In the treatment of hypertension and diabetes, the combination of blockers of the renin-angiotensin system with calcium channel blockers appears as one of the most effective options. Nevertheless, all calcium blockers do not behave similarly. Manidipine, unlike other third-generation dihydropyridine derivatives, blocks T-type calcium channels present in the efferent glomerular arterioles, reducing glomerular pressure and microalbuminuria. In addition, T-type channels are related with proliferation, inflammation, fibrosis, vasoconstriction and activation of the renin-angiotensin system. Inhibition of these factors could explain manidipine non-hemodynamic actions compared to other blockers.
En el tratamiento de la hipertensión y la diabetes, la combinación de bloqueantes del sistema renina-angiotensina y de los canales de calcio se presenta como una de las opciones más eficaces. Sin embargo, no todos los bloqueantes de calcio se comportan del mismo modo. Manidipino, a diferencia de otros derivados dihidropiridínicos de tercera generación, bloquea los canales de calcio T presentes en las arteriolas glomerulares eferentes, disminuyendo la presión intraglomerular y la microalbuminuria. Además, los canales T están relacionados con proliferación, inflamación, fibrosis, vasoconstricción y activación del sistema renina-angiotensina. La inhibición de estos factores podría explicar la acción no hemodinámica del manidipino frente a otros bloqueantes.
CALCIUM CHANNELS
Voltage-dependent calcium channels mediate the flow of calcium in response to the depolarisation of the cell membrane and regulate intracellular processes such as contraction, secretion, neurotransmission, and gene expression, in which calcium acts as a second messenger.
The channels are composed of multiple heteromeric subunits: α, β, γ and δ, which are coded for by several different genes. They are named using the chemical symbol of the principal ion they regulate the passage of (Ca) and the primary physiological regulator, voltage (v). The numerical identifier corresponds to the gene subfamily to which the channel corresponds (1-3), and the letter (A-I) indicates the order in which it was identified, except subunit a1S, which was assigned the letter S due to its presence in skeletal muscle.
Subunit α1, which is coded for by CACNA1, appears to be responsible for the main characteristics of these channels, since it is involved in ion selectivity and conductivity and sensitivity to voltage.1-3
The calcium currents registered in different cell types have multiple pharmacological and physiological properties, so it is possible to group calcium channels into L, P/Q, N, R, and T types.1
L-type (long-lasting) calcium channels are activated by strong depolarisations, mediated by the prolonged flow of calcium into a wide variety of cell types. In this manner, these channels play a central role in the contraction and excitation of skeletal, cardiac, and smooth muscle4 (Cav 1.2 [a1C]). They are responsible for muscle tone in arterial smooth muscles, and have become a target for drugs to treat hypertension and angina. In the kidney (Cav1.2 [a1C] and Cav1.3 [a1D]), these channels promote the dilation of preglomerular (afferent) arterioles, ostensibly increasing intraglomerular pressure. Additionally, other L-type channels are found in the skeletal muscle (Cav1.1 [a1S]), brain and kidney (Cav1.2 [a1C], Cav1.3 [a1D]), pancreas (Cav1.3 [a1D]), and retina (Cav1.4 [a1F]).1
Other lesser-known channel types are P/Q, N, and R, and these also require strong depolarisation in order to be activated.1
Contrary to the previously mentioned channel types, T-type (transient) channels are activated by weak depolarisations, and provoke a transitory flow of calcium.5 T-type channels are present in the nervous system (Cav3.1 [a1G]), brain (Cav3.1 [a1G,] Cav3.2 [a1H] and (Cav3.3 [a1D]), heart (Cav3.2 [(a1H]), kidneys (Cav3.1 [a1G], Cav3.2 [a1H]) and liver (Cav3.2 [a1H]).1 These are involved in heart rate, vascular smooth muscle contraction, and cell growth.4 T-type channels have been implicated on several occasions in the secretion of hormones such as renin, aldosterone, atrial natriuretic peptide, and insulin. Only T-type channels (not L-type) are found in postglomerular (efferent) arterioles, and so the tone of these vascular muscles must be controlled by T-type channels and angiotensin II AT1 receptors.
In principle, L-type channel blocking is considered to be the most important type in the regulation of vascular functioning, since Cav1.2 is the major route of calcium entry into skeletal muscle, heart, and kidney cells.1,6 However, the non-haemodynamic action of T-type channel blockers could have multiple beneficial effects by inhibiting inflammatory processes (inhibition of Rho kinases, NF-kB, leukocyte adhesion), blocking the renin-angiotensin system, and blocking the sympathetic nervous system (Table 1).7
TYPES OF CALCIUM CHANNEL BLOCKERS
Calcium channel blockers (CCB) are highly heterogeneous molecules that can be grouped into: derived from phenylalkylamine, such as verapamil; derived from benzodiazepines, whose prototype is diltiazem; and derived from 1,4-dihydropyridines, such as manidipine.8 These molecules mainly block L-type channels.
The first generation of dihydropyridine calcium channel blockers, such as nifedipine, are characterised by instantaneous release, a short lifetime, and quick absorption. In spite of a favourable metabolic profile, these drugs cause some adverse effects such as sharp drops in blood pressure, tachycardia, and sympathetic activation. In the second generation of drugs, including nimodipine, the release of the molecule is slower.9
The latest-generation CCB have a long lifetime and prolonged action that significantly reduces blood pressure, thus notably diminishing the secondary side effects of the drug (Table 2). In contrast with traditional blockers, this new group of molecules, including manidipine, blocks both L-type and T-type channels.10
THE EFFECTS OF CCB ON CARDIOVASCULAR MORBIDITY
Cardiovascular disease is clearly and consistently related to blood pressure. The main objective of antihypertensive therapy is to reduce both cardiovascular and renal morbidity and mortality. In order to achieve this, a target blood pressure level was established at below 140/90mm Hg, although in patients with diabetes or kidney disease, it appears that this limit should be less than 130/80mm Hg.11
According to the guidelines for the management of arterial hypertension,12 thiazide diuretics should be the treatment of choice, although certain high-risk cases could be treated with angiotensin II receptor antagonists (ARA-II), CCB, or angiotensin-converting enzyme (ACE) inhibitors as a first choice.11 Precise indications exist for the use of antihypertensive agents such as CCB, ACE inhibitors, and ARA-II in order to prevent the development of diabetes in hypertensive patients.11
The CAMELOT study concluded that, as a monotherapy, CCB are more efficient at diminishing cardiovascular events and slowing the progression of atherosclerosis than ACE inhibitors.13 With regard to CCB combined with other drugs, Fogari et al. demonstrated that manidipine (CCB) and delapril (ACE inhibitor) provide increased benefit over olmesartan (ARA II) and hydrochlorothiazide, as this combination reduces orthostatic blood pressure and does not cause adverse metabolic effects.14
Other studies, such as the one carried out by the Japanese Hypertension Society, affirm that CCB possess greater antihypertensive efficacy than all other preferred antihypertensive drugs available, without affecting blood flow to the body’s organs. This characteristic makes this group of drugs particularly indicated in elderly patients and those with complications such as left ventricular hypertrophy, tachycardia, angina pectoris, and chronic cerebrovascular disease.15
In this respect, one trial treated 30 obese hypertensive patients with amlodipine, manidipine, and cilnidipine, revealing that these long-acting CCB reduce blood pressure and also diminish resistance to insulin, suggesting valuable cardio-metabolic properties.16
However, other studies have not observed significant differences in the efficacy of reducing blood pressure between different latest generation calcium channel blockers.17,18
CCB AND INSULIN RESISTANCE. MANIDIPINE AND THE EXPRESSION OF AP2
In terms of cardiovascular morbidity and mortality, arterial hypertension and diabetes are key risk factors. These are interrelated in a complex and multifactorial manner. Hypertensive patients with metabolic syndromes (MS) have an elevated risk of diabetes mellitus (DM). The incidence of DM appears to increase in patients with AHT, partly due to the high percentage of obese patients in both groups. The UKPDS study demonstrated that high blood pressure and glycaemia independently and additively increase the risk of cardiovascular disease.19,20
In this respect, the MARIMBA study compared the effects of administering manidipine and amlodipine21 in non-diabetic MS patients, and found that blood pressure and C-reactive protein (CRP) levels decreased with both types of treatment, although manidipine also significantly reduced albuminuria and insulin resistance, associated with an increase in serum adiponectin levels. Manidipine also caused fewer adverse effects.
By comparing manidipine with another CCB with similar kinetic and lipophilic characteristics, such as lercanidipine, manidipine is more effective in reducing insulin resistance in obese and hypertensive patients.22 In other studies, manidipine proves itself to be just as effective as pioglitazone in reducing the expression of RAGE and the production of ROS, as well as reducing CRP levels. These experiments with specific inhibitors have concluded that the mechanism depends on PPAR-γ. This mechanism could explain the reduced inflammatory effect of hyperglycaemia and vascular damage.23
The “lipotoxicity hypothesis” correlates type 2 diabetes with a loss in capacity of adipose tissue to accommodate excess calories. The loss in adipocyte differentiation makes the excess calories accumulate primarily in the liver, pancreas, and muscles, contributing to the development of insulin resistance.24 We know that small adipocytes are sensitive to insulin, as opposed to mature cells, which undergo hypertrophy and become resistant to the hormone. For this reason, favouring adipogenesis would contribute to reducing insulin resistance in type 2 diabetes patients.
The improvement produced in insulin sensitivity by dihydropyrimidine CCB is almost imperceptible. In the case of nifedipine, which blocks only L-type channels, the body becomes even more desensitised to insulin, and glucose release is inhibited.16 However, studies with manidipine have reported surprising results in this respect. Although some of these studies were already taken into account, a clinical trial performed with 64 hypertensive MS patients. The patients were evaluated using NCEP/ATPIII criteria and randomly assigned to manidipine or amlodipine treatments for 12 weeks. Similar reductions in blood pressure were observed with both treatments, and the patients treated with manidipine also experienced significant reductions in insulin resistance.25 In this same manner, a more recent analysis on insulin sensitivity and plasma fibrinogen in obese and hypertensive patients compared the combination of manidipine and delapril versus olmesartan and hydrochlorothiazide. It demonstrated that the first combination significantly reduced insulin resistance and plasma fibrinogen levels, in spite of the fact that the reduction in blood pressure values would indicate similar efficacy between both combinations.26 A later study compared the combination of manidipine and delapril with losartan and hydrochlorothiazide in patients with diabetes and microalbuminuria, and concluded that the first combination was the more useful therapeutic option for these patients.27
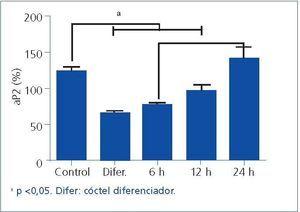
Experiments have shown that manidipine, but not amlodipine or lercanidipine, activates PPAR-γin 3T3-L1 rat adipocytes.23,28 In our studies, we have observed that NIH3T3 cells treated with manidipine29 experience increased PPAR-γ(Peroxisome Proliferator-activated Receptor gamma) expression and adipocyte differentiation 2 (aP2) gene expression, which can be considered as evidence of the expression of the first (Figure 1). These results indicate mechanisms that link manidipine to the increase observed in insulin sensitivity in hypertensive, diabetic patients and with de novo adipocyte formation. In this sense, the increase in intracellular calcium levels has been observed to inhibit preadipocyte differentiation.30 This contrasts with the normal role of calcium in faster processes, such as neurosecretion, excitation, and contraction.
Adipogenesis, as other differentiation processes, depends on stimulating transcription factors, such as PPAR-γ, and inhibitors such as the GATA family, which in turn is activated by extracellular signals. Calcium homeostasis has been studied with special emphasis on calreticulin, which is one of the main calcium binding proteins in the lumen of the endoplasmic reticulum, and is largely responsible for a rapid calcium exchange. One study performed with stem cells and 3T3-L1 preadipocytes demonstrated how calreticulin can modulate adipogenesis through a negative feedback mechanism. PPAR-γis a potent transcription activator for calreticulin, as it binds to its promoter. In this manner, it increases the expression of calreticulin, but once this protein is over-expressed, calreticulin inhibits the cis-bond of the PPAR-γ-RxR heterodimer to PPAR-γresponse elements (PPRE), thus cancelling the transcriptional activation of PPAR-γby fatty acids. Through this mechanism, calreticulin negatively regulates both the expression of PPAR-γand other critical proadipogenic transcription factors such as C/EBPa.31
Manidipine’s calcium channel blocking activity could impede the entrance of calcium into the cells, thus reducing calcium concentrations in the endoplasmic reticulum, and in turn, that of calreticulin, favouring the differentiation of adipocytes.
EFFECTS OF CCB ON MICROALBUMINURIA
The renal protection is associated with cardiovascular protection as well, and the evolution of albuminuria is an excellent predictor of both the evolution of renal function and the development of cardiovascular complications.32 The presence of microalbuminuria makes the use of ACE inhibitors or ARA-II necessary, even as CCB are still considered for combined therapy. In the absence of albuminuria, and with maintained or diminished glomerular filtration rate, CCB could be the first pharmacological option. However, a high percentage of patients do require ACE inhibitors and/or ARA-II.33
Contrary to other dihydropyridines, manidipine blocks T-type channels of efferent arterioles, which diminishes glomerular pressure and, consequently, albumin excretion, but at the same time it blocks L-type channels, thus favouring the dilation of the afferent arteriole. In this manner, T-type calcium channel blockers (CCB)1,7,34 influence haemodynamics through their antihypertensive effect. Thus, we could consider their effect as protective against kidney damage, since the kidney is one of the target organs in hypertensive and diabetic patients. The AMANDHA study (Efficacy and Safety Assessment of Manidipine in Type 2 Diabetic Patients with Hypertension and Microalbuminuria Uncontrolled with Renin-Angiotensin System Blockers)35 compared manidipine and amlodipine in diabetic patients with uncontrolled hypertension and microalbuminuria. Although both CCB are equally effective in reducing CRP and blood pressure, the first implies fewer adverse effects. Also, the reduction in albuminuria and insulin resistance was significantly higher in patients treated with manidipine. A recent study demonstrated yet again that manidipine is capable of significantly reducing albumin urine excretion in patients with essential hypertension without causing adverse effects, and so the combination of manidipine with renin-angiotensin antagonists could be beneficial in these cases.36
MANIDIPINE AND OXIDATIVE STRESS
Endothelial structure and function could improve considerably with the use of CCB. Research such as the INSIGHT study (International Nifedipine Intervention as a Goal in Hypertension Treatment) and MIDAS study (Myocardial Infarction Data Acquisition System) demonstrates the superiority of these drugs as compared to thiazide diuretics in terms of lower increases in intimal thickness.33,37,38
The beneficial effects of calcium blockers in macrovascular endothelial cells must be demonstrated and justified through mechanisms that do not include calcium channels, since these are not expressed in endothelial cells.39,40 To this end, some authors have postulated that the action of DHP in this type of tissue are related to their lipophilicity.41
Oxidative stress plays a fundamental role in the development of atherosclerosis. Toba et al. pointed out the antioxidant and anti-inflammatory effects of manidipine and other CCB such as amlodipine, mediated by the increased expression of endothelial nitric oxide synthase (eNOS) and the inhibition of angiotensin converting enzyme (ACE) expression, but not their possible activity in reducing blood pressure. In this study, the authors observed how manidipine normalises the reduction in the expression of both the eNOS gene and protein, and reduces the over-expression of NADPH oxidase, VCAM, and MCP-1 in hypertensive rat aortas.41 Furthermore, manidipine has another beneficial effect on atherogenesis, as it inhibits the expression of LOX-1, a low-density lipoprotein receptor induced into action by angiotensin II.43
Sun X et al.44 recently demonstrated that both in mature differentiated adipocytes and 3T3-L1 cells, and in co-cultures of both cell types, calcitrol increases the expression of inflammatory molecules such as MCP-1, MIF, M-CSF, MIP, IL-645, TNF and CD14. Treatment with nifedipine or dinitrophenol inhibits the activity of calcitrol, which could reveal a calcium-dependent mechanism that requires mitochondrial uncoupling. As such, we could expect the blockage of calcium channels with manidipine to also show anti-oxidative and · anti-inflammatory effect by reducing intracellular calcium levels.
Our preliminary studies on smooth muscle cells revealed an increase in endothelial nitric oxide synthase (eNOS) gene after being treated with manidipine. In response to many types of aggression, the expression of eNOS in cells treated with manidipine is essentially stable, compared to the control culture. This data may reveal a beneficial effect of CCB against endothelial dysfunction (Figure 2).
CONCLUSIONS
T-type calcium channel blockers provide protection to the kidneys, as they improve glomerular microcirculation due to their vasodilatory effect both on afferent and efferent arterioles. Manidipine stands out from among these types of calcium channel blockers due to its anti-inflammatory activity, which does not rely on the renin-angiotensin system, and because of its possible beneficial effects against endothelial dysfunction.
Acknowledgements
This manuscript was developed with the aid of authors from the Fundación Mapfre-Guanarteme (Mapfre-Guanarteme Foundation) and Laboratorios Chiesi (Chiesi Laboratories), who we thank for their support and collaboration. The authors declare a Research Agreement between Chiesi Farmaceutica S.p.A. and the Research Unit of the Dr. Negrín Gran Canaria
Figure 1. Exposure of NIH-3T3 preadipocyte cells to manidipine activates the expression of the aP2 gene in a time-dependent manner.
Figure 2. The Expression of eNOS in cells treated with angiotensin II and manidipine is significantly greater than in cells treated with just angiotensin II
10643_108_14367_en_10643tabla_1.doc
Table 1. Calcium channels
10643_108_14368_en_10643tabla_2.doc
Table 2. Calcium channel blockers