Sodium phosphate (Fosfosoda®) is an osmotic lavage solution used widely in colonoscopies. The liquid contains 29.7g of sodium phosphate in 45ml. According to the Summary of Product Characteristics, it offers good tolerance and allows little fluid intake.1 We present a series of cases of patients who started with normal kidney function prior to colonoscopy and in whom kidney deterioration with no clear cause was subsequently observed, and we reach the conclusion that preparation with Fosfosoda® contributed to the kidney damage.
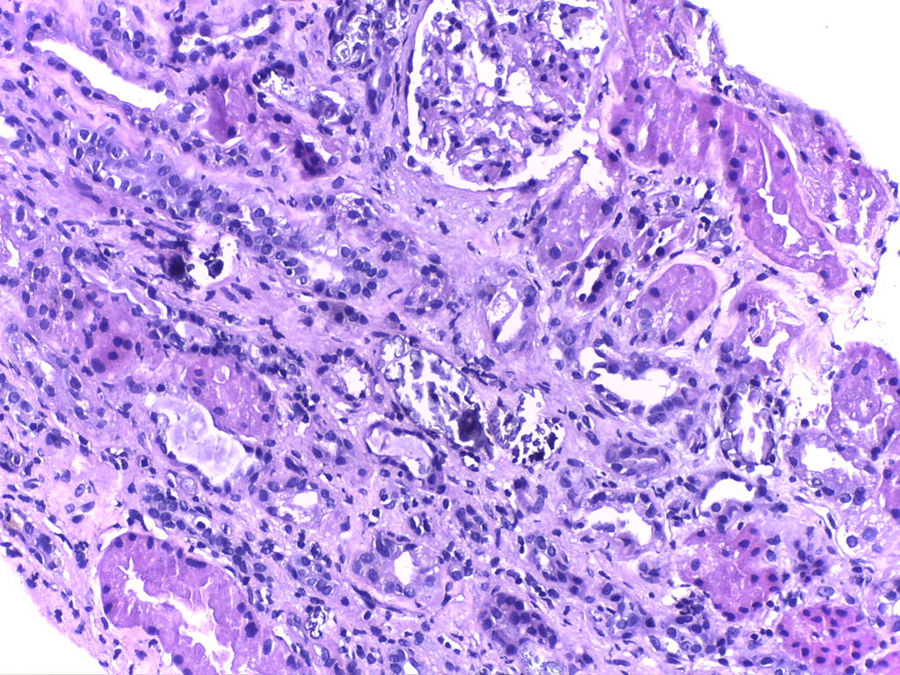
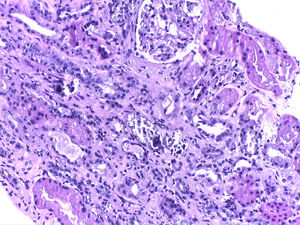
We present the case of a male, aged 72 years, who was referred to Nephrology after finding plasma creatinine of 6.2mg/dl, phosphorous of 5.6mg/dl and uric acid of 10.5mg/dl. Of particular importance in his medical history were arterial hypertension treated with olmesartan and hydrochlorothiazide, and prostate surgery, he takes nonsteroidal anti-inflammatory drugs. One month before, he had had a colonoscopy involving preparation with Fosfosoda®. The patient was asymptomatic and with preserved diuresis. The deferred study confirmed plasma creatinine levels of 5.6mg/dl, urea 184mg/dl, phosphorous 5.6mg/dl, calcium 9.5mg/dl, potassium 5.5mEq/l and uric acid 9.8mg/dl. Serological testing (hepatitis B, C and HIV) was negative. The immunological study (ANA/ANCA immunoglobulins A and M and complement) was normal. IgG was 2030mg/dl (751–1560). The serum protein electrophoresis test showed an IgG kappa monoclonal peak, monoclonal component 1.1g/dl. The urinalysis was normal and proteinuria was 0.5g/24h. The kidney ultrasound was normal. Haematology was consulted for the monoclonal gammopathy test: the kappa/lambda light chains were negative and the bone marrow puncture was non-specific, with no bone marrow plasma cell infiltration, and the diagnosis was reached of IgG kappa monoclonal gammopathy of undetermined significance. A kidney biopsy was carried out because of the kidney deterioration with no apparent cause, revealing normal glomeruli with slight mesangial expansion with no cell proliferation. Abundant intratubular calcium deposits were seen with occasional associated inflammatory infiltration (Fig. 1), for which the final diagnosis was nephropathy due to intratubular calcium deposits.
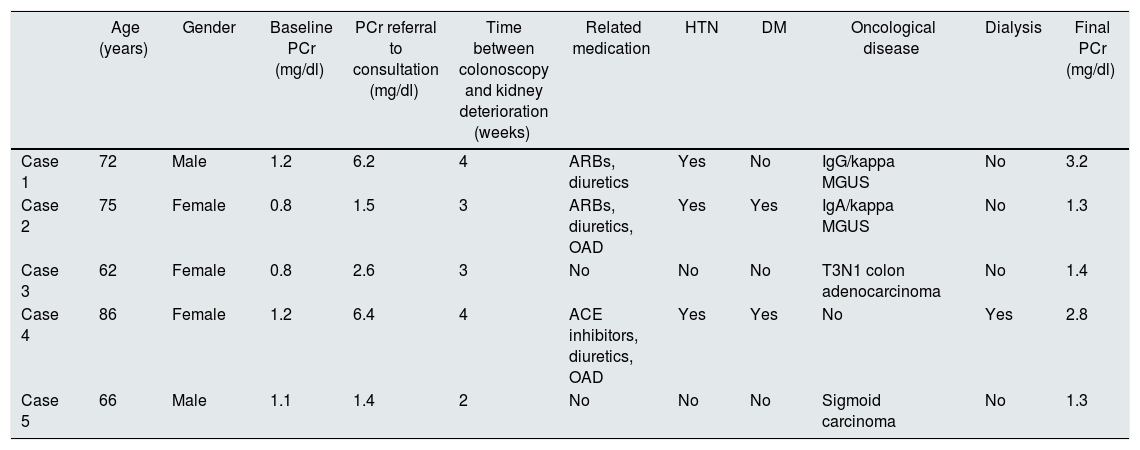
Table 1 gives a summary of case 1 and presents the characteristics of 4 other cases related to the use of Fosfosoda®. They were all asymptomatic, had preserved diuresis, a normal immunological study and normal urinalysis with/without mild proteinuria.
Cases of deferred nephropathy due to phosphates after exposure to Fosfosoda®.
| Age (years) | Gender | Baseline PCr (mg/dl) | PCr referral to consultation (mg/dl) | Time between colonoscopy and kidney deterioration (weeks) | Related medication | HTN | DM | Oncological disease | Dialysis | Final PCr (mg/dl) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 72 | Male | 1.2 | 6.2 | 4 | ARBs, diuretics | Yes | No | IgG/kappa MGUS | No | 3.2 |
| Case 2 | 75 | Female | 0.8 | 1.5 | 3 | ARBs, diuretics, OAD | Yes | Yes | IgA/kappa MGUS | No | 1.3 |
| Case 3 | 62 | Female | 0.8 | 2.6 | 3 | No | No | No | T3N1 colon adenocarcinoma | No | 1.4 |
| Case 4 | 86 | Female | 1.2 | 6.4 | 4 | ACE inhibitors, diuretics, OAD | Yes | Yes | No | Yes | 2.8 |
| Case 5 | 66 | Male | 1.1 | 1.4 | 2 | No | No | No | Sigmoid carcinoma | No | 1.3 |
ACE inhibitors: angiotensin-converting enzyme inhibitors; ARBs: angiotensin II receptor blockers; DM: diabetes mellitus; HTN: hypertension; MGUS: monoclonal gammopathy of undetermined significance; OAD: oral antidiabetics; PCr: plasma creatinine.
The use of osmotic lavage solutions for colonoscopy preparation, including Fosfosoda®, has facilitated a better view of the colon, improving the diagnosis of various diseases.1 However, its use is associated with complications related to phosphate overload. ‘Phosphate nephropathy’ can manifest as “acute”,2,3 the diagnosis of which can be established more easily, particularly in patients with a history of recent colonoscopy. However, in “outpatients”, the fact that no routine kidney control tests are carried out after a colonoscopy can result in ‘phosphate nephropathy’ going unnoticed, and it should be suspected in people with kidney deterioration of unknown cause. In our cases, kidney deterioration was found weeks after performing the colonoscopy, with the majority of them showing risk factors which contribute to their onset: female, elderly, arterial hypertension and diabetes, as well as the use of drugs to block the renin-angiotensin-aldosterone system.2,4
Colonoscopy is also used to screen for colorectal neoplastic disease.1 However, the use of agents for its preparation with the potential risk of causing kidney failure may affect the prognosis of these patients, as it limits the use of chemotherapy agents which may be required to treat their tumours. Among the cases we are presenting, two were diagnosed with gastrointestinal neoplasms, showing kidney deterioration after the colonoscopy. These negative aspects associated with the use of Fosfosoda® have led to us to modify the use of preparations for colonoscopies, administering other safer agents, such as polyethylene glycol (Casenglicol®),1,5 and recommending that the preparation agent used is recorded in colonoscopy reports. This would facilitate a diagnosis of ‘phosphate nephropathy’, particularly in those cases of unexplained kidney deterioration, as it allows a causal relationship to be established between the agent used in the colonoscopy and kidney deterioration.
In conclusion, sodium phosphate solutions (Fosfosoda®) represent a kidney “threat” in healthy patients undergoing a colonoscopy, causing ‘phosphate nephropathy’. Their damaging renal effects may be detected late and, therefore, have negative repercussions on the patient's prognosis, as they limit the use of drugs excreted by the kidneys, which may be required to treat neoplasms.
Please cite this article as: Garcia LC, Benito MH, Garcia AC, Gonzalez AS, Martinez RC, Gomez AR, et al. Nefropatía «oculta» tras preparaciones orales de fósforo para colonoscopia. Nefrologia. 2018;38:442–443.