The enemy to beat is the hepatitis C virus (HCV), a RNA virus of 30–38nm in size with icosahedral coat. It is a Hepacivirus of the Flaviviridae family, which has 7 genotypes.1 Its transmission is parenteral, therefore its transmission through transplanted organs has been well documented and it causes acute and chronic hepatitis.2 Given this risk, transplant from donors with antibodies against HCV has been totally contraindicated in noninfected recipients. It is estimated that more than 500 high quality kidney grafts are discarded annually in the USA for this reason.3 In 2016 we did not transplanted 15 kidneys for this reason.4. A number of organs could be transplanted to recipients infected with HCV.5 The efficacy of direct-acting antivirals eliminated HCV patients from the waiting lists6 making possible the use these organs, in a society clearly need them.4
At present we have tools to change this panorama that have been clearly stated in the Hepatitis C Consensus Conference of the American Society of Transplantion.7
The risk of HCV transmission must be defined; there is a clear distinction between donor carrying antibodies, not necessarily infectious, and the patient with viraemia that is infective. To carry out this separation, we use the Xpert® HCV Viral Load test with the Cepheid GeneXpert® system (distributed by Werfen), available in our hospital, that quantifies the viral load in 1h which allows the quick identification of the infective donor.
The efficacy, safety and tolerability of direct antivirals make feasible the transplantation of viraemic donors in seronegative recipients. Even more now with the current commercialization of the compounds glecaprevir and pibrentasvir effective against all genotypes, so there is no need to identify the genotype of the donor and are not contraindicated in renal failure, which allows its use in the peritransplant period.8
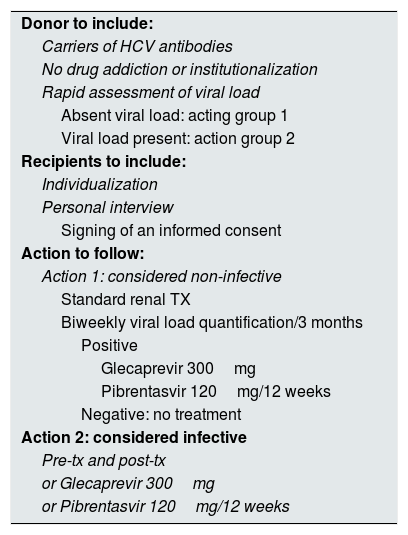
Having these tools and the successful experiences by Durand et al.9 and Goldberg et al.10 this year, we requested an ethical evaluation to implement an action protocol (Table 1) to the bioethics committee of our hospital, which gave its approval on July 18, 2017 and stressed the importance of explaining to the recipient the procedure for the subsequent signing of an informed consent.
Action protocol.
| Donor to include: |
| Carriers of HCV antibodies |
| No drug addiction or institutionalization |
| Rapid assessment of viral load |
| Absent viral load: acting group 1 |
| Viral load present: action group 2 |
| Recipients to include: |
| Individualization |
| Personal interview |
| Signing of an informed consent |
| Action to follow: |
| Action 1: considered non-infective |
| Standard renal TX |
| Biweekly viral load quantification/3 months |
| Positive |
| Glecaprevir 300mg |
| Pibrentasvir 120mg/12 weeks |
| Negative: no treatment |
| Action 2: considered infective |
| Pre-tx and post-tx |
| or Glecaprevir 300mg |
| or Pibrentasvir 120mg/12 weeks |
In the application of the protocol of action (Table 1), we transplanted 2 seronegative male HCV recipients of 45 and 58 years with grafts from a 40-year-old female donor and a 57-year-old male both seropositive, but with negative viral load, for what we apply the action 1. The patients today are carriers of a functioning graft and the viral load in them has been repeatedly negative.
Our initial experience, endorsed by other teams, should encourage the extensive, albeit meticulous, use of this type of donors and lead us to the conclusion that HCV, the enemy to beat, is very likely beaten.
Please cite this article as: Franco A, Balibrea N, Gimeno A, Merino E, Lopez MI, Santiago C, et al. Trasplantar receptores hepatitis C negativos con riñones de donantes seropositivos. ¿Por qué no? Nefrologia. 2018. https://doi.org/10.1016/j.nefro.2018.04.005