Waldenström macroglobulinemia (MW) is a low-grade malignant lymphoproliferative disorder, with well recognized manifestations such as kidney disease and type I cryoglobulinemia; however, cryoglobulinemic membranoproliferative glomerulonephritis (GNMP) has been describe in only few isolated cases.1,2
Here, we present the case of a patient with cryoglobulinemic glomerulonephritis associated with Waldenström's disease.
A 68-year-old woman consulted for new-onset oedemas, arthralgias and purpuric lesions in lower limbs. She had medical history of hypertension, dyslipidemia, monoclonal gammapathy of unknown significance, kappa IgM diagnosed in 2012, followed up by hematology department that did not require specific treatment and inactive infection of hepatitis B virus (HBV) without requiring treatment.
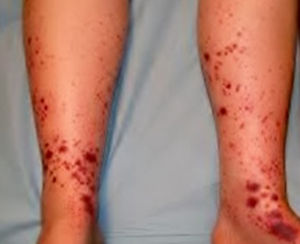
Physical examination revealed edemas with fovea and purpuric lesions on both legs (Fig. 1). Blood test showed, VSG: 82mm; creatinine: 1.2mg/dl; glomerular filtration rate: 55ml/min; LDH 604 UI/l; proteins: 5.7g/dl; albumin: 3.0g/dl; proteinuria 3.7g/day; hypogammaglobulinemia IgG and IgM; reduced complement, C3: 1.4mg/dl and C4: 0.06mg/dl.
The serologies of HIV, HCV, HBVsAg, anti-HBV, HBeAg were negative and anti-HBV positive, with a viral load of HBV-DNA 87 and a normal level of transaminases, being labeled as an inactive carrier of HBV without abnormalitiy in the liver function.
The immune study showed mixed monoclonal cryoglobulinemia IgM kappa with cryocrit of 7%. The serum immunofixation revealed a kappa IgM band, with quantification of the homogeneous component of 2.8g/L. The proteins in urine showed an electrophoretic image of glomerular type. Anti-nuclear, anti-DNA, anti-ENA, anti-glomerular basement membrane antibodies and ANCA were negative.
A thoracic and abdominal CT was performed, without evidence of adenopaties suggestive of a lymphoproliferative process. The bone marrow aspirate reported the presence of kappa monoclonal lymphocytosis B, CD5 negative, CD23 weak, CD10 negative, compatible with Waldenström macroglobulinemia.
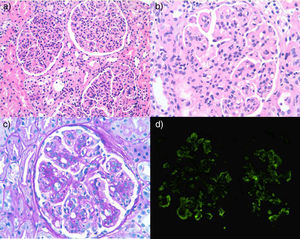
A renal biopsy was performed, the sample contained a total of 34 glomeruli, showing a lobulated appearance with marked endocapillary hypercellularity, with doubling of basement membranes and with subendothelial fuchsin deposits. Direct immunofluorescence was diffusely positive and globally for IgM, C3 and kappa, with a pattern of membranoproliferative glomerulonephritis. The histology described was compatible with cryoglobulinemic glomerulonephritis (Fig. 2).
(a) Lobulated glomeruli with marked diffuse endocapillar hypercellularity. (b) Marked endocapillary hypercellularity, occasional subendothelial fuchsin deposit and apparent thickening of basement membrane. (c) Occasional double contour. (d) Positive direct immunofluorescence with parietal pattern.
These results allowed us to establish the diagnosis of cryoglobulinemic MPGN associated with low-risk MW, suggesting active treatment for the associated renal involvement. Treatment with rituximab was agreed, after joint multidisciplinary evaluation. Previously, treatment of chemoprophylaxis with isoniazid due to latent tuberculosis and treatment of HBV carrier status with tenofovir was established.
The patient was started on rituximab, with progressive decrease of proteinuria, remission of edema, polyarthralgia and disappearance of skin purpura, after the 2nd dose of the drug. After 4 months, the patient remained asymptomatic and with a proteinuria of 0.4g/day.
Waldenström's disease is a malignant lymphoproliferative disorder characterized by bone marrow infiltration and monoclonal production of IgM B lymphocytes. In recent years, several authors have associated the presence of monoclonal processes with the histological pattern of MPGN. One study observed that the most frequent hematological disorder associated to MPGN was monoclonal gammopathy of uncertain significance, followed by: B cell lymphoma, Waldenström macroglobulinemia, chronic lymphatic leukemia and multiple myeloma.3
With the appearance of increasing number of cases, it is postulated that dysproteinemias could act as a mechanism that triggers the deregulation of the alternative pathway of complement.4 However, cryoglobulinemic glomerulonephritis, sometimes described in type II cryoglobulinemia, consisting of polyclonal IgG and monoclonal IgM, associated with Waldenström macroglobulinemia is a rare finding in the literature.5–7
The MW is a disease characterized by a relatively indolent course. However, in some patients the disease is more aggressive and it is associated with a worse prognosis. The indications for early treatment are mainly the presence of severe cytopenia, organomegaly or “bulky” adenopathy, symptomatic hyperviscosity, cryoglobulinemia, severe neuropathy, amyloidosis, cryoglutinin disease or progression of the disease. Recent studies recommend the combination of rituximab with analogs and/or alkylating agents or cyclophosphamide.8
The treatment of secondary mixed cryoglobulinemia will depend on the treatment of the disease to which it is associated. The use of rituximab seems to be a safe and effective therapeutic option, without forgetting the importance of concomitant treatment with antiviral HBV therapy, given the risk of reactivation of the infection during the course of rituximab therapy.9,10
This case illustrates the usefulness of renal biopsy in the diagnosis of certain hematological dysproteinemias such as Waldenström's macroglobulinemia, which can be expressed early at the glomerular level, being essential for adequate therapeutic management. The cryoglobulinemic membranoproliferative glomerulonephritis associated with MW is described as very infrequent renal manifestation.
Please cite this article as: Antón Vázquez V, Giménez Torrecilla I, Gomà Gallego M, Paredes Henao V. Macroglobulinemia de Waldenström presentándose como glomerulonefritis membranoproliferativa crioglobulinémica tipo II. Nefrologia. 2018;38:439–441.