Secondary hyperparathyroidism (SHPT) is a common complication in patients with chronic kidney disease (CKD) that is characterised by elevated parathyroid hormone (PTH) levels and a series of bone-mineral metabolism anomalies. In patients with SHPT, treatment with paricalcitol, a selective vitamin D receptor activator, has been shown to reduce PTH levels with minimal serum calcium and phosphorus variations. The classic effect of paricalcitol is that of a mediator in mineral and bone homeostasis. However, recent studies have suggested that the benefits of treatment with paricalcitol go beyond PTH reduction and, for instance, it has a positive effect on cardiovascular disease and survival. The objective of this study is to review the most significant studies on the so-called pleiotropic effects of paricalcitol treatment in patients with CKD.
El hiperparatiroidismo secundario (HPTS) es una complicación habitual en pacientes con enfermedad renal crónica que se caracteriza por unos niveles elevados de hormona paratiroidea (PTH) y una serie de anomalías en el metabolismo mineral-óseo. En pacientes con HPTS, el tratamiento con paricalcitol, un activador selectivo de los receptores de la vitamina D, ha demostrado reducir los niveles de PTH con mínimas variaciones del calcio y del fósforo séricos. El efecto clásico de paricalcitol es el de mediador en la homeostasis mineral y ósea. Sin embargo, estudios recientes han indicado que los beneficios del tratamiento con paricalcitol van más allá de la reducción de PTH, por ejemplo, ocasionando efectos positivos en la enfermedad cardiovascular y en la supervivencia. El objetivo del presente trabajo es revisar los estudios más significativos sobre los llamados efectos pleiotrópicos del tratamiento con paricalcitol en pacientes con ERC.
The classic physiological role of the vitamin D hormonal system is in the essential regulation of bone mineral metabolism. 1α,25-dihydroxyvitamin D3 binds with a high affinity to vitamin D receptors (VDR) and regulates serum levels of calcium (Ca) and phosphorus (P) by increasing their absorption in the intestine, increasing calcium reabsorption in the renal tubules, and suppressing secretion of parathyroid hormone (PTH), which in turn is a key regulator of mineral metabolism.1 The decrease in 1α,25-dihydroxyvitamin D3 levels is attributed to the increased production of FGF23 induced by the accumulation of phosphate, which stimulates FGF23 production, even without an evident increase in serum P concentration. Therefore, vitamin D deficiency plays a central role in the development of secondary hyperparathyroidism (SHPT), a common early complication in patients with chronic kidney disease (CKD)2–4 that progresses as glomerular filtration rate decreases.4 SHPT is characterised by high PTH levels and the presence of bone and mineral abnormalities.1,2 SHPT and abnormal mineral metabolism lead to clinical consequences at 2 levels: in the musculoskeletal system and in the cardiovascular system. The consequences in the musculoskeletal system are due to increased bone remodelling, the most common condition being osteitis fibrosa. The consequences on the vascular system are related to the increased risk of vascular calcification.2
The usual treatment for SHPT involves dietary phosphorus restriction and drugs such as phosphate binders, VDR activators (selective and non-selective), and/or calcimimetics such as cinacalcet.2,3
The first commercial VDR activator (VDRA) was calcitriol.2 Calcitriol is an important drug in the treatment of SHPT in patients with CKD. However, due to its potent effects on intestinal Ca and P absorption, this molecule frequently induces hypercalcaemia, hyperphosphatemia, and renal calculi formation, and increases the likelihood of calcification.
In 1998, paricalcitol was approved for the treatment of SHPT.5 This third-generation vitamin D analogue emerged in view of the need for treatments that could inhibit high PTH concentrations in patients with SHPT, with a minimal effect on serum concentrations of Ca, P, and the calcium-phosphorus product (Ca×P), without renal toxicity.4 Recently, paricalcitol has been reclassified by the World Health Organisation to H05BX (other antiparathyroid agents) rather than A11CC (vitamin D and analogues).
The beneficial effect of paricalcitol in reducing PTH levels in patients with CKD is widely established.4 Its therapeutic efficacy is due to the tissue selectivity of its mechanism of action.4 Paricalcitol is considered a selective VDR activator. The term “selective” refers to the differential binding of the ligand to the VDR. The synthesis of selective vitamin D receptor activators (sVDRA) such as paricalcitol and maxacalcitol came about because of the clinical need to broaden the therapeutic window of the classic vitamin D forms and to try to reduce the risk of hypercalcaemia and hyperphosphatemia associated with the non-selective derivatives calcitriol and alfacalcidol. Selective VDRAs allow more efficient inhibition of PTH synthesis and secretion, with a lesser effect on intestinal absorption of calcium and phosphorus.6 The selectivity of paricalcitol is explained at a biochemical level by the C-terminal of the vitamin D receptor, which is the region that binds specifically to the ligand.6
Although current clinical practice guidelines for CKD limit the use of VDRAs to the treatment of SHPT,7 the VDR has been identified in more than 30 different human tissues (Table 1).8 This suggests the different actions that vitamin D may have, in addition to those related to bone and mineral homeostasis, giving what are known as the “non-classical” effects of vitamin D. Such effects include an anti-inflammatory immunomodulatory effect on cardiomyocyte remodelling,9 a renal protective effect, and to a lesser degree, an effect on the progression of vascular remodelling, particularly in some derivatives.
Tissue distribution of vitamin D receptors.
| System | Tissue |
|---|---|
| Endocrine | Parathyroid, pancreatic B cells, thyroid C cells |
| Cardiovascular | Arterial smooth muscle cells, cardiac myocytes |
| Musculoskeletal | Osteoblasts, chondrocytes, striated muscle |
| Gastrointestinal | Oesophagus, stomach, intestine |
| Hepatic | Liver parenchymal cells |
| Renal | Tubules, juxtaglomerular apparatus (renin), podocytes |
| Reproductive | Testicles, ovaries, uterus |
| Immune | T cells, B cells, bone marrow, thymus |
| Respiratory | Alveolar cells of the lung |
| Epidermis | Keratinocytes, hair follicles |
| Central nervous system | Neurons |
The wide dissemination of VDRs in human tissues explains some potential effects in terms of improved cardiovascular structure and function, reducing the risk of cardiovascular disease and mortality, particularly in patients with CKD.6
Paricalcitol and survival in haemodialysis patientsSHPT affects to, at least, half of all patients with end-stage renal disease on dialysis10 and is associated with different diseases and comorbidities, such as increased risk of fracture, vascular complications, and infections. Many of these comorbidities may lead to hospital admission. Dobrez et al. observed that patients treated with paricalcitol had a lower risk of all-cause hospitalisation (–14%; P<.0001), fewer hospitalisations per year (–0.642; P<.001), and fewer hospital days per year (–6.84; P<.001) than patients treated with calcitriol.10
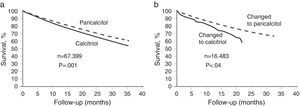
Furthermore, several epidemiological studies have shown the significant systemic effect of VDR activation on survival, in patients with SHPT on haemodialysis.5,11 The results of the first study demonstrated that treatment with paricalcitol led to a mortality rate that was 16% lower than with the VDRA calcitriol (95% CI).5 In that retrospective cohort study, the difference in survival was significant at 12 months of treatment and increased over time (P<.001) (Figure 1a).5 Within that study, there was one subgroup of patients that changed from treatment with calcitriol to treatment with paricalcitol. At 2 years, that subgroup of patients had a higher survival rate than the subgroup who changed from treatment with paricalcitol to treatment with calcitriol (73% vs 64%, P<.04) (Figure 1b).5 The survival advantage for patients treated with paricalcitol over those treated with calcitriol appears to be independent of baseline serum levels of calcium, phosphorus, and PTH.5
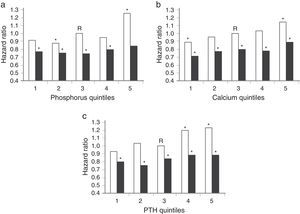
In a second retrospective study, patients who received i.v. vitamin D (calcitriol or paricalcitol) showed a survival rate 20% higher than those who did not receive vitamin D (HR, 0.80; 95% CI).11 Survival at 2 years was 75.6% in the group of patients who received vitamin D, vs 58.7% in the group of patients who did not receive vitamin D (P<.001).11 The survival advantage for patients treated with i.v. vitamin D over with those not treated with vitamin D also appears to be independent of baseline calcium, phosphate, and PTH levels (Figure 2).11
Hazard ratios of death according to quintiles of P (a), Ca (b), and PTH (c). The dark bars represent the effect of receiving injectable vitamin D, and the light bars represent the effect of not receiving injectable vitamin D. R, reference category. *P<.05.
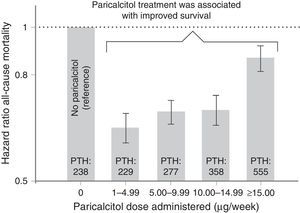
Another published study by Kalantar-Zadeh et al. also associated treatment with paricalcitol with improved survival, in a cohort of patients on maintenance haemodialysis.12 The authors observed that treatment with the vitamin D receptor activator paricalcitol at any dose was associated with improved survival compared with those patients who were not treated with paricalcitol.12,13 Subsequently, Lee et al. compiled the results of the study by Kalantar-Zadeh et al. and similarly demonstrated that the administration of paricalcitol at any dose was associated with improved survival in subgroups of patients on maintenance haemodialysis (Figure 3).13
Relationship between paricalcitol dose and mortality risk.
Vascular protection: paricalcitol in vascular calcification, atherosclerosis, and cardiac structure and function.
Patients with stage 5 CKD on dialysis have a high rate of mortality due to cardiovascular complications.14–17 Several studies have shown that vitamin D deficit and activation of the vitamin D receptor are associated with vascular calcification and dysfunction and cardiovascular mortality, in both the general population and in patients with CKD.1 Calcification of vascular tissue is common in patients with CKD14,16 and has haemodynamic consequences, such as loss or arterial elasticity, increased pulse wave velocity, development of left ventricular hypertrophy, reduced coronary artery perfusion, myocardial ischaemia, and heart failure.17 This calcification occurs at 2 distinct levels in the vessel: in the intimal layer, were it is associated with atherosclerosis, and in the medial layer, where it is associated with arteriosclerosis due to factors such as age, diabetes, and end-stage renal failure.14,17
The exact mechanisms responsible for the development and progression of vascular calcification in patients with CKD are not completely known.16 For many years, it has been proposed that vascular calcification is the result of a passive process, due to high phosphate levels and a high Ca×P product.17 However, recent studies have shown a relationship between vascular calcification and osteogenesis,17 indicating that vascular calcification is an actively-regulated process.17,18
Calcitriol and other vitamin D analogues are used for the treatment of SHPT, but there is some disagreement regarding whether or not these VDR activators directly accelerate the vascular calcification process.17 For example, in vitro and in vivo studies have demonstrated that calcitriol induces calcification at high doses. However, other VDRAs, such as paricalcitol, are less likely to cause hypercalcaemia and hyperphosphatemia or to induce vascular calcification.16
VDRAs can have differential effects on vascular calcification, and these effects are independent of the Ca×P product,14,16 serum levels of Ca and P,14 and PTH.16 Unlike paricalcitol, calcitriol15,16 and doxercalciferol14,16 appear to increase aortic calcium content in studies with uraemic post-nephrectomy rats. Even more importantly, calcitriol and doxercalciferol appear to increase the expression of Cbfa-1 (RunX-2), a transcription factor that initiates the active mechanisms of vascular calcification.16
It is known that vascular smooth muscle cells (VSMC) have VDRs and contain the enzyme 25-hydroxyvitamin D3 1α-hydroxylase, indicating that vitamin D and its analogues play an important role in the function and physiology of this cell type.17 In a study by Cardús et al., high doses of calcitriol, but not of paricalcitol, increased calcification in VSMCs of post-nephrectomy rats.18 The same study showed that treatment with hypercalcaemiant doses of calcitriol increased pulse pressure (the difference between systolic arterial pressure and diastolic arterial pressure), probably due to the extensive arterial calcification observed. In contrast, in rats treated with paricalcitol, no increase was observed in aortic calcification or pulse pressure.18
Noonan et al. quantified the functional consequences of vascular calcification and observed that paricalcitol had no effects on pulse wave velocity, whereas high doses of doxercalciferol increased pulse wave velocity in post-nephrectomy rats at 6 weeks of study.
While the activation of VDRs can slow vascular calcification in induced uraemia, recent data also support the role of VDR activation in the prevention or slowing of atherosclerosis.8 VDRAs have shown a beneficial effect on thrombosis, inflammation, and vasodilatation, risk factors associated with endothelial function and the atherosclerotic process.19 According to a study performed in primary cultures of human aortic smooth muscle cells, VDRs may play a role in atherosclerosis by regulating plasminogen activator inhibitor-1, thrombospondin-1, and thrombomodulin. Thus, VDRs could contribute to the therapeutic benefit that vitamin D analogues have in reducing morbidity and mortality risk in patients with stage 5 CKD.20
A subsequent study assessed the protector effect of paricalcitol, an angiotensin-converting enzyme inhibitor (ACEI) (enalapril), and a combination of both, on oxidative damage in atherosclerotic mice aortas.21 In the study, paricalcitol alone, enalapril alone, and a combination of paricalcitol plus enalapril all prevented atherosclerotic plaque formation in ApoE21-deficient mice and showed a protector effect against cardiac oxidative stress in uraemia.22 This protection against the inflammation and oxidative damage of atherosclerosis was greater with the combined therapy (enalapril+paricalcitol) than with monotherapy.21 In the results of the study, it was observed that both paricalcitol and enalapril as monotherapies, as well as the combination of the two, reduced the concentration of the enzyme aortic malondialdehyde (MDA) and increased levels of the enzyme glutathione peroxidase (GSH-Px).21 Both the reduction of MDA levels and the increase in GSH-Px levels are indicative of the protector effect of paricalcitol, enalapril, and the combination of both, in this model of atherosclerotic mice.21 In addition, paricalcitol also appears to improve endothelial function in uraemic rats in a dose-dependent manner and independently of PTH levels and blood pressure.19
In addition, it is thought that paricalcitol is associated with a lower cardiovascular morbidity, possibly due to modification of cardiac structure and function.23,24 The PRIMO study dealt with determining the effects of paricalcitol on left ventricular mass over a period of 48 weeks in patients with CKD and left ventricular hypertrophy.23,24 During the study period, paricalcitol did not reduce left ventricular mass or improve certain Doppler measurements of diastolic function, but it did appear to be associated with fewer hospitalisations for cardiovascular causes.23 In a post hoc analysis of the PRIMO study, there was a significant reduction in left ventricular volume index in the group of patients treated with paricalcitol compared with those in the placebo group (P=.002).24
Renal protection: reduction of proteinuria and renal fibrosisSeveral studies have demonstrated the renoprotective effect of vitamin D analogues in experimental animal models with glomerular disease.25
Diabetic nephropathy, which is characterised by glomerular lesions, inflammatory infiltration, tubular atrophy, and interstitial fibrosis,26 is one of the most complex renal diseases and is often associated with cardiovascular complications. Tubulointerstitial damage or fibrosis in CKD leads to end-stage renal failure.27 In a mouse model with interstitial obstructive nephropathy due to unilateral ureteric ligation,27 paricalcitol reduced interstitial inflammation and the production and accumulation of interstitial matrix components (collagen and fibronectin). In this model, paricalcitol also inhibited the transition of epithelial cells to mesenchymal cells, a process that is partly responsible for renal fibrosis.27 In addition, paricalcitol restored the number of VDRs in obstructive nephropathy.27 Paricalcitol also blocked in vitro and in vivo expression of the Snail gene, Snail being a transcription factor that plays a crucial role in initiating epithelial to mesenchymal transition. These results indicate that the beneficial effect of paricalcitol is associated with its capacity to preserve the tubular epithelial phenotype by suppressing transition.27 Likewise, paricalcitol has a beneficial inhibitory effect on renal inflammation thanks to signalling-sequestration of nuclear transcription factors such as nuclear factor kappa B (NF-KB).26
The urinary protein/albumin ratio is an important marker of renal and cardiovascular disease28,29 that is associated with CKD progression.1 Increased urinary protein reflects a higher degree of glomerular disease or underlying renal tubular dysfunction,1 and plays a direct role in progression of renal and cardiovascular disease.1,28,29
The renin–angiotensin–aldosterone system (RAAS) has been identified as an important mediator of progressive renal damage in diabetic nephropathy. The drugs that affect the RAAS, such as ACEIs and angiotensin receptor blockers (ARBs), can delay cardiovascular and renal morbidity and mortality.30 However, residual proteinuria may be high in patients that receive these treatments1; therefore, different treatment strategies are needed to reduce that residual risk.1 Recent studies have shown that the selective activation of VDRs could be of relevance in the reducing proteinuria in patients with CKD.29
In a post hoc analysis of a randomised trial that assessed the efficacy of oral paricalcitol in reducing PTH in patients with CKD,3,4 a reduction in proteinuria was observed in more than 50% of the patients treated with paricalcitol. This reduction was independent of concomitant treatment with RAAS inhibitors (P=.004).28 In another randomised double-blind study, paricalcitol significantly reduced protein excretion measured by the urinary protein-creatinine ratio at baseline and at the end of the study in 2 groups of patients: one treated with paricalcitol and the other with placebo.29 In one short study, 24 patients were randomly assigned to receive placebo, 1μg of paricalcitol, or 2μg of paricalcitol. Following evaluation, a reduction in proteinuria was observed in patients on paricalcitol, independently of haemodynamic changes or reduction in PTH levels.31
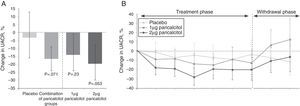
The VITAL study showed that treatment for 24 weeks with 2μg of paricalcitol reduced residual albuminuria in patients with type 2 diabetes mellitus and kidney disease that were being treated with stable doses of ACEIs or ARBs.30 The change in the urinary albumin-creatinine ratio (UACR) from baseline level to the end of treatment was greater in the groups of patients treated with paricalcitol (1 or 2μg) than in the placebo group (P=.071). Fig. 4 shows how the differences in UACR appeared to have a dose-response relationship with the use of paricalcitol, indicating that paricalcitol could be an important adjuvant treatment in the reduction of residual albuminuria.30
Changes in urinary albumin-creatinine ratio from baseline level to end of treatment (A) and during treatment and on withdrawal (B).
A recently-published meta-analysis of 9 studies (n=832) by Cheng et al. confirmed the beneficial effect of paricalcitol in reducing proteinuria (P=.001) without increased risk of adverse effects.32
Paricalcitol as an immunomodulatory and anti-inflammatory agentSeveral epidemiological studies have associated vitamin D deficiency with an increased susceptibility to immune-mediated diseases, including chronic infections and auto-immune diseases.33 Systemic inflammatory processes are common in patients with CKD and constitute a marker of poor prognosis.1 It is also known that immune cells express vitamin D activator enzymes, allowing local conversion of inactive vitamin D to its active form 1,25(OH)(2)D(3).33 These data indicate that vitamin D plays a role in maintaining immune homeostasis.33 Several studies have evaluated the immunomodulatory effect of paricalcitol and other VDRAs in this area.25,30,33
Paricalcitol and calcitriol have been demonstrated to reduce differentiation of immature dendritic cells from monocytes and their activation capacity.25 The prevention of dendritic cell maturation may play an important role in the prevention of autoimmune diseases, including atherosclerosis.25 The immunomodulatory action of vitamin D also occurs through regulation of the activity of different types of immune cells, such as macrophages, dendritic cells, and T cells,33,34 and regulation of expression of the pro-inflammatory cytokines RANTES and TNF-α.34
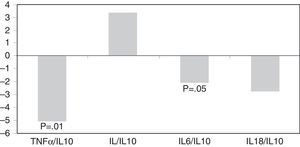
Tan et al. also observed a beneficial effect from paricalcitol on the inhibition of renal inflammation, due to sequestration of NF-KB signalling.34 Another short, randomised double-blind study in patients with CKD showed a significant reduction of serum concentration of C-reactive protein (CRP), measured using a high-sensitivity method.31 Likewise, paricalcitol was associated with inflammation modulation in patients on haemodialysis by a reduction in inflammatory biomarkers such as CRP and TNF-α and improved IL-6/IL-10 and TNF-α/IL-10 ratios.35 These anti-inflammatory effects of paricalcitol were independent of iPTH concentration (Figure 5).35
In another recently published study by Sánchez-Niño et al., an anti-inflammatory effect was observed for both paricalcitol and calcitriol in an experimental model using rats with streptomycin-induced diabetes. Calcitriol and paricalcitol reduced the expression of the anti-inflammatory mediators fibronectin and renin in cultivated podocytes. These VDRAs reduced glomerular inflammation even at sub-antiproteinuric doses.36
Importance of paricalcitol in transplant patientsA normal-functioning renal transplant corrects the endocrine and metabolic imbalances secondary to renal function deterioration.37,38 However, after renal transplant, persistent elevation of PTH is observed in a considerable percentage of patients. Whilst it usually returns to normal slowly in patients with normal graft function, in patients with mild or moderate deterioration of renal function it can persist indefinitely. The persistence of high PTH is a risk factor for loss of bone mass and increased risk of fracture. Reduced bone mineral density has also been associated with an increased risk of atherosclerosis.39 Therefore, better control of PTH may improve both aspects.
Furthermore, several studies have shown that at one year post-transplant, proteinuria is present in up to 45% of patients; in two thirds of these patients, the level is below 500mg/day.40,41 Proteinuria is one of the factors that significantly predisposes to reduced renal graft survival.42
As there is a certain degree of similarity between chronic graft disease and chronic kidney disease, is can be assumed, logically, that antiproteinuric therapeutic approaches could be beneficial in renal transplant patients.43,44 As has been seen throughout this review, in patients with CKD, paricalcitol has been shown to be capable of reducing proteinuria in both patients with SHPT and those with normal PTH levels.
Experience with paricalcitol in renal transplant patients is limited; however, in the few studies that have been published, paricalcitol appears to have been effective in improving control of PTH and proteinuria in this group of patients.
In a retrospective study that included 58 renal transplant patients with secondary hyperparathyroidism, treatment with paricalcitol significantly reduced serum iPTH levels and proteinuria (35% decrease relative to baseline levels). Renal function remained stable during treatment with paricalcitol, with a significant difference compared with the deterioration observed in the 2 years prior to treatment.45 In another, prospective study in a population of 46 transplant patients that assessed urinary peptidome, treatment with 1μg/day of paricalcitol for 3 months effectively reduced PTH levels and notably modified the urinary peptide profile.37 In another study, published only as an abstract, that compared the use of different vitamin D analogues in patients with renal transplant, treatment with paricalcitol was associated with a greater reduction in proteinuria.46
Toxicity from calcineurin inhibitors is one of the most significant causes of chronic graft disease. At an experimental level, it has been observed that paricalcitol attenuates cyclosporine-induced nephropathy, suppressing interstitial inflammation and fibrosis and epithelial tubule cell fibrosis.47 This could be, therefore, an additional beneficial effect of paricalcitol in renal transplant patients on calcineurin inhibitors.
ConclusionsParicalcitol, a third-generation VDRA, provides effective control of SHPT with minimal hypercalcaemic and hyperphosphatemic effects.4 Its therapeutic efficacy is due to the tissue selectivity of its mechanism of action, as it does not up-regulate the intestinal vitamin D receptor and is less active than calcitriol at a bone level.4
Recent studies have indicated that the benefits of paricalcitol go beyond the reduction of PTH and bone and mineral homeostatic control. This review shows that the pleiotropic effects of paricalcitol on cardiovascular structure and function, proteinuria, and immune function, amongst others,1,8,9 may explain the increased survival rate observed in patients with CKD treated with paricalcitol.5
Although more experience is needed, use of paricalcitol in renal transplant patients appears to be a safe and promising option for improving control of high PHT levels and proteinuria.
Conflicts of interestThe authors declare the following potential conflicts of interest:
Dr Jordi Bover has given talks sponsored by Abbott (Abbvie), Amgen, Genzyme, and Shire. He has also participated in national and international advisory committees for Abbott (Abbvie), Amgen, and Genzyme.
Dr Alberto Martínez has received research grants and has given talks sponsored by Abbott (Abbvie), Amgen, Boëhringer-Ingelheim, Esteve, Janssen-Cilag, Novartis, Roche, and Shire. He has also participated in advisory boards for Abbott (Abbvie), Amgen, Esteve, Roche, and Shire.
Dr Egido has performed work as scientific advisor, and has received research funding and given takks sponsored by Abbott (Abbvie).
Dr Elvira Fernandez-Giraldez has received research funding and has given talks sponsored by Abbott (Abbvie).
Dr Carlos Solozabal has given talks sponsored by Abbott (Abbvie).
Please cite this article as: Egido J, Martínez-Castelao A, Bover J, Praga M, Torregrosa JV, Fernández-Giráldez E, et al. Efectos pleiotrópicos del paricalcitol, más allá del metabolismo óseo-mineral. Nefrología. 2016;36:10–18.