Antecedentes: La eritropoyetina beta pegilada (PegEPO) está indicada en el tratamiento de la anemia por enfermedad renal crónica. Su larga semivida permite su administración mensual en fases de mantenimiento. Objetivo: Evaluar el uso, efectividad y coste de PegEPO en un grupo de pacientes con insuficiencia renal crónica en estadio prediálisis. Método: Estudio observacional retrospectivo en pacientes prediálisis que iniciaron tratamiento con PegEPO entre mayo de 2008 y febrero de 2009. Se recogieron: edad, sexo, niveles de hemoglobina (Hb) y dosis y frecuencia del agente estimulante de eritropoyesis (AEE) utilizado. El período de seguimiento fue de 12 meses. Resultados: Se incluyeron 198 pacientes. La Hb media al inicio de PegEPO en pacientes sin tratamiento previo fue de 10,8 g/l y de 11,6 g/l a los 90 días (p < 0,0001). En pacientes con AEE previo, la Hb media al inicio de PegEPO fue de 11,2 g/l y de 11,4 g/l a los 12 meses de tratamiento (p = 0,846). El 25% de los pacientes presentaron valores de Hb superiores a 12 g/l (p < 0,0001) a los 12 meses del inicio del tratamiento, de los cuales un 45% superó los 13 g/l. Se observa la utilización de dosis un 39% menores de las indicadas en ficha técnica y, como consecuencia, un coste inferior respecto al teórico esperado. Conclusiones: Las dosis utilizadas de PegEPO en pacientes con AAE previo fueron inferiores a las indicadas en ficha técnica manteniéndose estable la Hb a los 12 meses del inicio. Una mayor proporción de pacientes superan el límite de 13 g/l.
Background: Methoxy polyethylene glycol-epoetin beta (PEG-EPO) is indicated for the treatment of anaemia due to chronic kidney disease. Its long half-life allows it to be administered once per month in maintenance therapy. Objective: To evaluate the use, effectiveness and cost of PEG-EPO in a group of pre-dialysis chronic renal failure patients. Method: Retrospective observational study in pre-dialysis patients who began treatment with PEG-EPO between May 2008 and February 2009. The following data were gathered: age, sex, haemoglobin levels (Hb) and erythropoiesis-stimulating agent (ESA) dose and frequency. The follow-up period was 12 months. Results: We included 198 patients. Mean Hb upon starting PEG-EPO in patients who had received no prior treatment was 10.8g/l, and 11.6g/l at 90 days (P<.0001). In patients previously treated with ESA, mean Hb before starting PEG-EPO treatment was 11.2g/l, and 11.4g/l at 12 months (P=.846). Hb values were higher than 12g/l (P<.0001) after 12 months of treatment in 25% of patients; of these, 45% had values above 13g/l. We observed doses 39% lower than those indicated on the drug leaflet, resulting in a reduction in the originally expected theoretical costs. Conclusions: The doses of PEG-EPO administered to patients with a prior history of ESA treatment were lower than those indicated by the drug leaflet, and Hb remained stable after 12 months of treatment. A large portion of the patients had levels above the 13g/l threshold.
INTRODUCTION
Anaemia is a common complication of chronic kidney disease (CKD) that is associated with decreased patient quality of life and increased risk of other clinical complications, including an important increase in cardiovascular risk. Anaemia appears in the early stages of the disease in the form of decreased haemoglobin (Hb) levels when the glomerular filtration rate is around 70ml/min (males) or 50ml/min (females). In more advanced stages, and in patients on dialysis, approximately 90% of patients have anaemia.1-3
Several non-PEGylated erythropoiesis stimulating agents (ESA) are currently available for clinical use, including epoetin alpha, epoetin beta, darbepoetin alpha, and epoetin theta. In addition to these drugs, the European Union has issued a centralised authorisation for the use of several different epoetin biosimilars, with epoetin alpha being the reference agent. The short half-life of these molecules requires frequent administrations, one to three times per week, in order to achieve Hb levels within the established limits. In 2007, methoxy polyethylene glycol epoetin beta (PEG-EPO) was authorised for use in Europe. The longer half-life of this compound allows for a bi-weekly administration during the induction phase of treatment, and a monthly administration during the maintenance phase.
The efficacy, safety, and tolerance of PEG-EPO has been analysed and compared to other ESA in six different phase III trials, which evaluated the efficacy and safety of treating CKD-related anaemia with this drug. These trials demonstrated that PEG-EPO has a similar safety profile to other ESA, and that its most common adverse side effects are arterial hypertension, diarrhoea, and nasopharyngitis. The incidence of severe adverse side effects (pneumonia and myocardial infarction) was slightly lower in the group treated with PEG-EPO than in the other groups.
The aim of our study was to evaluate the use and effectiveness of PEG-EPO in a group of pre-dialysis patients at our hospital, as well as to perform a cost analysis, comparing this to previous treatments with other ESA.
METHODS
We performed a retrospective observational study including all pre-dialysis (not currently receiving dialysis treatment) patients at a tertiary hospital with stage 3, 4, or 5 CKD who started treatment with PEG-EPO between 1 May 2008 and 28 February 2009. We excluded all patients that were on dialysis.
We obtained our data from the outpatient pharmacy programme and the hospital clinical workstation. We compiled data for the following variables: age, sex, Hb at start of treatment with PEG-EPO and after 1, 3, 6, and 12 months, and treatment with previous ESA (ESA used, dose, and frequency) and PEG-EPO (dose and frequency). For patients that had received treatment with other ESA before starting treatment with PEG-EPO, Hb at the start of the study was calculated as the mean Hb value of the 6 months prior to treatment with PEG-EPO.
All statistical analyses were performed using SPSS software version 19.0.
RESULTS
Our study included a total of 190 pre-dialysis patients (101 males) with stage 3 (50 patients), stage 4 (85 patients) or stage 5 (55 patients) CKD, excluding stage 5 D. The mean age for all patients was 65 years old (range: 22-93 years).
During the study period, no significant differences were observed in iron supplements or the administration of potentially anaemia-causing agents, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Of the 190 patients included in the study, 127 (66.8%) had previously received treatment with another ESA: 67 patients received darbepoetin (52.7%) and 60 received epoetin beta (47.3%).
The median dose of PEG-EPO was 75µg/month (range: 50-150µg/month) in patients with and without previous treatment with ESA. We observed no statistically significant differences in the dose of PEG-EPO received based on previous treatment or stage of CKD. In patients previously treated with darbepoetin, the median dose of PEG-EPO was 75µg/month (range: 75-250µg/month). In patients that previously received epoetin beta, the median dose of PEG-EPO was also 75µg/month (range: 50-250µg/month).
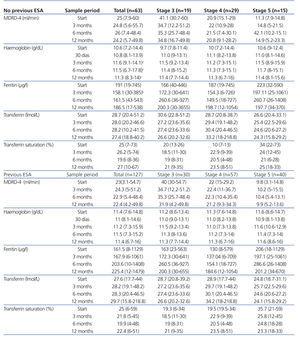
Mean Hb at the start of treatment with PEG-EPO in patients without previous treatment with ESA was 10.6g/dl (range: 7.2-14.4g/dl); after 3 months, the mean was 11.6g/dl (range: 9.1-14.1g/dl) (P<.0001), and 11.3g/dl (range: 8.3-14g/dl) (P<.0001) after 12 months. Ferritin levels were significantly different 3 months after the start of treatment with PEG-EPO, with a mean 158.1µg/l as compared to 191µg/l at the start of treatment. After 12 months, the mean ferritin value was 187µg/l. The ferritin saturation value did not vary significantly 12 months after the start of PEG-EPO treatment, nor did glomerular filtration rate after 3, 6, or 12 months. The analysis of patient values by stage of CKD only showed a statistically significant decrease in ferritin values for stage 4 patients after 3 months.
Mean Hb during the 6 months prior to treatment with PEG-EPO in the group of patients that had previously received ESA treatment (132 patients) was 11.4g/dl (range: 7.2-14.8g/dl) and this value was 11.5g/dl (range: 7.3-15.2g/dl) after 6 months of treatment with PEG-EPO (P=.476) and 11.4g/dl (range: 6.7-16g/dl) after 12 months (P=.846). Ferritin, transferrin, and transferrin saturation values were stable throughout the follow-up period. Glomerular filtration rate did not vary between measurements taken after 3, 6, and 12 months of treatment in any stage of CKD (Table 1), but there was a non-statistically significant drop in glomerular filtration rate at the end of the first year (approximately 6-7ml/min) in stage 3 patients (both in patients with and without previous treatment with ESA).
The PEG-EPO dose was adjusted in 13% of patients, 44% of which (n=11) required increased doses (mean increase: 58%), and 56% (n=14) required reduced doses (mean reduction: 32%). These changes in dosage did not cause any variation in the median dose prescribed. PEG-EPO treatment was suspended in 20% of patients, of which 35% required transfer to treatment with another type of ESA due to poor control of Hb, 25% died, and the remaining 40% received transplants. The causes of death were: lung cancer (2), ischaemic heart disease (1), congestive heart failure (2), pulmonary oedema (1), complicated clavicle fracture (1), pneumonia (1), and subarachnoid haemorrhage (1). No deaths were attributed to treatment with ESA.
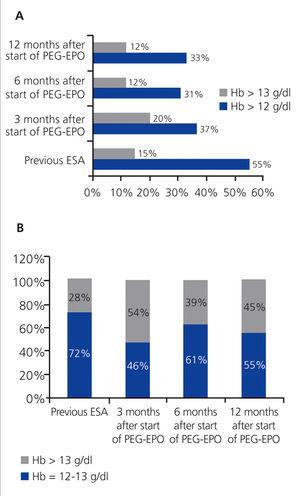
Hb values were above 12g/dl during treatment with ESA prior to PEG-EPO in 55% of patients (n=77). Values were above 13g/dl in 28% of these patients. During treatment with PEG-EPO, 19% of patients (n=37) had Hb values higher than 12g/dl after 3 months, 54% of which (n=20) had values above 13g/dl (P<.0001). After 6 months, 31% (n=41) had values above 12g/dl (39% of which were above 13g/dl), and after 12 months, 25% (n=33) had values above 12g/dl (45% of which were above 13g/dl) (Figure 1).
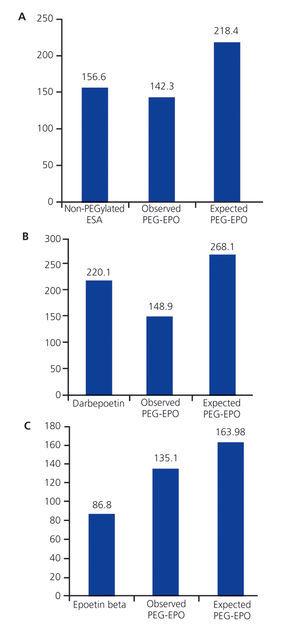
The mean manufacturer’s sale price (MSP) of non-PEGylated ESA was 156.5€/patient/month, and 142.3€/patient/month for PEG-EPO (P>.05). The mean cost of darbepoetin was 220.1€/patient/month; after switching to PEG-EPO, the mean cost was 148.9€/patient/month (P<.0001). The mean cost of epoetin beta was 86.8€/patient/month; after switching to PEG-EPO, this cost increased to 135.1€/patient/month (P<.0001).
DISCUSSION
The NKF-KDOQI 2006 guidelines recommend maintaining Hb levels between 11g/dl and 12g/dl, without exceeding 13g/dl,2,3 in contrast to the 2000 guidelines, in which no upper limit was established. These changes are based on the results from 22 randomised and controlled studies, including the CREATE study (Europe) and the CHOIR study (United States). The CREATE study analysed cardiovascular events, the need for dialysis, and quality of life in CKD patients that did not receive dialysis treatment and that were administered epoetin beta and randomised into two different treatment groups: patients with Hb at 13-15g/dl (n=301) and patients with Hb at 10.5-11.5g/dl (n=302). The authors observed that normalisation of Hb levels improved patient quality of life, especially in terms of physical function and mental health, but with no differences in the appearance of cardiovascular effects. In contrast, these patients required more dialysis and had more episodes of hypertension and headache.4
In the CHOIR study, all patients received epoetin alpha and were randomised into two different treatment groups, one for patients with Hb levels equal to or greater than 13.5g/dl (n=715) and one for patients with Hb values of 11.3g/dl (n=717). The authors concluded that normalisation of Hb values was associated with an increased risk of cardiovascular events or others, but with no improvement in quality of life, and so it was recommended that patients maintain Hb levels between 11g/dl and 13g/dl.5 A review of the use of ESA performed by Ortega et al6 supported these conclusions. The results from these studies led the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to issue a safety warning on the use of ESA and high Hb values. The FDA recommends using the lowest possible doses of ESA, with gradual increases in order to avoid the need for transfusions, but without exceeding Hb concentrations of 12g/dl.7 The EMA, in a post-authorisation report, stated that patients with CKD and an Hb>12g/dl had more severe cardiovascular complications and all-cause mortality. So, Hb values were recommended to be kept below 12g/dl.8
The 2008 KDIGO guidelines concluded that Hb levels greater than 13g/dl are harmful to the patient, 9.5-11.5g/dl provide more benefits than risks, and there is no evidence of the cost-benefit ratio in patients with Hb values between 11.5g/dl and 13g/dl.9 The S.E.N. recommends maintaining levels between 11g/dl and 13g/dl and not to exceed 13g/dl except for certain specific cases, such as in non haemodialysis young patients or those with chronic obstructive pulmonary disease.10
In our study, 12% of all patients developed Hb levels >13g/dl in the first 6 months of treatment with PEG-EPO, as compared to 15% with the previous non-PEGylated ESA treatment. Within the group of patients with Hb>12g/dl, 45% of patients exceeded the limit of 13g/dl within 12 months vs 28% in the last six months of treatment with other non-PEGylated ESA. Figure 2 shows the percentages of patients with Hb>13g/dl.
In the ARCTOS study (diabetic patients with stage 3 or 4 CKD), mean Hb with PEG-EPO treatment every two weeks was 11.8g/dl, and 11.7g/dl in patients that received treatment every 4 weeks, as compared to an Hb of 11.2g/dl in patients that received darbepoetin. In our study, Hb in patients treated with non-PEGylated ESA was 11.19g/dl (range: 7.2-14.8g/dl) and with PEG-EPO, mean Hb was 12.9g/dl (range: 6.84-15.9g/dl). The mean Hb in the 6 months prior to treatment with PEG-EPO was not significantly different from Hb values during the 12 months following the start of treatment with PEG-EPO.
In the CORDATUS study, 307 patients with stage 3-4 CKD were randomised to receive either monthly PEG-EPO or darbepoetin alpha every 2 or 4 weeks. The increase in Hb was faster in patients in the darbepoetin group but more progressive in the PEG-EPO group. The mean response time of Hb=12g/dl was 43 days for PEG-EPO patients and 29 days for the darbepoetin alpha group. Fewer patients developed Hb>12g/dl in the PEG-EPO group (14.3%) than in the darbepoetin group (23.1%) (P<.05).11 In our study, mean haemoglobin values at the start of treatment with PEG-EPO in the 66 patients with no previous treatment with ESA was 10.6g/dl (range: 7.2-14.4g/dl); one month after starting treatment with PEG-EPO, mean Hb was 10.8g/dl (range: 7.3-15.9g/dl), 11.6g/dl (range: 9.1-14.1g/dl) after 3 months, and 11.3g/dl (range: 8.3-14g/dl) after 12 months (P<.0001).
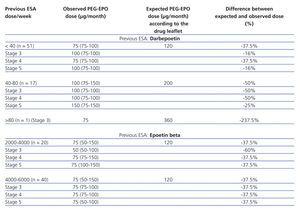
The drug leaflet for PEG-EPO establishes the dosage required for switching from a non-PEGylated ESA to PEG-EPO. Table 2 summarises the PEG-EPO doses administered, on accordance with the previous ESA dose, and the theoretical (expected) PEG-EPO dose that should be used according to the leaflet for switching from non-PEGylated ESA to PEG-EPO in our patients. The table also includes the difference between the expected and the observed (actual) PEG-EPO doses. Both the group of patients that previously received darbepoetin and the group that received epoetin beta showed clear differences between these two values (expected vs observed), with a tendency for using lower doses than those recommended in the drug leaflet, with good Hb control during the first three months following the switch to PEG-EPO. The observed doses were 39.2% lower than expected doses (43.5% lower for the group that was previously treated with darbepoetin, and 34.5% lower for the group previously treated with epoetin beta) (P<.0001; 95% CI: -68.18-[-51.21]).
As regards the other variables analysed in our study, there was a significant decrease in ferritin levels after three months in patients with no previous treatment with ESA, which could be explained by the high variability in tolerance to oral iron supplements, since intake remained stable.
In the case of patients that previously received ESA, ferritin, transferrin, and transferrin saturation values remained constant. Renal function remained stable in all patient groups.
Figure 2 displays the economic analysis comparing the observed cost of switching to PEG-EPO with the expected cost based on the theoretical dosage for PEG-EPO. Using the doses indicated in the drug leaflet, the monthly cost of PEG-EPO would be 218.4€/patient/month, whereas the observed cost was 142.3€/patient/month (P<.0001; 95% CI: -91.77-[-60.45]). The mean cost of PEG-EPO for patients that had previously received darbepoetin and epoetin beta would theoretically be 268.1€/patient/month and 163.98€/patient/month, respectively (P<.0001 as compared to observed cost).
Our study has the primary limitation of being a retrospective study; even so, we were able to evaluate the usefulness and effectiveness of PEG-EPO during the first months of treatment, as well as the economic impact caused by the switch.
In this study, although the doses used were lower than those recommended in the PEG-EPO drug leaflet, we achieved good control of Hb. In light of our results, we would advise to reduce the initial doses of PEG-EPO recommended in the drug leaflet by 44% for patients previously treated with darbepoetin and by 35% in patients treated with epoetin beta. However, given that ours was a retrospective study, we also believe that specific studies should be designed to determine the most adequate dose of PEG-EPO based on Hb values or previous doses of other ESA.
CONCLUSIONS
The mean time elapsed until reaching an Hb>11g/dl in the group of patients that started PEG-EPO with no previous medication was 3 months, and the mean value of Hb remained stable for 12 months.
In our study, the change from non-PEGylated ESA to PEG-EPO caused no significant difference in the mean haemoglobin level reached. Despite the fact that the total number of patients that reached HB>12g/dl was similar between treatments with PEG-EPO and previous non-PEGylated ESA, the percentage of patients with Hb>13g/dl was higher.
The doses used in the switch from non-PEGylated ESA to PEG-EPO were lower than the recommendations from the drug leaflet, with good control of Hb within 12 months after making the switch and a mean cost that was lower than expected.
Our results may be useful in the management and optimisation of treatment with PEG-EPO.
Conflicts of Interest
Dr A. Martínez-Castelao has collaborated with the laboratories Novartis, Boëhringer, Abbott, Shire, Amgen and Roche, and is a consultant for the laboratories Abbott, Amgen and Roche.
All other authors affirm that they have no conflicts of interest related to the content of this article.
Figure 1. Percentage of patients with haemoglobin above 12g/dl
Figure 2. Comparison of the observed costs of switching to PEG-EPO and expected costs according to PEG-EPO dosage
Table 1. Mean values of haemoglobin, glomerular filtration rate, ferritin, transferrin, and transferrin saturation 1, 3, 6, and 12 months after the start of PEG-EPO
Table 2. Observed and expected (based on previous ESA) doses of PEG-EPO