Introducción: Existen pocos estudios sobre el pronóstico individual del paciente añoso que inicia hemodiálisis (HD) crónica, pese a que frecuentemente se plantea el dilema entre el posible beneficio y la carga que supone el propio tratamiento. Objetivos: Evaluar la utilidad del índice pronóstico del Registro REIN (REIN) y del modelo predictivo de mortalidad precoz del Registre de Malalts Renals de Catalunya (RMRC) en pacientes añosos incidentes en HD al compararlos con la supervivencia observada. Métodos: Se estudiaron los pacientes mayores de 75 años que iniciaron y siguieron HD en nuestro Servicio entre 2004-2009. Se recogieron variables sociodemográficas, clínicas, comorbilidad, mortalidad y si el inicio de HD fue planificado o no. Se calculó el índice REIN y la probabilidad de mortalidad precoz del RMRC. Resultados: Se analizaron 63 pacientes de una edad media de 80,4 ± 3,9 años, con un número de enfermedades añadidas de 3,4 ± 1,8. Un 59% iniciaron HD por un catéter, un 57,1% tenían enfermedad cardiovascular, el 15,9% neoplasia, el 31,2% enfermedad pulmonar obstructiva crónica y el 19% nefropatía diabética. La supervivencia observada a los 6 y a los 12 meses fue de 79,4 y 73%, respectivamente. Los pacientes que no se valían por sí mismos (21%) presentaban una mayor mortalidad a los 6 meses. El análisis de las curvas ROC (Receiver Operating Characteristic) mostró una escasa concordancia entre la mortalidad observada y los índices REIN (área 0,681, p = 0,046) y RMRC (área 0,594, p = 0,255). Conclusiones: El índice de probabilidad de mortalidad al año del RMRC es poco útil en la práctica clínica para el pronóstico individual. El índice REIN es sólo ligeramente concordante con la mortalidad observada en los primeros 6 meses de HD. Una pobre autonomía funcional fue el principal factor de riesgo de mortalidad precoz en los pacientes añosos que inician HD.
Introduction: Few studies address the individual prognosis of an elderly patient beginning chronic haemodialysis (HD), despite the fact that doctors must frequently weigh the possible benefits and disadvantages of prescribing this treatment. Objectives: Evaluate the usefulness of the REIN Registry’s prognosis score and the predictive index for early mortality proposed by the Catalan Registry of Renal Patients (RMRC, Registre de Malalts Renals de Catalunya) in elderly patients beginning HD by comparing indices with observed survival rates. Methods: We studied patients aged 75 years and older who started and continued HD treatment in our Department between 2004 and 2009. Socio-demographic, clinical, co-morbidity and mortality data were recorded, in addition to whether or not initiating HD was planned. We calculated the REIN score and the RMRC probability of early mortality. Results: We analysed 63 patients with a mean age of 80.4±3.9 years and a mean of 3.4±1.8 additional illnesses. Of these patients, 59% began HD with a catheter; 57.1% had cardiovascular disease, 15.9% neoplasia, 31.2% chronic obstructive pulmonary disease and 19% diabetic nephropathy. Survival rates observed at 6 and at 12 months were 79.4% and 73%, respectively. Patients who began HD on an emergency basis (47.7%) or who were unable to care for themselves (21%) had higher 6-month mortality rates. Analysis of ROC curves (Receiver Operating Characteristic) showed slight concordance between the observed mortality rates and both the REIN score (area 0.681, P=.046) and the RMRC index (area 0.594, P=.255). Conclusions: The RMRC 1-year mortality probability model is not well adapted for individual prognoses in clinical practice. The REIN score only shows slight concordance with the mortality rates observed in the first 6 months of HD. Poor functional independence was the main risk factor for early mortality in elderly patients beginning HD treatment.
INTRODUCTION
Chronic kidney disease (CKD) is very prevalent in the elderly population. In fact, more than 40% of patients initiating chronic dialysis in Catalonia are more than 70 years old.1 The peak incidence rate is among patients older than 75 in the United States,2 and as a result, increasing numbers of octogenarians and nonagenarians are starting dialysis.3 On the other hand, the elderly population tends to suffer from other chronic diseases which worsen short term vital and functional prognoses, particularly in individuals who were already unable to perform basic daily activities unaided.4 The above considerations raise a debate over the theoretical benefit of chronic dialysis and its drawbacks in terms of the complications and reduced quality of life for elderly patients with multiple co-morbidities and advanced stages of CKD.5-7 It would therefore be useful to have access to the best possible prognosis predictors in order to make the right decision for the patient.8
Several co-morbidity scores have been used in order to estimate the prognosis of CKD, such as those created by Charlson,9 Khan,10 Davies,11 Liu,12 and the modified Charlson index13; the latter probably being the most commonly used and recommended for CKD.14,15 Although these scores are useful for comparing co-morbidity in different groups or populations, they are not particularly effective in determining individual prognoses. In addition, other factors including age, sex, primary kidney disease, type of treatment or starting dialysis on an unplanned, emergency basis may significantly influence mortality in these patients.16,17
Some individual prognostic models were published recently for elderly patients starting dialysis,18 haemodialysis (HD),19 and prevalent patients already undergoing HD.20 These prognostic models for incident patients on dialysis draw on a wide array of clinical and demographic variables, and they were elaborated after analysing databases containing thousands of patients from the French and Catalan kidney replacement therapy registries, the Renal Epidemiology and Information Network (Réseau Epidémiologie et Information en Néphrologie or REIN)18 and the Registre de Malalts Renals de Catalunya (Catalan Registry of Renal Patients or RMRC).19
The aim of our study was to evaluate the clinical usefulness of the REIN prognostic index (REIN score) and the Catalan registry's predictive index for early mortality (RMRC) by comparing them with individual mortality rates observed in a cohort of elderly patients who started HD and continued their treatment in a hospital nephrology department. If these models were valid in clinical practice, they would be useful tools for helping decide whether or not KRT should be indicated in these patients.
MATERIAL AND METHOD
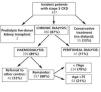
Observational, retrospective, comparative study of different individual prognostic indices for early mortality in patients 75 years of age and older who began chronic HD treatment in Hospital de Sabadell between 2004 and 2009. The nephrology department provides care to a population of 429 000 inhabitants and offers different alternatives for the initial management of end-stage chronic renal failure (ESCRF): conservative treatment, kidney transplant, peritoneal dialysis and HD. We excluded patients referred to other centres and those treated with peritoneal dialysis. Figure 1 shows clinical management details for our ESCRF patients. Of a total of 437 patients, 13% underwent conservative treatment without dialysis and 87% started chronic dialysis treatment, with 11% undergoing peritoneal dialysis. Of the 339 patients who started chronic HD, 12% were referred to other centres due to lack of available beds in our hospital. Consequently, during the study period 297 incident patients were treated with chronic HD; of which 63 (21%) were more than 75 years old and were included in the study. Follow-up ended on 1 December 2010. The mean follow-up time was 27.4±20.7 months. All patients alive at the end of the study were monitored for more than 12 months.
Treatment was undergone in a hospital HD unit, following a programme of 3 weekly sessions with a total duration of 10.5–12 hours per week to maintain eKt/V≥1.2. During the study period, we used medium-to-low-flux biocompatible synthetic membranes and low-molecular-weight heparin as anticoagulant. Since 2006, our HD unit has undergone internal and external audits and it is accredited and certified with a quality management system in accordance with ISO standards 9000-2001 and 2008.
Study variables
The following parameters with prognostic significance were gathered from the REIN18 (Table 1) and RMRC19 (Table 2) predictive indices for mortality: age, sex, primary renal disease (categorised as standard, diabetic or systemic), degree of functional independence (categorised as normal, limited but able to care for themselves, and unable to provide self-care) body mass index, type of vascular access (catheter, arteriovenous fistula or graft), type of HD onset (planned or unplanned) and co-morbidities (diabetes mellitus, NYHA class III and IV congestive heart failure, ischaemic heart disease, arrhythmias, stage III and IV peripheral vascular disease, active neoplasia, chronic obstructive pulmonary disease, chronic liver disease and severe behaviour disorders). The total number of accompanying diseases was calculated. Systemic primary renal disease was defined as a disease secondary to vasculitis, amyloidosis, myeloma, lupus or haemolytic-uraemic syndrome, and standard primary renal disease was defined as a disease not caused by diabetes or a systemic condition.19 Severe behaviour disorder was classified according to the definition given by Couchoud et al,18 that is, one affecting a patient's independence or treatment compliance. It included patients with severe dementia, psychosis or neurosis. Onset of HD was considered unplanned in cases in which the first session was administered under emergency conditions requiring that dialysis be initiated in less than 24 hours.18 The date of HD onset was recorded, as were dates and causes of death where applicable.
Calculating REIN and RMRC prognostic scores
For each patient in the study cohort, we determined real mortality at 6 months and 12 months after starting HD (Table 3), predicted mortality at 6 months according to the REIN registry's prognostic clinical score for elderly patients18 (Table 4) and probability of death at 12 months following HD onset according to the Catalan registry RMRC’s predictive model.19 The REIN score was calculated by totalling the points awarded according to each of the model’s risk factors (Table 1).18 The REIN score does not include age as a mortality factor since the model was created using data from patients in the French registry who were aged 75 years and older, and it therefore only applies to elderly patients. RMRC score was calculated by applying the equation shown in Figure 2.19
In this equation, Π (x) is the probability of death during the first 12 months of HD treatment; β is the constant for the logistic regression model (−5.799) and β1x is the sum of the different â coefficients applicable according to the model’s risk factors present in a specific patient (Table 2).
Statistical analysis
We compared prognoses observed in our cohort with predictions made using the two study methods in order to determine whether or not the models would be useful in predicting individual prognoses in clinical practice. Statistical analysis was performed using SPSS software for Windows, version 15. We used descriptive statistics with a 95% confidence interval (CI) for means and proportions; Fisher’s exact test and the Mann-Whitney U test as non-parametric tests for comparing qualitative and quantitative variables, respectively; Kaplan-Meier curves to analyse survival data; and ROC (Receiver Operating Characteristic) curves to analyse for concordance with prognostic indices. Values of P<.05 were considered statistically significant.
RESULTS
We studied 63 patients with a mean age of 80.4 ± 3.9 years (range: 75.4-91.8); 60% were male. The most frequent cause of primary kidney disease was vascular (37%), followed by diabetic nephropathy (19%). The mean length of the pre-dialysis follow-up was 3.9+4.1 years (range, 0–15.8). Patients who started chronic HD had not undergone prior nephrological monitoring in 19% of all cases.
REIN score
The mean score on the REIN index was 2.9±2.3 (range: 0–8) and it was significantly higher in patients who died during the first 6 months (4.0 vs 2.6; P=.046). Table 1 shows the frequency of different REIN index prognostic factors in our study sample. We observed high incidence rates of heart disease and unplanned onset of HD (52.4%). In many cases, this is due to chronic renal failure becoming acute. Table 4 compares predicted mortality with actual observed mortality for each of the REIN scores.
RMRC index
Table 5 shows the frequency of early mortality risk factors included in the Catalan register RMRC’s predictive model for our study cohort. It should be stated that many patients have poor functional independence (21% of patients were unable to provide self-care) and 59% of the patients needed a catheter as their first vascular access for HD. The mean number of co-morbidities, excluding CKD, was 3.4±1.8 (range: 0–8).
Prognostic factors for mortality
We used the Fisher’s exact test to examine the distribution of the different factors included in a patient’s REIN and RMRC prognostic scores, according to whether or not early death occurred, and we only observed a statistically significant association between poor functional independence (need for special care) and 6-month mortality (14% vs 46% mortality rates at 6 months, P=.02).
Observed and predicted mortality rates
Mortality rates observed in our population were 20.6% at 6 months and 27% at 12 months, respectively (Figure 3). Table 3 shows the causes of death. Analysis of the average for the entire cohort did not reveal significant differences between observed mortality (27%) and the mean mortality at 1 year mortality predicted by the RMRC index (23.5; 95% CI:19.9%–27.1%). However, there were differences between observed mortality at 6 months (20.6%) and mean mortality at 6 months predicted by the REIN index (17.5%; 95% CI: 14.8%–20.1%).
ROC curves
When ROC curves were analysed in order to study how the two predictive models for mortality (REIN and RMRC) might be applicable to individual patients, we found no statistically significant concordance (area under the curve 0.594, P=.255) between the RMRC index for predicting mortality within 12 months following HD onset and observed mortality (Figure 4). Slight concordance was observed (area under the curve 0.678, P=.049) between the REIN index and observed mortality at 6 months (Figure 5).
DISCUSSION
Upon evaluating the two predictive models of early mortality in incident patients on HD aged 75 and older and their applicability to daily clinical practice, we did not find good concordance between predicted and observed mortality that would enable us to use these models as prognostic tools to aid in deciding whether or not to start KRT on an individual basis. With regard to the probability of death at 12 months estimated using the Catalan registry’s model (RMRC),19 observed survival rates for the entire study cohort were similar to calculated predicted rates, but when individual prognostic value was analysed using ROC curves, concordance between observed and predicted mortality was very slight. Concordance of the REIN18 index’s predictions with observed 6-month mortality rates was only slightly significant, making it of little use for clinical practice. We must point out that in our study sample, there were no patients with a REIN score of 9 or more points; and the predicted 6-month survival rate of such patients is only 38%.18 On the other hand, the main factor in our cohort indicating poor survival prognosis within 6 months following HD onset was very poor functional independence (being unable to provide self-care), and this has already been described by other authors.17,21
A remarkable 52.4% of patients in our cohort started HD on an unplanned basis according to the criteria employed by the French REIN model, meaning that the first session was administered during an emergency in which the patient needed dialysis in less than 24 hours.18 Other authors prefer to speak of “suboptimal dialysis initiation” and define it as in-hospital dialysis initiation of dialysis through a central venous catheter or on a dialysis modality that was not planned in advance.22 In any case, our data are not surprising if we consider the fact that some registries, including the Catalan registry, report that approximately 35% of all adult patients and 40% of those older than 75 start chronic HD treatment during an episode of acute or acute on chronic renal failure.1 On the other hand, the high percentage of unplanned HD initiation in our cohort is similar to that published for Spain23 and other countries,24 between 40% and 50%.
Another interesting finding is that only 41% of our elderly patients started chronic HD treatment by means of an arteriovenous fistula. The progression of non-proteinuric CKD in the elderly is usually slow, especially if it is due to nephroangiosclerosis.24 Many elderly patients with stage 4 CKD die before KRT25 is needed, so creating fistulas for HD is often delayed in such patients until they reach glomerular filtration rates26 of close to 15ml/min/1.73m2. In this context, it is not surprising that many elderly patients with acute on chronic renal failure have no usable fistula for HD initiation.
A complex dilemma arises in cases of very elderly CKD patients with numerous co-morbidities as treatment with chronic HD is burdensome, but may produce benefits in terms of survival and quality of life. The matter is open to interpretation and although there are observational studies that show that in patients older than 75 with multiple co-morbidities, especially with ischaemic heart disease, dialysis does not offer better survival rates than conservative treatment without dialysis when considering the survival time from the moment when the glomerular filtration rate27 falls below 15ml/min/1.73m2.
The problem arises when we estimate an individual patient prognosis to help decide whether or not to start chronic dialysis. Tools for such a task must be evaluated in the specific contexts (countries, hospitals and concrete clinical practices) in which they will be used. With this in mind, our study shows a poor level of concordance between early mortality observed and early mortality predicted by the two prognostic models in the study, one of which was developed in Spain.
This study has certain limitations. Firstly, it is a retrospective study with a relatively small patient sample. On the other hand, we must point out that for very advanced renal failure, our hospital explicitly offers the possibility of conservative palliative treatment without dialysis28 in addition to KRT techniques, which could introduce a selection bias as patients receiving such treatment were excluded from the study. Likewise, patients were referred to other nearby dialysis centres during the study period due to lack of beds in our hospital, and additionally, the patients we refer to other centres are generally in better clinical condition than those which our department accepts for HD treatment. For these reasons, our early mortality rates would have been lower if we had not referred these patients to other hospitals, thereby losing them to follow-up. Despite these limitations, we believe that an interesting aspect of our study is that it was carried out under the normal conditions of clinical practice in a nephrology department with an active education and information programme for patients with advanced CKD.
In conclusion, after having evaluated the usefulness of two new prognostic indices predicting the probability of early mortality in patients older than 75 who started chronic HD in our hospital, we observe that neither the 1-year mortality index developed by the Catalan registry RMRC nor the 6-month mortality index developed by the French registry REIN were useful as prognostic tools in our clinical practice.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Prognostic index developed by the French registry REIN
Table 2. Prognostic model for mortality developed by the Catalan Registry RMRC
Table 3. Early mortality observed in patients older than 74 years in our cohort
Table 4. Mortality during first 6 months on dialysis and REIN index score
Table 5. Frequency of different RMRC index mortality risk factors in our cohort
Figure 1. Kidney replacement therapy in our patients with stage 5 chronic kidney disease (2004-2009)
Figure 2. Formula for calculating the RMRC index
Figure 3. Survival curve of patients older than 75 years on chronic haemodialysis
Figure 4. ROC curve for the predictive model of early mortality during haemodialysis, developed by the Catalan Registry RMRC
Figure 5. ROC curve for the French registry REIN¿s predictive model of mortality