Checkpoint inhibitors (CPI) have revolutionised cancer treatment. They act by inhibiting T-lymphocyte (TL) receptors, cytotoxic T-lymphocyte antigen 4 (CTLA4) or programmed cell death 1 (PD1) receptors or their ligands, triggering TL dysregulation and hyperactivation, which causes immune-related adverse events (irAE). In the kidneys, the most common is immune-mediated interstitial nephritis, but there is also pauci-immune glomerulonephritis, podocytopathies and C3 glomerulopathy, which worsen the prognosis.1–6 We present a case of pauci-immune glomerulonephritis (PIGN) during treatment with durvalumab, antiPD-L1, in a patient with squamous cell carcinoma of the lung.
This was a 71-year-old male with a history of hypertension, type 2 diabetes, dyslipidaemia and squamous cell carcinoma of the lung (G2 pT3 pN1, PD-L1<1%) diagnosed in 2018; surgical treatment and chemotherapy with four cycles of cisplatin and vinorelbine. Maintenance treatment with durvalumab, 12 cycles, until 14/11/2019, remaining disease free (Figs. 1 and 2).
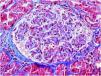
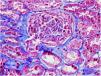
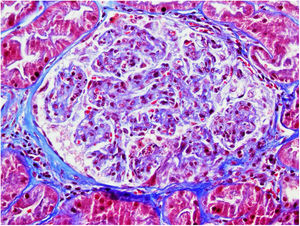
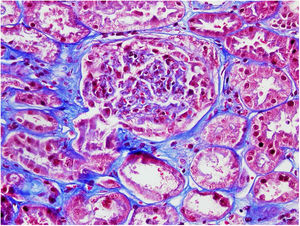
On 17/11/2019 he was admitted for acute renal failure; creatinine 4.5mg/dl, microhaematuria (300 red blood cells/μl), clinical and analytical nephrotic syndrome (proteinuria 6g/day, albumin 2.2mg/dl). Immunological study normal/negative; renal biopsy: 21 glomeruli, 6globally sclerotic. Inflammatory hypercellularity in capillary lumens, mononucleated, focally neutrophils, fragmented red blood cells and leucocytoclastic phenomenon. Four glomeruli with epithelial crescents. Interstitial fibrosis, tubular atrophy and mild chronic inflammation. The arteries and arterioles appeared normal. Direct immunofluorescence no IgA, IgG, IgM, C3, C1q, kappa, lambda deposits. Diagnosis: pauci-immune glomerulonephritis with extracapillary proliferation in 27% of glomeruli. He was started on intravenous steroids (500mg×3 days) orally at a dose of 1mg/kg/day and adjusted intravenous cyclophosphamide (500mg/m2).
After one month, outpatient follow-up with oral steroids and cyclophosphamide; creatinine 3–3.5mg/dl, CKD-EPI 16.5–18ml/min, nephrotic proteinuria and microhaematuria.
Readmission after second bolus of cyclophosphamide; creatinine 7.14mg/dl, urea 319mg/dl and proteinuria 4.18g/24h without nephrotic syndrome, severe haematuria. Renal replacement therapy (RRT) was started; intermittent haemodialysis, maintaining a diuresis of 1500–2000cc/day. Subsequently, he continued intravenous cyclophosphamide and a regular haemodialysis programme, with close follow-up. We have noted a decrease in proteinuria to <1.5g/day, diuresis 2l and a CrCl 12–18ml/min with no need for RRT until now and oncological disease in complete remission.
Checkpoints are regulators of the TL immune response. Their blockade with immunotherapy promotes a state of lymphocyte dysregulation, leading to hyperstimulation of TL and better control over tumour cells. However, their main drawback is irAE, exacerbating autoimmune diseases such as PIGN. PIGN is characterised by positive serum antineutrophil cytoplasmic antibodies (ANCA), sometimes, as in our patient, they are negative, speculating that they are ANCA against another epitope or other undetected autoantibodies.3
PIGN has been associated with aberrant PD1 expression in some subjects and increased TL hyperactivity, with the risk of developing PIGN increased by immunotherapy treatment. In our case we could postulate that there was this aberrant expression which triggered PIGN,7 and stopping the immunotherapy combined with immunosuppressive treatment was able to control the TL hyperactivity, which then led to remission of the PIGN.
Polymorphisms in PDCD1 (the gene that encodes PD1) have also been described, which increase susceptibility for developing PIGN; therefore, a genetic analysis could be useful to prevent the development of PIGN in patients requiring treatment with CPI and to consider other treatments.8,9
The close relationship between oncology and nephrology leads to early renal biopsy and assessment by nephrology, with early diagnosis increasing renal and overall survival.