have shown that HF management guided by BNP was associated with more frequent medical evaluations, more frequent treatment adjustment and reduction of hospitalisations, particularly in patients <75 years.27-29 Finally, in a cohort study of 182 patients consecutively admitted with ADHF, Bettencourt et al.30 stratified patients into three groups based on the relative changes in the values of NT-proBNP at the time of admission and discharge (≥30% decrease, no significant change; ≥30% increase). In multivariate analysis, clinical evidence of fluid overload and changes in NT-proBNP were the only factors independently associated with death or re-hospitalisation over the next 6 months.
BNP measurement is playing an increasingly important role in contributing to diagnosis, risk identification and follow-up of treatment in patients with HF. However, BNP values are probably context-specific and require individualization because of variability among subjects. More studies are needed to further our understanding of the role of BNP in HF.
Recently, we have developed new technologies, such as implantable devices and cardiac non-invasive impedance, in order to better monitor water balance, fluid redistribution and early detection of fluid accumulation in patients with HF. This allows us to guide treatment and reduce hospitalisations. In a prospective pilot study of 32 patients with chronic HF, Adamson et al.31 implanted a unicameral pacemaker in the right ventricle (RV) like a continuous haemodynamic monitor. It was supposed to assess whether changes in right ventricular haemodynamics can guide HF therapy and predict clinical deterioration. The pressure increase measured by the device predicted HDAF approximately four days before the event.31 More specifically, for 36 incidents of volume overload, right ventricular systolic pressures increased by 25% (P<.05) and heart rate by 11% (P<.05) compared to baseline. In 33 class III and IV patients of the New York Heart Association (NYHA), Yu et al.32 implanted a pacemaker capable of measuring intrathoracic impedance as a diagnostic complement to detect pulmonary fluid accumulation. Patients were monitored serially and, during admittance, water status and pressure of pulmonary artery wedge were registered. In 10 patients who required hospitalisation for fluid overload, intrathoracic impedance was shown to be reduced by 12%, on an average of 18 days before acute decompensation and hospitalisation. This change in impedance correlates inversely with the pressure of pulmonary artery wedge and fluid balance.32 In a prospective observational study of 212 patients with stable chronic HF, Packer et al.33 performed a serial clinical evaluation and a blind cardiac impedance to obtain markers for lung fluid accumulation every two weeks for 26 weeks. They recorded the appearance of ADHF, hospitalisation due to HF or death. They found that three cardiac impedance parameters (speed index, LV ejection time, thoracic fluid content index) were combined in a powerful predicting score for an event to occur during the first 14 days (P<.001). The water balance and/or redistribution, measured and defined by these devices, demonstrated the diagnostic and therapeutic potential of dynamic continuous and/or serial monitoring of HF.
In a small clinical study of patients with critical pulmonary oedema, defined as a high extravascular lung water (>7ml/kg) measured by pulmonary artery catheterization, it was found that a positive fluid balance greater than 1l in over 36h is associated with increased mortality, longer duration of mechanical ventilation and length of stay in the ICU and hospital34. This study was one of the first to show that water balance measurement is clinically relevant. It also suggested that adopting a strategy to achieve a negative or neutral balance in this population may improve clinical outcomes without risking the haemodynamic profile of the patient or precipitating organ dysfunctions such as ARF. This has been subsequently confirmed in larger studies of critically ill patients with acute lung injury.35,36
ACUTE RENAL FAILURE
Epidemiology
ARF is also a very common clinical problem that portends a significant increase in morbidity, mortality and health resource utilization.37-42 Numerous epidemiological studies have provided a wide range of estimates on the incidence of ARF; however, these inferences have often been limited due to the lack of a standard definition and the selection process of the studied populations.43-45 Several recent multicentre cohort studies have used the ARF RIFLE criteria (acronym for risk, injury, failure, loss, end stage renal disease, a new definition agreed upon for the classification scheme) and have reported that the incidence of ARF is 36%-67% of all patients admitted to intensive care.38,46-50 Subsequent research has found that the incidence of ARF keeps growing1,3,5, which is consistent with results (32.5 ARF/month) in our hospital (unpublished observations). It is likely that the increasing numbers of ARF cases is partly attributed to a demographic change (older population, risk modification by concomitant comorbid conditions), presenting also more severe diseases (multiple organ failure) and to the development of ARF with new and more complex interventions (cardiac surgery, organ transplant).51 ARF leads to deterioration of the fluid and electrolyte homeostasis, and is frequently associated with fluid overload (FO) or it can cause it.
Pathophysiology
Several mechanisms contribute to positive fluid balance in ARF, particularly in the context of critical illnesses. A "trigger" event, that in critical situations may be multifactorial (sepsis, nephrotoxins, intra-abdominal hypertension), causes ARF which is characterised by a rapid and sustained decrease in GFR. This is manifested clinically by an increase in serum creatinine and a progressive diuresis reduction, which alters electrolyte homoeostasis and markedly reduces the capacity to excrete free water and solutes. However, this reduction may go unnoticed unless a rigorous balance control is performed. This retention may be exacerbated by increased activation of the SNS, the RAAS and stimulation of non-osmotic release of arginine vasopressin. In critical illnesses, shock and systemic inflammation contribute to less effective circulation, reduced oncotic pressure gradient (hypoalbuminemia) and capillary permeability alterations which leads to an active increase of the inputs (resuscitation, intravenous medication) and a major leak from the vascular compartment (Figure 1). Recent data have also shown that the ARF can contribute to systemic inflammation and lead to distant organ dysfunction.52-53
In an experimental model of ARF by ischaemia/reperfusion injury (IRI), Rabb et al.53 showed significant suppression of the expression of sodium channel lung receptors, Na-K-ATPase and aquaporin in ARF versus controls. In a similar model IRI, Kramer et al.52 found that the ARF is associated with increased pulmonary vascular permeability within 24 hours of the injury, which correlates with changes in renal function. Both studies have important implications on how ARF may influence or exacerbate acute lung injury, contributing to the accumulation of extravascular lung water (in addition to fluid therapy in the management of ARF). In a small cohort study with critically ill septic patients with ARF, Van Biesen et al.54 showed that in patients with apparently optimal haemodynamics, intravascular volume restoration and a high rate of diuretics usage failed (after fluid therapy) to improve renal function and led to unnecessary fluid accumulation and worsening gas exchange. Fluid accumulation and overload can also affect renal function, worsening ARF. For example, fluid overload can contribute to or worsen intra-abdominal hypertension, particularly in critically ill patients with trauma or burn patients. It can lead to further reductions in renal blood flow, venous return, renal perfusion pressure and diuresis.55 Mechanical ventilation and positive end-expiratory pressure can alter renal function by increasing intra-thoracic pressure. This can contribute to the accumulation of fluid through the stimulation of an array of haemodynamic responses, neural and hormonal acting on the kidney to reduce perfusion, GFR and inhibit excretion function.56,57 Furthermore, the injury by mechanical ventilation (barotrauma, biotrauma, volutrauma, atelectrauma) can induce renal tubular cells to apoptosis and ARF.58 Finally, the positive balance of fluid in risk patients may precipitate acute reductions in cardiac function and exacerbate HF.59
Fluids accumulation in acute renal failure
Several clinical studies in critically ill children with ARF have consistently identified water overload as an important independent factor associated with mortality60-63 (Table 2). Furthermore, the severity of the overload has been shown to correlate with poor clinical outcomes. Goldstein et al.62 evaluated 21 children with FRA and found a higher percentage of water overload at the time of initiation of the continuous renal replacement therapy (CRRT), independent of the severity of the disease, and independently associated with poor survival. The formula used to calculate the percentage of fluid overload was the following:
% WO=[(total incomes-total outcomes)/weight at admittance x 100]
This finding was later confirmed in additional studies (a retrospective single-centre study and a prospective observational multicentre study) in critically ill children with multiple organ failure and ARF.60,63 In another retrospective review, Gillespie et al.61 showed that WO%>10% at the beginning of the CRRT is associated independently with mortality (hazard ratio [HR] 3.02, confidence interval [CI] 95% 1,5 - 6.1, P=.002). In a recent follow-up of 51 children who received a stem cell transplantation, and who ended up requiring admission to Intensive Care Unit (ICU) and ARF, 88% required CRRT for handling fluid overload (average percentage of WO at the beginning of 12.4%).64 These data make a strong case regarding the survival benefit that supports the early initiation of CRRT to prevent WO.
In a secondary analysis of the onset of sepsis in acutely ill patients, Payen et al.65 examined the influence of fluid balance on survival of critically ill patients with ARF. In that study, the comparison was based on whether patients developed ARF (defined by a score ≥ 2 in the sequential evaluation of renal failure) or a urine output <500 ml/day. Of the 3147 patients included, 1120 (36%) developed ARF; 75% of them did so within the first 2 days of ICU admission. The 60-days mortality was higher for patients with ARF (36% vs. 16%, P<.01). In patients with both early and late ARF, the average daily water balance during the first 7 days in ICU were significantly more positive compared to patients without ARF (P<.05 for each day). Similarly, the average daily balance was significantly more positive in those with oliguria (620ml vs. 270ml, P<.01) and those receiving renal replacement therapy (RRT) (600ml vs. 390ml, P<.001). The average daily balance was significantly higher for non-survivors compared to survivors (1000ml vs. 150ml, P<.001). By multivariate analysis, a positive fluid balance (l/24h) was independently associated with 60-day mortality (HR 1.21, 95% CI 1.13 to 1.28 P<.001). While no data were available on water balance during RRT, those who received it early (<2 days after admission to ICU) had a lower 60-days mortality (44.8% vs. 64.6%, P<.01 ), despite more prominent oliguria and increased severity of disease. This study has limitations such as the fact that it was not a randomized trial therefore the observed associations are prone to selection bias, random error and confusion.
In a study of 610 critically ill patients with ARF included in the PICARD database,39 Bouchard et al.66 evaluated the association of fluid overload with renal recovery and mortality. Complete data on fluid intake, output and balance from the 3 days prior to inclusion to hospital discharge were available for 542 patients (88.9%) of the cohort. The accumulated balance was standardized by weight at time of hospital admission and defined as described by Goldstein et al.62 Fluid overload was defined as the percentage of fluid accumulation above 10% of the baseline body weight. The areas of interest were the proportion of patients classified as patients with WO when diagnosed with ARF, initiation of RRT, along with the duration (i.e., number of days) of the WO. The main outcomes assessed were 60-days mortality and recovery of renal function stratified by fluid overload. Patients classified as overloaded had greater disease severity and intensity of treatment (mechanical ventilation). They were mostly patients post-surgery and had less diuresis and serum creatinine at the time of inclusion. The gross 60-days mortality was significantly higher for patients with ARF and fluid overload (48% vs. 35%,P<.006). The adjusted odds ratio for death from fluid overload at the time of diagnosis of ARF was 3.1 (95% CI 1.2-8.3). In patients receiving RRT, average fluid accumulation was significantly lower in survivors compared with non-survivors (8.8% vs. 14.2%, P=.01) and the adjusted odds for death due to overload equivalents at the beginning of the RRT was 2.1 (95% CI 1.3-3.4). In addition, there was evidence of an almost linear increase in mortality when stratified by WO accumulated over the duration of admission, along with increased mortality for patients with volume overload of longer duration (P<.0001). Fluid overload at the time of diagnosis of ARF or the start of the RRT was not independently associated with renal recovery. This study also has recognised limitations, such as being a post-hoc secondary analysis of data collected prospectively, which makes it potentially prone to bias due to residual confounding and selection. In addition, the formula for calculating the percentage of adult patients with WO in weight income has not been validated prospectively and may predispose to poor ratings. Finally, this study was unable to compare the association of water balance and results in critically ill controls without ARF. However, data from two observational studies, together with previous studies in critically ill adult and children, provide compelling evidence that attention to the prevention of volume overload and water balance, particularly in FRA, can be an important underestimated determinant of survival.
CONCLUSION
ARF and HF are common and more frequent every day in clinical practice. Overload and fluids accumulation have a common pathophysiology and clinical course. Water balance represents an important biomarker or unit to serially measure these patients. It can provide important diagnostic, prognostic, and therapeutic information. Determining the water balance in HF can be complex. It depends largely on the underlying pathophysiology. However, besides the simple balance (inflows minus outflows), new biomarkers (BNP) and new technologies (cardiac impedance) are proving to be useful for the early detection and identification of ADHF. This advantage may allow early intervention which can account for better clinical outcomes. Several observational studies in paediatric and adult patients centered on ARF show data supporting the importance of water balance as a modifiable biomarker, determinant of clinical outcome. Sometimes, the impact of water balance in both syndromes, especially the ARF, has not been adequately assessed. There is little or no data specifically on the water balance in the cardio-renal syndrome where the acute/chronic heart disease can directly contribute to acute/chronic worsening of symptoms.
Conflicts of interest
The authors declare that they have no potential conflicts of interest related to the contents of this article.
Figure 1. Mechanisms involved in water overload and its consequences
Table 1. Summary of clinical symptoms of water overload in patients hospitalised due to decompensated acute heart failure
Table 2. Clinical data and mortality in paediatric patients with acute renal failure and water overload