Autosomal dominant polycystic kidney disease (ADPKD) is a main cause of end-stage renal disease. Today, knowledge of its genetic basis has made it possible to develop strategies that prevent the transmission of the disease.
ObjectivesThe objective of the study was to analyze the natural history of ADPKD in the province of Córdoba and to design a database that allows grouping families with different mutations.
Patients and methodsAll patients (n = 678) diagnosed with ADPKD followed by the Córdoba nephrology service are included. Various clinical variables (age and sex), genetic variables (mutation in PKD1, PKD2) and the need for renal replacement therapy (RRT) were retrospectively analyzed.
ResultsThe prevalence was 61 cases per 100,000 inhabitants. Median renal survival was significantly worse in PKD1 (57.5 years) than in PKD2 (70 years) (log-rank p = 0.000). We have genetically identified 43.8% of the population, detecting PKD1 mutations in 61.2% and PKD2 mutations in 37.4% of cases, respectively. The most frequent mutation, in PKD2 (c.2159del), appeared in 68 patients belonging to 10 different families. The one with the worst renal prognosis was a truncating mutation in PKD1 (c.9893 G > A). These patients required RRT at a median age of 38.7 years.
ConclusionsRenal survival of ADPKD in the province of Córdoba is similar to that described in the literature. We detected PKD2 mutations in 37.4% of cases. This strategy allows us to know the genetic basis of a large proportion of our population while saving resources. This is essential to be able to offer primary prevention of ADPKD through preimplantation genetic diagnosis.
La Poliquistosis renal autosómica dominante (PQRAD) es una de las principales causas de insuficiencia renal terminal. Hoy día el conocimiento de sus bases genéticas están permitiendo desarrollar estrategias que eviten la transmisión de la enfermedad.
ObjetivosEl objetivo del estudio fue analizar la historia natural de la PQRAD en la provincia de Córdoba y diseñar una base de datos que permita agrupar a las familias y a las diferentes mutaciones.
Pacientes y métodosSe incluye todos los pacientes (n = 678) diagnosticados de PQRAD seguidos en el servicio de nefrología de Córdoba. Se analizaron de manera retrospectiva diversas variables clínicas (edad y sexo, necesidad de terapia renal sustitutiva (TRS) y genéticas.
ResultadosLa prevalencia fue de 61 casos por 100.000 habitantes. La mediana de supervivencia renal fue significativamente peor en PKD1 (57.5 años) que en PKD2 (70 años) (log-rank p = 0.000). Tenemos identificadas genéticamente al 43.8% de la población, detectando mutaciones en PKD1 en el 61.2% y en PKD2 en el 37.4% de los casos. La mutación más frecuente fue detectada en PKD2 (c.2159del) en 68 pacientes pertenecientes a 10 familias diferentes. La de peor pronóstico renal fue una mutación truncante detectada en PKD1 (c.9893 G > A).
ConclusionesLa supervivencia renal de la PQRAD en la provincia de Córdoba es similar a la descrita en la literatura. Con nuestra metodología y estudiando genéticamente al 43.8% de la población, detectamos mutaciones en PKD2 en una mayor proporción de pacientes, en el 37.4% de los casos. Esta estrategia permite conocer las bases genéticas de gran parte de nuestra población con ahorro de recursos. Esto es fundamental para poder ofrecer prevención primaria de la PQRAD mediante diagnostico genético preimplantacional.
Autosomal dominant renal polycystosis (ADPKD) is the most common inherited kidney disease, with a global prevalence of 1 in 1000 people, making it one of the leading causes of end-stage renal failure (ESRD)1,2,3 worldwide. The most characteristic clinical manifestation is the formation of renal cysts that increase in number and size throughout life. The cysts replace normal renal parenchyma such that lithiasis, arterial hypertension, hematuria and renal failure, the most severe manifestation of the disease, appear from the second to fifth decades of life. Around 50% of patients with ADPKD require renal replacement therapy (dialysis or transplantation) at a mean age of 57 years.4,5
ADPKD is an autosomal dominant genetic disease6 with complete penetrance. It is a heterogeneous disease, and the main genes are well-known: PKD1, on chromosome 16p13.3, and PKD2, on chromosome 4q21-23.7,8 85% of described cases are PKD1 and 15% are PKD27,8 although this proportion could vary depending on the geographical area and the proportion of patients studied. Individuals with mutations in PKD1 tend to have a more serious clinical presentation, albeit with great inter-family and intra-family variability. Most individuals with PKD1 mutations develop ESRD at a mean age of 54.3 years; by contrast, more than 50% of individuals with PKD2 mutations have adequate renal function at that age (median age of ESRD is 74.0 years).9,10
Kidney ultrasound is the imaging study commonly used due to its safety and low cost. Currently, the modified Ravine ultrasound criteria are the most accepted.11 Renal cysts in ADPKD begin to develop from the embryonic stage and continue to increase in size throughout life. The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP)12,13: has provided the best clinical information on renal growth and long-term renal prognosis.
As already discussed, the development of renal failure is variable, and depends mainly on the presence of the PKD1 or PKD2 genes. Other factors that influence the clinical course of the disease include male sex, diagnosis before age 30, first episode of hematuria before age 30, onset of hypertension before age 35, hyperlipemia and low HDL cholesterol.14 ADPKD is a multisystem disease; in addition to renal cysts, there are other extrarenal manifestations including liver cysts and vascular,15 cardiac, digestive and musculoskeletal abnormalities.16 Polycystic liver disease (PLD) is the most frequent extrarenal manifestation and is associated with both genotypes: PKD1 and PKD2. The only treatment that has shown some benefit in delaying the deterioration of chronic kidney disease is Tolvaptan.17
It is essential to know the genetic basis of diseases, especially considering that today it is possible to avoid their primary transmission through preimplantation genetic diagnosis (PGD). All patients with ADPKD should have a genetic study and PGD before reproductive age. Genetic studies are expensive and are not routinely indicated for all patients, so it is necessary to design strategies to obtain improved health results at the lowest possible economic cost.
The objective of our study was to analyze the natural history of ADPKD in the province of Córdoba. For this we designed a database that would allow us to group families and different mutations with the idea of making genetic studies affordable and offering primary prevention to our population.
Patients and methodsFor this study, we used a database that includes all patients with ADPKD followed by the nephrology service of Córdoba. This database is updated periodically; most recently in January 2021. Patients are included if they meet the modified Ravine criteria10 or carry a pathogenic genetic mutation.
VariablesThe following variables were analyzed: date of birth, sex, date of RRT, date of death and date of genetic sequencing. We performed sequencing using the Next-Generation Sequencing (NGS) method for the PKD1, PKD2 and GANAB genes. Additionally, we used Multiplex Ligation-dependent Probe Amplification (MLPA) technique for deletions/duplications of PKD1 and PKD2, when indicated.
We considered a mutation responsible for the disease when it was pathogenic, probably pathogenic or the family presented a segregation study. Patients were grouped according to the affected gene (PKD1 or PKD2), the specific mutation and the family to which they belonged.
We performed a descriptive study of the quantitative and qualitative variables. We calculated renal and patient survival. All contrasts are bilateral and we considered significant those where p < 0.05. The data have been collected, processed and analyzed with the statistical program SPSS v.17.
Ethical aspectsThe data was handled anonymously, in accordance with data protection law. We followed all ethical and legal requirements applicable in our country to ensure both confidentiality and good practice in applied clinical research. Likewise, the study complied with the ethical precepts contained in the Declaration of Helsinki.
ResultsDescriptive studyDuring the study period (1990–2021), 678 patients were diagnosed with ADPKD in the province of Córdoba. There were 350 females (51.6%) and 328 male (48.4%). In this period 197 (29.1%) died. In January of 2021, 481 patients were still followed by the nephrology service of Córdoba. Given that the National Institute of Statistics (INE) reported that the population of Córdoba on January 1, 2020 was 784,256 inhabitants, the prevalence of ADPKD in the province of Córdoba is 61 per 100,000 inhabitants.
Of these 481 patients, 168 (34.9%) are in RRT. Of the 168 patients in RRT, 50 (29.7%) are in some form of dialysis and 118 (70.2%) have a functioning kidney transplant. Another 313 patients (65.1%) are followed in outpatient nephrology clinics. The mean age of patients in RRT was 53.0 +/− 11.6 versus 51.6 +/− 16.1 years (p < 0.0001) of outpatients without RRT.
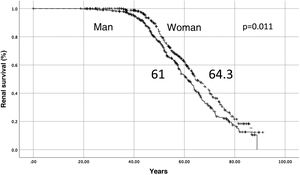
Renal and patient survivalThe median renal survival for the entire cohort of 678 patients was 63.2 years. Renal survival was higher in women than in men, 64.3 vs. 61.0 years (log-rank, p = 0.011) (Fig. 1). The median survival of the patient was 78.9 years, 75.6 years in men and 81.04 years in women (log-rank, p = 0.026).
Genetic studyWe have genetically classified 297 of the 678 patients because they had genetic studies available or belong to families with a probable pathogenic mutation or relevant segregation analysis result. Only 381 patients have not been studied or genetically classified (56.2%). Of the 297 genetically studied cases, we found mutations in PKD1 in 182 (61.2%), in PKD2 in 111 (37.4%) and 2 in GANAB gene (Gene encoding alpha II glucosidase subunit) (0.7%). There were 74 genetic variants in our population, with 43 (58.1%) pathogenic, 22 (29.7%) probably pathogenic and 9 (12.2%) of uncertain significance. Of the 9 variables of uncertain significance, 1 was described in the databases and the remaining 8 were not.
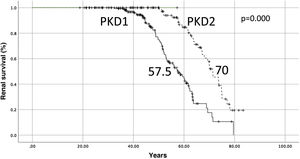
The median renal survival for PKD1 was 57.5 years and 70 years for PKD2 (log-rank p = 0.000) (Fig. 2). The median renal survival for un-sequenced patients (n = 381) was 62.7 years.
Family and mutation groupingWe conducted genetic studies of 100 different families and found 62 different mutations. Table 1 shows the most frequently detected mutations. We only include mutations with at least 10 cases detected. The most frequent mutation, which occurred in 68 cases, was the truncating mutation c.2159del in PKD2.
Table 2 presents the mutations that were associated with the development of end-stage chronic kidney disease with greater severity. The table only describes those mutations for which at least 3 patients needed RRT. We note that of these 6 mutations, 5 belong to PKD1 and 1 to PKD2 and that 4 of the 6 are truncating mutations.
Mutations with the worst renal prognosis.
| Mutation | Description | Category | Type | RRT (n, %) | Mean age at RRT |
|---|---|---|---|---|---|
| Pathogenic | Truncating | 38.7 (32−51) | |||
| Prob. Pathog. | Duplication | 46.1 (43−47) | |||
| Pathogenic | Truncating | 3/7 (42.8%) | 48.8 (46−56) | ||
| Pathogenic | Truncating | 6/11 (42.8%) | 61.6 (51−71) | ||
| Prob. Pathog. | Missense | 5/15 (33.3%) | 59.3 (53−68) | ||
| Pathogenic | Truncating | 20/68 (29.4%) | 63.8 (51−78) |
PKD1 and 2: Polycistic Kidney Disease 1 and 2, RRT: renal replacement therapy, n: number of patients.
The mutation with the worst prognostic of our cohort is located in PKD1, c.9893 G > A. Some 40% of patients in this family need RRT at a median age of 38.7 years (range 32−51).
Grouping the cases by mutations and families, we observed the same mutation in 68 members belonging to 10 families. It is a truncating mutation in PKD2 (c.2159del) already described and considered pathogenic. Some 29.4% of patients with this mutation required RRT at an average age of 65.2 +/- 7.6 years. This mutation is grouped in a specific geographical area of Córdoba.
DiscussionThis study arose from the necessity to know the epidemiology, distribution and natural history of ADPKD in the province of Córdoba and the need to design efficient genetic health strategies. This study has the strength that it includes all patients diagnosed with ADPKD in our province, so it allows us to make very accurate estimates of the prevalence, distribution of mutations and natural history.
ADPKD is the most frequent hereditary disease, with a described prevalence of 1/1000–2000 (50–100 per 100,000 inhabitants). The most rigorous study, conducted in 2020 by the Mayo Clinic (Olmsted County), calculated the prevalence of the “definitive/probable” disease at 68 per 100,000 inhabitants.18 These authors speculate that the diagnosis of “possible” disease of ADPKD could reach up to 234 per 100,000 inhabitants. In our study, the prevalence was very similar, at 61 per 100,000. In Andalusia, an analysis carried out in Granada19 estimated the prevalence at 58 per 100,000 inhabitants. Knowledge of this data is essential for programming the health needs of this population, especially the more serious cases. According to the Andalusian Transplant Coordination Information System (SICATA in Spanish), more than 10% of all patients in RRT (dialysis and transplantation) in Andalusia have ADPKD. The annual cost of these patients (1068 patients in 2019) can range between 30 and 50 million euros. The health system needs strategies that offer these families a response including primary prevention of the disease with PGD techniques.20 This is especially important in our environment since these techniques are not effectively reaching the population that needs it.
ADPKD is an autosomal dominant genetic disease caused mainly by mutations in 2 genes, PKD1 and PKD2.21 All studies find that PKD1 mutation are more frequent (85%) than the PKD2 mutation (15%).22 Probably these values reflect the population studied genetically, not their actual prevalence. Patients with PKD1 mutations have more severe disease and are therefore likelier to be studied than patients with PKD2 mutations. Cornec-le Gall et al.,23 in a multicenter study of 22 centers which included 741 patients, detected PKD1 mutations in 75.5% and PKD2 mutations in 18.3% of patients. In our study involving 678 patients from a single center, we detected mutations in PKD1 in 61.2% of patients and in PKD2 in 37.4%. This difference could be due to our strategy of sequencing 43.8% of cases. Mutations in PKD2 may be more frequent than average in our province. This hypothesis would be supported by the fact that many cases (n = 68) are in a specific geographical area that suggests a mutation with a founder effect. We will only be able to answer this question when most health centers have sequenced a majority of their patient populations. Obviously, there are other minority genes and some not yet described.24 In two cases we detected mutations in the GANAB gene (0.7%) and in two cases we did not detect any mutations.
Renal prognosis depends on the mutation, patients with mutations in PKD1 have terminal CKD at a median age of 54.3 years (compared to 74 years in PKD2). Our data are overlapping: the median renal survival for PKD1 was 57.5 years and 70 years for PKD2 (log-rank, p = 0.000). As in most studies, patient survival was significantly higher in women than in men (75.6 vs. 81.0, p = 0.026). Of note perhaps is the fact that renal survival was also significantly better in women than in men (64.3 vs. 61.0 (log-rank, p = 0.011)). This should come as no surprise as being male is a risk factor for kidney disease progression in most kidney diseases and has also been described recently as a risk factor in patients with ADPKD.25
With our strategy of grouping mutations and families we identified 74 mutations in 100 unrelated families. This allows us to avoid duplicate genetic studies within an identified family. Remarkably, we found the same mutation in 68 members of 10 different families concentrated in a single geographical area of Córdoba. This is a truncating pathogenic mutation in PKD2. It produces a deletion of an adenine (c.2159del) which results in a premature stop codon. We also identified the mutations with the worst renal prognoses. As expected, five of the six mutations were in PKD1 and four of the six were truncating. The most severe mutation (median age of RRT 38.7 years) was in PKD1. This family had a nucleotide change (c.9893 G > A) in heterozygosity in exon 29 of the PKD1 gene. This mutation results in a protein made of 3298 amino acids instead of the 4302 in the native protein.
In summary, the renal survival of ADPKD in the province of Córdoba is comparable to that published in the literature and is significantly worse in PKD1 (57.5 years) than in PKD2 (70 years). The prevalence of ADPKD was 61 per 100,000. We designed a strategy to group families and mutations by genetically identifying 43.8% of the population, detecting mutations in PKD1 in 61.2% and in PKD2 in 37.4 cases. We identified the most frequent mutations in our population and those with the worst renal prognoses. This strategy can be very useful for future approaches by human reproduction techniques in this common and severe disease.
Conflicts of interestThe authors have no conflicts of interest to declare.