Chronic kidney disease (CKD) is caused by several cardiovascular disease (CVD) risk factors such as diabetes mellitus (DM), hypertension, chronic glomerular nephritis and ageing. Although the estimated glomerular filtration rate (eGFR) is believed to be helpful in detecting kidney function, it is generally difficult to assess the pathogenesis of CKD and predict renal prognosis accurately using only eGFR.1 Therefore, to treat CKD patients according to their individual pathogenesis and prevent worsening of renal function, a more useful index is used that can assess both renal function and the severity of atherosclerosis, which reflects kidney and CVD prognosis better than eGFR.
The vascular resistance index (VRI) of the renal segmental arteries, measured by renal Doppler ultrasound, is believed to be a good indicator of renal vascular resistance caused by atherosclerosis. It is elevated in patients with significant arteriosclerosis or polycystic kidneys. It has classically been used as a predictor of renal function impairment in the non-transplanted population.2–4 Previous reports have shown that it is associated with prognosis of renal disease.5–9
The objective of our study was to determine the VRI in kidney grafts and assess whether it has prognostic implications for graft survival. To do this, we reviewed the Doppler ultrasound of transplanted patients and collected the results of the blood test performed as close as possible to the transplant. We also reviewed antihypertensive medications and those related to calcium and phosphorus metabolism.
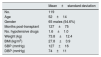
Our study population consists of 119 kidney transplant patients with the characteristics shown in Table 1.
Characteristics of the study population.
| Mean±standard deviation | |
|---|---|
| No. | 119 |
| Age | 52±14 |
| Gender | 65 males (54.6%) |
| Months post-transplant | 127±75 |
| No. hypotensive drugs | 1.6±1.0 |
| Weight (kg) | 73.8±12.4 |
| BMI (kg/m2) | 27.8±3.9 |
| SBP (mmHg) | 127±16 |
| DBP (mmHg) | 74±11 |
BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
The average resistance index was 0.69±0.08. No correlation was observed with gender, hepatitis C virus (HCV) or months after transplantation. Differences in the VRI could be attributed to age difference rather than the underlying nephropathy.
The following medication had no influence on VRI: calcitriol, bisphosphonates, diuretics and hypotensive drugs. However we found a relationship of VRI with the number of hypotensive drugs administered. Patients receiving cinacalcet had somewhat lower indexes. Regarding immunosuppressants, neither ciclosporin nor tacrolimus had any influence, but patients receiving sirolimus had lower indexes, although the difference was not significant.
Type II diabetic patients exhibited higher VRIs but did not reach significance.
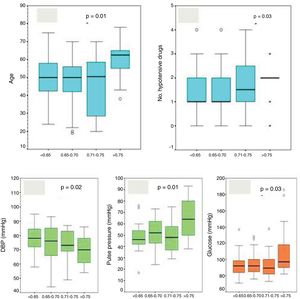
We classified the patients in quartiles of VRI(<0.65; 0.65-0.70; 0.71-0.75; >0.75) to analyse differences. We observed higher VRIs in patients with more age (p=0.01, Fig. 1A), on a higher number of hypotensive drugs (p=0.031, Fig. 1B), with lower diastolic blood pressure (DBP) (p=0.02, Fig. 1C), with higher pulse pressure (p<0.001, Fig. 1D) and with higher serum glucose level (p=0.003, Fig. 1E), and with a progressive increase in proteinuria and microalbuminuria although this was not statistically significant. There were no differences in diuresis, nor did we find differences between the different quartiles in terms of weight, BMI, renal function (Cr, cystatin C, ClCr, MDRD), serum sodium, cholesterol or triglycerides, or serum insulin levels.
After a follow-up of 25±15 months, we reviewed the progression of renal function or proteinuria, without finding any relationship between the VRI and the degree of renal function impairment or change in proteinuria. In the multivariate analysis, we found independent predictors to be glucose levels, as well as DBP and systolic blood pressure (SBP) figures (p<0.001, Table 2).
It has been reported that the resistance index (RI) measured by Doppler ultrasound can be a useful predictor of the progression of kidney dysfunction5–9 and can provide useful diagnostic information non-invasively for various kidney diseases. A higher RI is associated with poor kidney graft survival.10
The VRI has also been reported to be significantly correlated with damage at varying levels. Previous studies revealed that the VRI measurement in addition to low-grade albuminuria was useful for screening for target organ damage in patients with resistant hypertension,2 and VRI values were independently correlated with carotid intima-media thickness in patients with untreated essential hypertension3 and metabolic syndrome.4 These results suggest that the renal vascular resistance indicated by the resistance index may reflect the degree of systemic atherosclerosis and may be a useful marker for detecting and evaluating atherosclerotic diseases due to CVD risk factors such as hypertension, diabetes, dyslipidaemia and metabolic syndrome.
In our study, the renal VRI is elevated in a small proportion of transplant patients, mainly related to systemic blood pressure and pulse pressure. It is not related to the use of specific hypotensive medication. Patients with DM or with poor glycaemic control exhibit a higher VRI. It is not related to the degree of proteinuria or renal function, nor does it appear to have prognostic implications.
Please cite this article as: Merino García E, Borrego Utiel FJ, Polaina Rusillo M, García Cortés MJ. El índice de resistencia vascular renal no tiene implicaciones pronósticas en el trasplante renal. Nefrologia. 2021;41:69–71.