Amiodarone-induced thyroiditis is a type of thyrotoxicosis that may appear in the context of the treatment of different tachyarrhythmias with this drug. We can distinguish between two types: type 1 is an iodine-induced hyperthyroidism, whereas type 2 is a destructive thyroiditis.1
The treatment of type I includes thioamides (methimazole and propylthiouracil). Other alternatives could be potassium perchlorate, lithium carbonate, iopanoic acid, cholestyramine and radioiodine.2–5 The definitive option is a thyroidectomy.6
Type 2 is treated with prednisone. Mixed forms are treated with prednisolone and thioamides.1
Thyroid plasmapheresis was first described in 1970 in three cases of thyroid storm.7 The therapeutic benefit that it delivers is due to the fact that it eliminates the free thyroid hormones and the binding proteins (thyroxine-binding globulin [TBG], transthyretin, albumin), replacing them with new unsaturated proteins, and also eliminates thyroxine 5-deiodinase, cytokines, antibodies, catecholamines and toxins, etc.8
We present the case of a 48-year-old man referred by the Cardiology Department for thyrotoxicosis: thyrotropin (thyroid-stimulating hormone [TSH]) <0.01 mIU/l (0.3–4.2), free thyroxine (T4l) 3.9 ng/dl (0.85−1.75), triiodothyronine (T3l) 8.2 pg/mL (2−4.4), anti-thyroid peroxidase (anti-TPO) antibodies <9 IU/mL (0–34) and TSH antireceptor antibodies (thyroid stimulating immunoglobulin [TSI]) <0.8 IU/l (0–2.4). He had a background of dilated cardiomyopathy with severe ventricular dysfunction following viral myocarditis and anticoagulated chronic atrial fibrillation. No exposure to iodine sources, except for a three-month treatment with amiodarone one year previously. He reported a sensation of restlessness with continuous palpitations, excessive perspiration, dyspnoea on minimal exertion and weight loss in the previous weeks.
Amiodarone is a class III anti-arrhythmic drug structurally similar to the thyroid hormones and containing an abundant amount of iodine. Due to its lipophilicity, it is deposited on the fatty tissue and has a very long half-life, which explains that side effects may occur months after it is discontinued.1
The thyroid ultrasound showed a normal thyroid and the thyroid scan was consistent with thyroiditis, so antithyroid treatment was initiated with methimazole and prednisone.
Controls were performed every 2–4 weeks without achieving a response to the antithyroid treatment, and with clinical worsening. Despite potassium perchlorate being added, the hormones increased and renal function deteriorated.
As an exception, we considered performing thyroid plasmapheresis prior to a preferential thyroidectomy. Five (5) sessions were performed with an exchange of 3500 mL of pasteurised liquid plasma proteins (PLPP), which reduced hormone levels and yielded a notable clinical improvement.
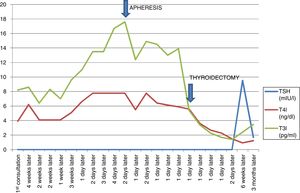
Table 1 presents the analytical evolution.
Analytical evolution.
| T4l (0.85−1.75 ng/dl) | T3l (2−4.4 pg/mL) | Methimazole (mg/day) | Prednisone (mg/day) | Perchlorate (mg/day) | Apheresis | |
|---|---|---|---|---|---|---|
| 1st consultation | 3.9 | 8.2 | 15 | 30 | ||
| 4 weeks later | 6.2 | 8.6 | 30 | 60 | ||
| 2 weeks later | 4.1 | 6.4 | 30 | 60 | ||
| 2 weeks later | 4.1 | 8.3 | 40 | 90 | ||
| 1 week later | 4.1 | 7 | 40 | 75 | ||
| 3 weeks later | 5.09 | 9.6 | 40 | 90 | 600 | |
| One day later | 6.62 | 11 | 40 | 90 | 600 | |
| 2 days later | >7.77 | 13.5 | 40 | 90 | 600 | |
| 3 days later | >7.77 | 13.5 | 40 | 60 | 800 | |
| 4 days later | >0.77 | 16.7 | 40 | 45 | 600 | |
| 2 days later | >7.77 | 17.6 | 40 | 30 | — | 1st session |
| One day later | 5.49 | 12.4 | 40 | 30 | — | 2nd session |
| 2 days later | >7.77 | 14.9 | 40 | 30 | — | 3rd session |
| One day later | 6.41 | 14.5 | 40 | 30 | — | 4th session |
| One day later | 6.14 | 13 | 40 | 30 | — | 5th session |
| One day later | 5.89 | 13.9 | 40 | 30 | — | — |
Fig. 1 shows the evolution of hormone levels with conventional anti-thyroid treatment; the arrows indicate the beginning of the plasmapheresis sessions and time of surgery.
A total thyroidectomy was performed the day after the final plasmapheresis session. The patient presented haemodynamic stability during surgery. Six (6) days after surgery, the thyroid hormones were within normal limits and the patient was discharged with levothyroxine 100 mcg/day.
The definitive pathological anatomy was diffuse hyperplasia of the thyroid gland.
In the subsequent controls, the patient was asymptomatic and euthyreotic.
Plasmapheresis is the fastest way to reduce the thyroid hormones and achieve clinical improvement. The replacement solution includes fresh frozen plasma and albumin. Its effect is transient for 24−48 h, and sometimes a discordance is observed between clinical improvement and biochemistry, with a moderate reduction in hormone levels but a clear improvement in symptoms. In some cases, a reduction of up to 65% of T4l levels has been achieved.9
Our patient was given antithyroid treatment as a mixed thyroiditis with methimazole and prednisone. The addition of potassium perchlorate exacerbated renal function. In view of the absence of a clinical and analytical improvement with conventional antithyroid treatment, the decision was taken to perform plasmapheresis sessions, which were well tolerated. This led to a rapid reduction in hormone levels of at least 25%, somewhat moderate in comparison with other published cases, although a notable clinical improvement was achieved and the patient was stabilised in order to minimise perioperative morbidity prior to the thyroidectomy.
Thyroid plasmapheresis is therefore a technique without a clearly defined indication and is seldom used in hyperthyroidism, although, as in our case, it could prove highly useful in the following situations: presence of severe symptoms (cardiological, neurological manifestations, severe myopathy), rapid clinical deterioration, contraindication of other therapies (agranulocytosis, kidney failure, asthma, heart failure, etc.), the failure of conventional treatment and prior to emergency thyroidectomy.7–10
FundingThe authors declare that they have not received any funding to carry out this work.
Please cite this article as: Rojo Álvaro J, Conchillo Fernández C, Agea Díaz L, Bilbao Garay I, García Delgado C, del Río JM, et al. Plasmaféresis tiroidea. Nefrologia. 2020;40:566–568.