The recently published Heart and Kidney Audit (HAKA) study1 revealed the current situation of medical care of cardiorenal patients with advanced chronic kidney disease (ACKD) being followed in specialized clinics of advanced chronic kidney patients in Spain. This study included 29 ACKD units in the country with a total of 5012 patients (77% with CKD stage G4 and 23% with CKD stage G5), between January and March of 2021. The aim was to analyze the diagnosis and treatment of heart failure (HF) according to the 2016 European Society of Cardiology guidelines, then in force.2 These guidelines recommended diagnosis based on clinical findings, circulating biomarkers and imaging. Likewise, the guidelines recommended treatment of HF with reduced ventricular ejection fraction (EF) based on the combination of a renin-angiotensin system inhibitor (RAASI) or an antagonist of angiotensin receptor and a neprilysin inhibitor (ARNi), with a beta-blocker (BB) and with a antagonist of mineralocorticoid receptor (MRA). Key trials of sodium-glucose cotransporter type 2 (SGLT2) inhibitor drugs demonstrating a reduction in cardiac and renal events in patients with HF and reduced EF had been published already, prompting their addition to the three types of drugs mentioned above to treat these patients.3,4
Results from the HAKA studies are worrisome. First, the diagnosis of HF in patients with ACKD is not based on the obligatory combination of the three classic criteria: clinical manifestations, biomarkers (natriuretic peptides [NP]), and echocardiographic parameters. Since NPs levels can be elevated in ACKD due to the reduction in renal clearance,5 they lose diagnostic specificity, and it is understandable that in the HAKA study they were measured only in 50% of patients.1 However, it should not be forgotten that NPs continue to be predictors of cardiac events in patients with ACKD, so to exclude their measurement is not a valid option.6 In these circumstances, the most reasonable approach is a correct interpretation of the measured values that includes variations over the baseline values and includes different reference concentrations for patients with ACKD.7
Given the diagnostic limitation of the NPs, the use of the other two types of criteria for the diagnosis of HF in patients with ACKD are of greater relevance. The HAKA study1 showed that the assessment of clinical signs and symptoms and echocardiographic parameters was only performed in 84% and 75% of patients, respectively. It is also true that there are difficulties in clinical and echocardiographic assessment in patients with HF and renal failure8; still they should be performed in 100% of cases.
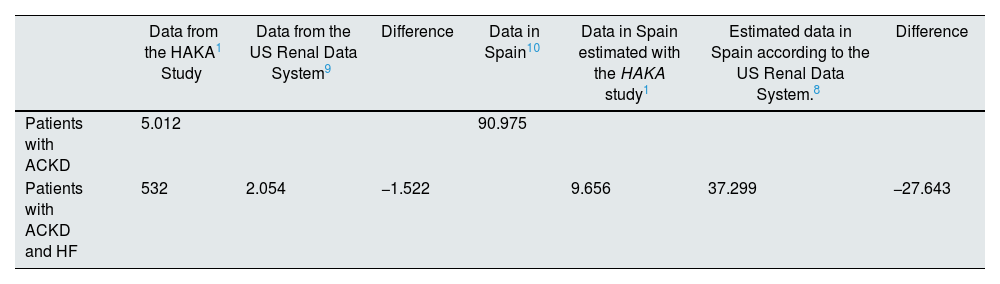
Secondly, the prevalence of HF diagnosed in patients in ACKD units in Spain is very low. Specifically, the diagnosis of HF in the HAKA study1 was 13% (median), a percentage three times lower than that obtained in the largest series published to date such as the 2020 report of the US Renal Data System.9 Of note is the great diagnostic variability observed in the HAKA study,1 with 10 centers with a diagnosed incidence equal to or lower than 10% and four centers with incidence equal to or greater than 30%. In view of the overall prevalence data and as shown in Table 1, it could be stated that the HAKA study1 shows that in the period evaluated and in the participating centers as a whole, of the 5012 patients with ACKD included, 1522 patients were not diagnosed with HF and likely they had it. Taking into account the most recent study on CKD epidemiology in Spain, which establishes that there were 90,975 patients with ACKD,10 the extrapolation of the above data to the entire country as a whole means that 27,643 patients with ACKD who most probably had HF were not diagnosed with this syndrome in the period during which the study was carried out (Table 2).
Actual data and estimated data on the diagnosis of heart failure in patients with advanced chronic kidney disease.
| Data from the HAKA1 Study | Data from the US Renal Data System9 | Difference | Data in Spain10 | Data in Spain estimated with the HAKA study1 | Estimated data in Spain according to the US Renal Data System.8 | Difference | |
|---|---|---|---|---|---|---|---|
| Patients with ACKD | 5.012 | 90.975 | |||||
| Patients with ACKD and HF | 532 | 2.054 | −1.522 | 9.656 | 37.299 | −27.643 |
ACKD: advanced chronic kidney disease; HAKA: Heart And Kidney Audit; HF: heart failure.
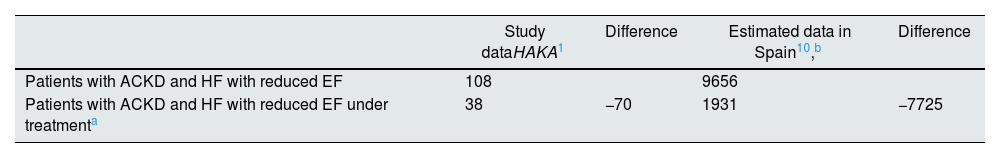
Actual data and estimated data for treatment of heart failure with reduced ejection fraction in patients with advanced chronic kidney disease.
| Study dataHAKA1 | Difference | Estimated data in Spain10,b | Difference | |
|---|---|---|---|---|
| Patients with ACKD and HF with reduced EF | 108 | 9656 | ||
| Patients with ACKD and HF with reduced EF under treatmenta | 38 | −70 | 1931 | −7725 |
ACKD: advanced chronic kidney disease; EF: left ventricular ejection fraction; HAKA: Heart And Kidney Audit; HF: heart failure.
Thirdly, with regard to the treatment of HF, focusing the analysis on patients with HF and reduced EF, the data from the HAKA study1 are also striking. Out from the 108 patients diagnosed, only 35% received the triple combination RAASI/ARNi + BB + MRA and only an additional 8% received SGLT2 inhibitors in addition to these three drugs. We must add that a minority of patients were treated with the optimal recommended doses of the different drugs (in stage 4 patients 6% received ARNi and 32% received BB, and in stage 5 patients a 6% received ARNi and 39% were on BB). Thus, in the HAKA study1 more than half (57%) of the patients with ACKD and HF with reduced EF were not treated adequately. The limitation in this interpretation lies in the fact that in this population it is especially difficult to achieve full doses of some drugs and side effects are more frequent. In any case, if this result is extrapolated to the data in Table 2, it could be estimated that out of the 1931 patients with ACKD diagnosed with HF and reduced EF on medication, only 831 would have been appropriately treated. All these data should be weighted taking into account that patients with ACKD have been systematically excluded from the large randomized clinical trials that have made an impact in the treatment of HF with reduced EF.11,12 However, in the most recent trials with SGLT2 inhibitors the diagnosis of ACKD (especially in stage G4) is no longer a major exclusion criterion given the cardiac and renal efficacy and safety demonstrated in these more recent trials.13
In summary, even with the limitations of an observational and retrospective study such as HAKA,1 the results discussed in this manuscript indicate that more than 3 years ago there was a significant underdiagnosis and very suboptimal treatment of HF in patients with ACKD in Spain. The clinical and health consequences of this situation can be easily deduced from the published evidence on the prognostic impact and economic costs of HF in patients with CKD in general, and particularly in patients with ACKD. For example, over a 2-year period the probability of survival (adjusted for multiple factors) of patients with CKD and HF is 77.85%, 90.2% in patients with CKD without HF and 93.7% in subjects without CKD or HF.14 Specifically, patients with CKD and HF have an increased relative risk of cardiovascular mortality and hospitalizations due to HF itself, which is higher in patients with ACKD than in those without ACKD.15 Additionally, between 2020 and 2030 the cost of care for patients with CKD and HF will increase by 143%, without taking into account patients on renal replacement therapy, which would increase the cost even further.16
Therefore, it can be concluded that three years ago cardionephrology had a limited implementation, which had a direct impact on the inadequate management of patients with cardiorenal involvement, specifically patients with ACKD and HF. It is therefore necessary and urgent to develop new cardiorenal units aiming to improve the diagnosis, treatment and prognosis of these patients, as well as reducing the costs of their care.17 These units should also aim to prevent the onset and progression of cardiovascular disease from the early stages of CKD. As has just been shown in an study that examined data from 61 cohorts including 24,603,016 subjects from all over the world, the relative risk of HF essentially increases by 200% in subjects with estimated glomerular filtration rate (eGFR) ≥90 mL/min/1.73 m2 and urine albumin-to- creatinine ratio(Au/Cru) values between 30 and 299 mg/g, and increases by up to 40% in subjects with Ao /Cro < 30 mg/g and GFR values between 60 and 89 mL/min/1.73 m2.18
The medical and health care of cardiorenal patients is a medical and health care need that has not been adequately accomplished in our country; thus the authors of this editorial, on behalf of the Cardiorenal Medicine Working Group of the Spanish Society of Nephrology (CaReSEN), call for the urgent implementation of cardionephrology in undergraduate programs in medical schools and postgraduate programs in specialist training, and in hospitals and health care centers. We are convinced that these measures will make it possible that in a future HAKA 2 study, the results of the diagnosis and treatment of HF in patients with ACKD will be comparable with what the evidence available indicates they should be.
FinancingThe authors declare that they have not received funding for this article.
The CaReSEN Working Group is formed by: Javier Díez, Borja Quiroga, Rafael Santamaría, Patricia de Sequera, Alberto Ortiz, Juan Navarro, M. Fernanda Slon and Gregorio Romero.