Androgen deficiency is an endocrine abnormality that is common in male patients with chronic kidney disease and affects 50%–75% of patients treated with haemodialysis.1,2 Its clinical significance is not well known, although various cross-sectional studies have linked low testosterone levels to sexual dysfunction, anaemia, loss of muscle mass, increase in cardiovascular risk and greater mortality.2–5
Androgens in pharmacological doses have effects on anaemia and nutritional parameters in patients treated with regular haemodialysis.6 However, few studies have analysed the effect of treating hypogonadism from renal failure with physiological doses of testosterone, and their results have been controversial.7,8
In this study we present the preliminary data for the results obtained following correction of androgen deficiency in patients treated with haemodialysis.
We defined androgen deficiency as a total serum testosterone concentration lower than 300ng/dl and an unbound testosterone concentration lower than 225pMol/l. Testosterone circulates in plasma bound to proteins (especially albumin and a transport globulin [sex hormone-binding globulin, or SHBG]); only 1%–3% circulates unbound. A uraemic patient may have an abnormal SHBG concentration.9 Therefore, in patients with chronic kidney disease it is advisable to confirm testosterone deficiency by determining the concentration of unbound testosterone, especially in cases with a total testosterone level that is low but close to the lower limit of the normal range. Unbound testosterone was calculated based on levels of total testosterone, SHBG and albumin.10
The study was conducted in male patients treated with regular haemodialysis for more than 6 months who were in a stable clinical situation and had not required admission in the last 3 months. All draws for laboratory testing were performed immediately before the first haemodialysis session of the week.
Of the 39 patients analysed, 20 (51%) had a total testosterone concentration lower than 300ng/dl, all had an unbound testosterone concentration lower than 225pMol/l, and all were diagnosed with androgen deficiency. Twelve of these 20 patients agreed to participate in the study and granted their written consent. Patients were randomly assigned to the treatment group (6 patients who received testosterone by the transdermal route: one sachet of 5g of gel containing 50mg of testosterone daily for 3 months) or the control group (the other 6 patients). The dose of testosterone administered to the treatment group was the minimum initial dose recommended in the medicine's summary of product characteristics.
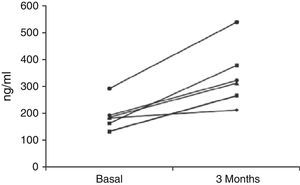
Patients in the treatment group had a younger age (67±4 vs 75±9 years; p=0.182), and at baseline there were no differences between the two groups in terms of the other parameters analysed. Table 1 shows the changes in these parameters. The unbound and total serum testosterone concentration increased in all patients treated, but it only reached the normal range in 4 (Fig. 1). Neither of the 2 groups of patients showed a change in concentrations of total cholesterol, HDL cholesterol, LDL cholesterol or triglycerides (data not shown).
Effect of testosterone replacement therapy.
| Treatment group | Control group | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | p | Baseline | 3 months | p | |
| Total testosterone [300–900ng/dl] | 188 (54) | 335 (113) | 0.005 | 174 (75) | 189 (77) | 0.105 |
| Unbound testosterone [>225pMol/l] | 147 (48) | 279 (109) | 0.034 | 102 (49) | 114 (71) | 0.310 |
| FSH [1–12mIU/l] | 10.4 (12.7) | 7.4 (10.7) | 0.035 | 13.1 (7.8) | 13.9 (9.3) | 0.746 |
| LH [1.1–8.8mIU/l] | 14.9 (15.9) | 7.5 (10.6) | 0.026 | 14.6 (8.2) | 15.3 (8.5) | 0.324 |
| Haemoglobin [g/dl] | 11.4 (1) | 11.2 (0.8) | 0.562 | 11.6 (1.8) | 10.6 (0.4) | 0.180 |
| EPO alfa [IU/kg/week] | 103 (82) | 69 (76) | 0.044 | 124 (104) | 121 (114) | 0.889 |
| Albumin [g/dl] | 4 (0.3) | 4.5 (0.4) | 0.011 | 3.9 (0.4) | 4.1 (0.5) | 0.242 |
The normal value is in square brackets and the standard deviation is in parentheses.
The prevalence of androgen deficiency in male patients from the haemodialysis unit was 51%. This figure was similar to that observed in other studies. Transdermal administration of testosterone, in the minimum dose recommended in the summary of product characteristics, increased the serum level of the hormone and had an inhibitory effect on the hypothalamic–pituitary axis. In 4 of the 6 patients treated, the total testosterone concentration exceeded the lower limit of the range considered to be normal, but the dose administered was not sufficient to reach the mean levels of this range (300–900ng/ml).
The increases in testosterone concentration achieved, though small, were associated with a reduction in needs for erythropoietin alfa and a modest but significant increase in albumin concentration.
This was a preliminary study, conducted in a small number of patients during a short follow-up period, but its results suggest that androgen deficiency in patients with chronic kidney disease may be clinically significant, with repercussions for anaemia and nutrition, as some cross-sectional studies have suggested. They also suggest that replacement therapy may have beneficial effects on these complications.
Please cite this article as: Pampa Saico S, Teruel Briones JL, Fernández Lucas M, Delgado Yagüe M, García Cano AM, Liaño García F. Tratamiento de la deficiencia androgénica del enfermo dializado con suplementos de testosterona. Resultados preliminares. Nefrologia. 2016;36:462–463.