Aunque el bloqueo del sistema renina-angiotensina ha sido citado como el tratamiento inicial para la nefropatía diabética (ND), en un número significativo de pacientes el avance de la enfermedad renal no se ve frenado en su totalidad por estos agentes. Hemos realizado un ensayo clínico doble ciego para valorar el efecto acumulativo de la pentoxifilina (PTX) en la reducción de la proteinuria en pacientes con diabetes tipo 2 (DM2) con bloqueo del sistema de angiotensina. La dosis de PTX utilizada en nuestro ensayo fue una cantidad baja de 400 mg diarios y, en nuestra experiencia, no logramos encontrar ningún artículo que evaluara el efecto antiproteinúrico de la PTX con esta dosis. De forma aleatoria, se dividieron en dos grupos 100 pacientes con ND y proteinuria persistente a pesar del tratamiento con losartán y enalapril durante al menos tres meses antes de ser incluidos en el estudio. El grupo de control (n = 50, 26 hombres y 24 mujeres) fueron tratados con losartán y enalapril, mientras que el grupo de tratamiento (grupo de PTX: n = 50, 28 hombres y 22 mujeres) recibieron losartán, enalapril y 400 mg/día de pentoxifilina durante 6 meses. Al comienzo del estudio no se encontraron diferencias significativas en las características demográficas y clínicas de los pacientes, incluida la creatinina sérica, HbA1c, presión arterial y excreción urinaria de proteínas entre los dos grupos (p > 0,05). En el grupo de PTX, la tasa media de excreción urinaria de proteína ha disminuido significativamente de 616,66 a 378,24 mg tras 3 meses (p = 0,000) y a 192,05 mg tras 6 meses (p = 0,000), mientras que en el grupo de control no se han observado cambios significativos. El beneficioso efecto antiproteinúrico del PTX no estuvo asociado a la intensidad del cambio metabólico ni a la reducción de la presión arterial. Además, al final del estudio, el aclaramiento medio de creatinina fue significativamente más elevado en el grupo de PTX (p = 0,04). En conclusión, la PTX puede aportar en gran medida un efecto antiproteinúrico acumulativo y ralentizar el grado de filtración glomerular en pacientes con DM2 con bloqueo del sistema de angiotensina.
Although blockade of renin-angiotensin system have been cited as the first line of therapy for the management of diabetic nephropathy (DN), however in a substantial number of patients, progression of renal disease are not completely halted by these agents. We have conducted a double blinded clinical trial to assess the additive effect of pentoxifylline on reduction of proteinuria among patients with type 2 DM under blockade of angiotensin system. The dosage of PTX used in our trial was at a low dosage of 400mg daily and to our knowledge, we did not found article which evaluated the antiproteinuric effect of pentoxifylline in this dosage. One hundred patients with DN and persistent proteinuria despite treatment with losartan and enalapril in at least 3 months before inclusion in the study were randomly assigned to two groups. Control group (n=50, 26 males and 24 females) received losartan and enalapril, while treatment group (PTX Group) (n=50, 28 males and 22 females) was given losartan, enalapril and pentoxifylline 400mg/day for 6 months. At the beginning of the study there were no significant differences in demographic and clinical characteristics of patients including serum creatinine, HbA1c, blood pressure and urinary protein excretion between two groups (P>.05). In the PTX group, the mean rate of urinary protein excretion have significantly decreased from 616.66mg to 378.24 after 3 months (P=.000) and to 192.05mg after 6 months (P=.000) whereas no significant changes were observed in the control group. The beneficial antiproteinuric effect of PTX was not associated to the degree of metabolic control and a reduction of blood pressure. In addition, at the end of study, the mean clearance of creatinine was significantly higher in PTX group (P=.04). In conclusion, PTX can significantly provide additive antiproteinuric effect and slow the decrease in GFR among patients with type 2 DM under blockade of angiotensin system.
INTRODUCTION
Diabetes mellitus (DM) is the most common cause of end-stage renal disease (ESRD) in the world and proteinuria is the most distinctive predictor of diabetic nephropathy (DN) in these patients.1-3 In addition, proteinuria appears to be associated with increased cardiovascular and coronary heart disease mortality in patients with DM.4 Many studies have shown that proteinuria even at the level of 500mg per day can cause deterioration of GFR and destruction of kidney. It is also demonstrated that in diabetic patients, the urine albumin excretion rate depends on disease progression and there is a very strong association, as shown in many observational and meta-analyses, between decreased proteinuria and slowing of progressive DN and decreased risk of ESRD.5-12 The importance of early detection of proteinuria in patients with DM, led to the recommendation in the 2007 K/DOQI guidelines that these patients should be screened for proteinuria and if not found at the initial screening, yearly screening is also recommended.1
Although a number of investigations have shown that the blockade of renin-angiotensin system (RAS) with angiotensin converting enzyme inhibitor (ACEI) and or angiotensin II receptor blockers (ARB) in patients with DN prevents and/ or delays the progression of renal disease, however in a substantial number of patients, albuminuria and progression of renal disease are not completely halted by these agents and in large studies, blockade of RAS with ACEI or ARB alone and or in combination, attenuate the decrease of kidney function by no more than 15% to 40%.8-10 In addition blockade of RAS with these agents cannot be used in many patients because of their side effect including refractory hyperkalemia, angioedema and etc. Therefore, it is necessary to evaluate other agents with potential antiproteinuric and renoprotective effects among these patients.
There now are some data that Pentoxifylline (PTX), a non selective phosphodiesterase inhibitor, reduces urinary protein excretion in individuals with diabetes. However, there are few and short period human studies about its benefits and therefore in the present study, we have conducted a clinical trial to assess the effect of PTX on reduction of proteinuria among these patients.
MATERIALS AND METHODS
Study Design
The study was a double blind, randomized and controlled clinical trial that was approved by the Research Center of Ahvaz Jundishapur University of Medical Sciences. The study performed at the outpatient diabetic clinic of the Naft Hospital, Ahvaz, Iran and the drugs were provided to the patients free of cost by the hospital. The period of study was nine months from March 2011 to December 2011. The nature of the trial was explained to the patients and written informed consents were obtained. Primary end points of the study was reduction of proteinuria in both control and treatment groups.
Patients
Patients with Type 2 diabetes mellitus (according to the American Diabetes Association criteria) who referred to our diabetic clinic because of proteinuriawere evaluated. The inclusion criteria for selection of patients was diabetic nephropathy, defined by persistent proteinuria >150mg/24h in three consecutive measurements, no other kidney or renal tract disease and insufficient response to adequate therapy with lozartan and enalapril in at least 3 months before inclusion in the study.
A standardized questionnaire was used to collect general information such as age, gender, Body mass index (BMI), the record of previous diseases, type and dose of medication, and drugs, vital signs and the results of laboratory data.
We were randomized our patients by using a computer-generated random-number table in two groups. Patients in control group (n=50) received losartan and enalapril and in the treatment group (n=50), PTX (400mg daily) was added to the afore-mentioned regimen for a six-month period. Patients were followed monthly for side effects of medications, blood pressure monitoring, and we performed laboratory tests including fasting blood sugar, blood urea nitrogen, creatinine, potassium, urinalysis, clearance of creatinine, and 24-hour urinary protein excretion rate. The glycated hemoglobin (HbA1C) level was also measured on the first visit, at the third month and the end of the study.
Fasting blood samples were obtained in the in the morning (between 8 and 11 a.m.) and in the sitting position. All of biochemical analyses on blood and urine samples were performed free of cost in the Naft Hospital. Serum creatinine, urinaryprotein excretion and HbA1c were determined by using the modified Jaffé method, colorimetry (TBA-120; Toshiba, Tokyo, Japan) and high-performance liquid chromatography.
Patients with the following characteristics were excluded: Loss to follow up, pregnancy, creatinine clearance less than 30cc/min, uncontrolled hypertension, a history of hypersensitivity reactions to losartan, enalapril and/or pentoxifylline and/or intolerance of medications, a history of cardiac, cerebral or peripheral vascular disease within the past year, current acute illnesses or co-morbid diseases (including unstable angina, myocardial infarction, symptomatic heart failure, retinal hemorrhage, infectious diseases and malignancies), hyperkalemia (serum potassium more than 5/5meq/lit) before and or during the period of study.
Sample Size
Sample-size analysis for the primary outcome estimated that 50 participants in each group would be sufficient on the base of comparing means formula in case of α=.05, β=.05, µ1=129, µ2=125, ð1=26 and ð2=12.
Statistical analysis
At the end of the study Statistical analyses were carried out usingstatistical package for social sciences (SPSS) version 15 software. Continuous variables with normal distribution were expressed as mean±SD and differences in quantitative and qualitative variables between groups were analyzed by the student t and Chi-square tests. Comparisons between two groups for urinary protein excretion rate were performed by using by using Mann-Whitney U test. Statistical significance was assessed at P<.05.
RESULTS
Baseline Characteristics and Follow-up
In overall, 100 patients with DM type 2 (54 males and 46 females) met eligibility criteria and were randomly assigned to control group (50 patients, 26 males and 24 females) and PTX group (50 patients, 28 males and 22 females).
The mean age of patients in control and PTX group were 58.24±8.06 and 56±7.63 years with no significant difference (P=.15).
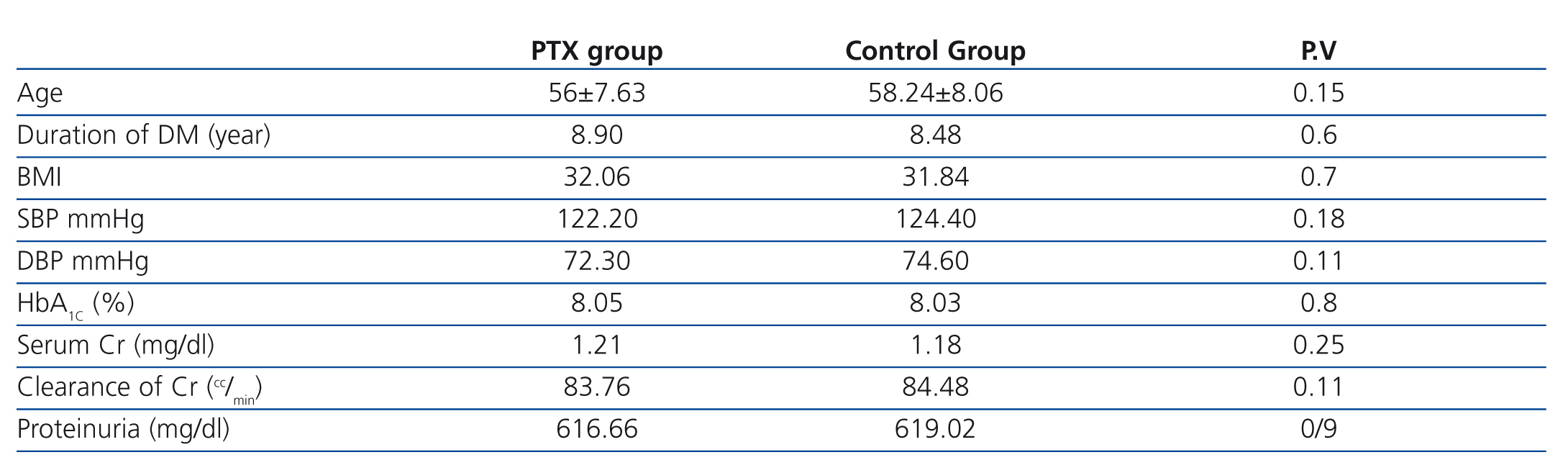
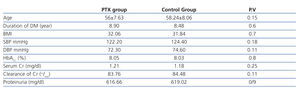
There was also no significant difference between males and females in both groups (P=.6).Other baseline general information, clinical characteristics, and laboratory data of the two groups were also similar and there was no significant difference between BMI (P=.7), systoloic blood pressure (P=.18), diastolic blood pressure (P=.11), duration of DM (P=.6), HbA1C (P=.8), serum creatinine (P=.25), creatinine clearance (P=.11) and 24-hour urinary protein excretion rate (P=.9) in both group at the beginning of the study (Table 1).
All of the patients in both groups were received losartan and enalapril at the recommended dosage. Mean dosage of losartan and enalapril in two groups were 50 and 15 mg respectively.
Through the study, 6 patients from PTX group were excluded because of chest pain and dyspnea in one, retinal hemorrhage in another and intractable nausea and vomiting in four patients. Therefore forty four patients completed the trial.
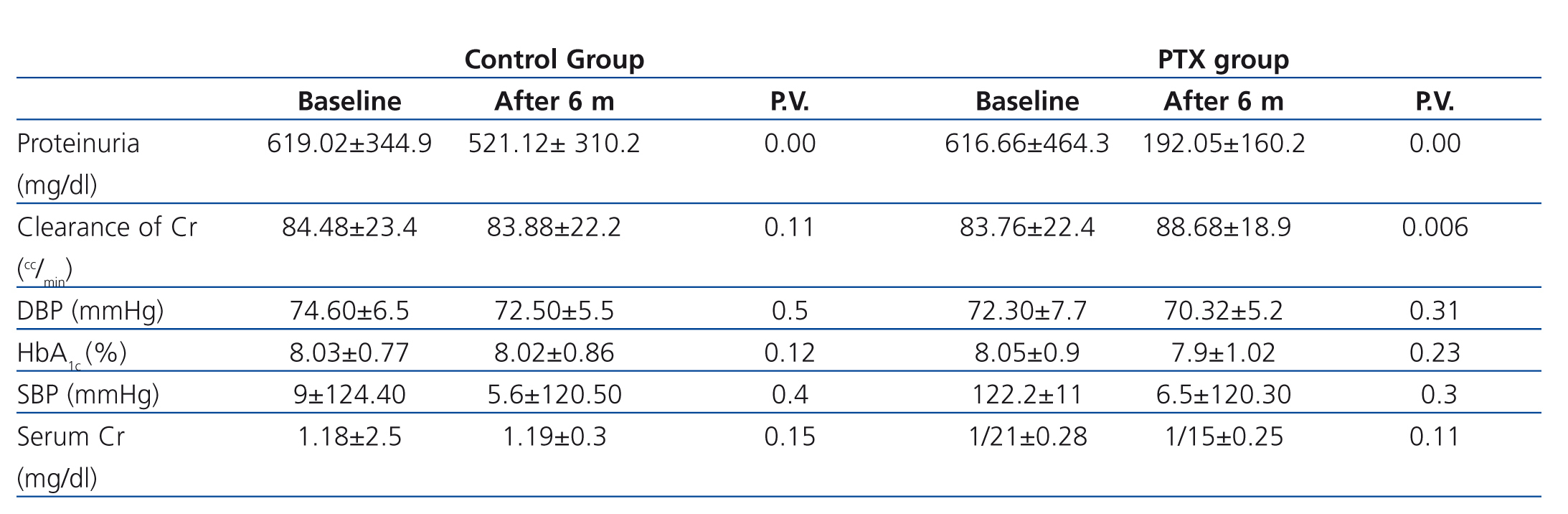
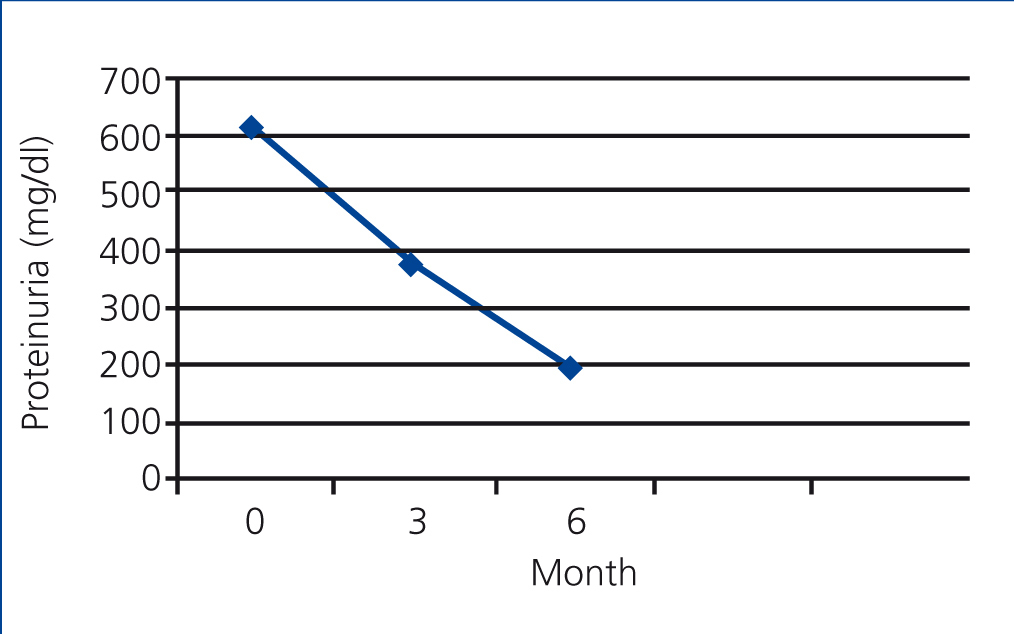
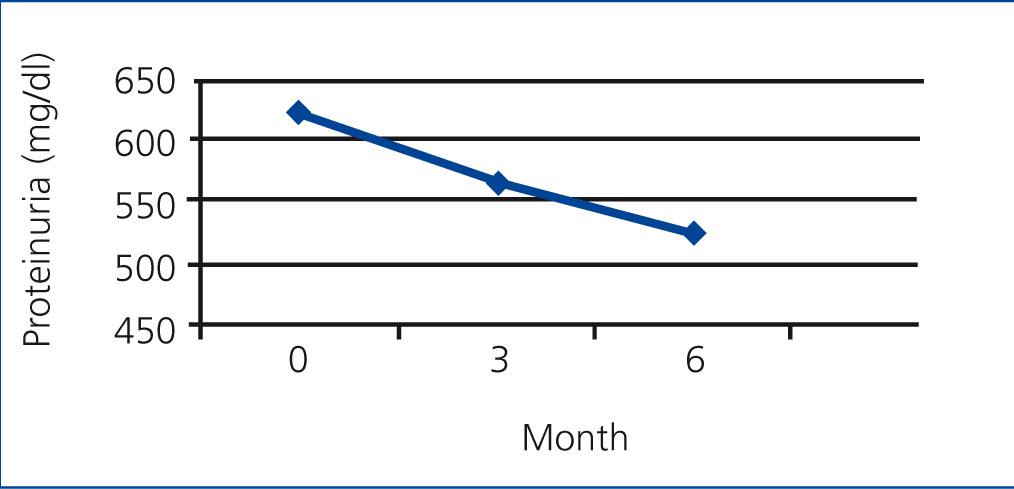
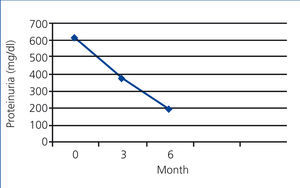
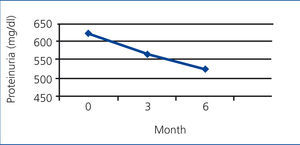
In the PTX group, the mean rate of 24-hour urinary protein excretion have significantly decreased from 616.66mg to 378.24 after 3 months (P=.000) and to 192.05mg after 6 months (P=.000) but in control group these values were 619.02mg, 561.86mg and 521.12mg respectively without a significant changes between them (Figure 1 and Figure 2).
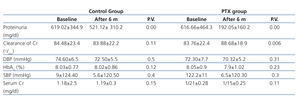
We compared the variation of systolic and diastolic blood pressure and HbA1C between PTX and control group at the end of the study and there was no significant difference between them (Table 2). In addition to losartan and enalapril, 25 and 9 patients in control group and 28 and 7 patients in PTX group were received spironolactone and amlodipine respectively with no significant difference between them (P=.5)
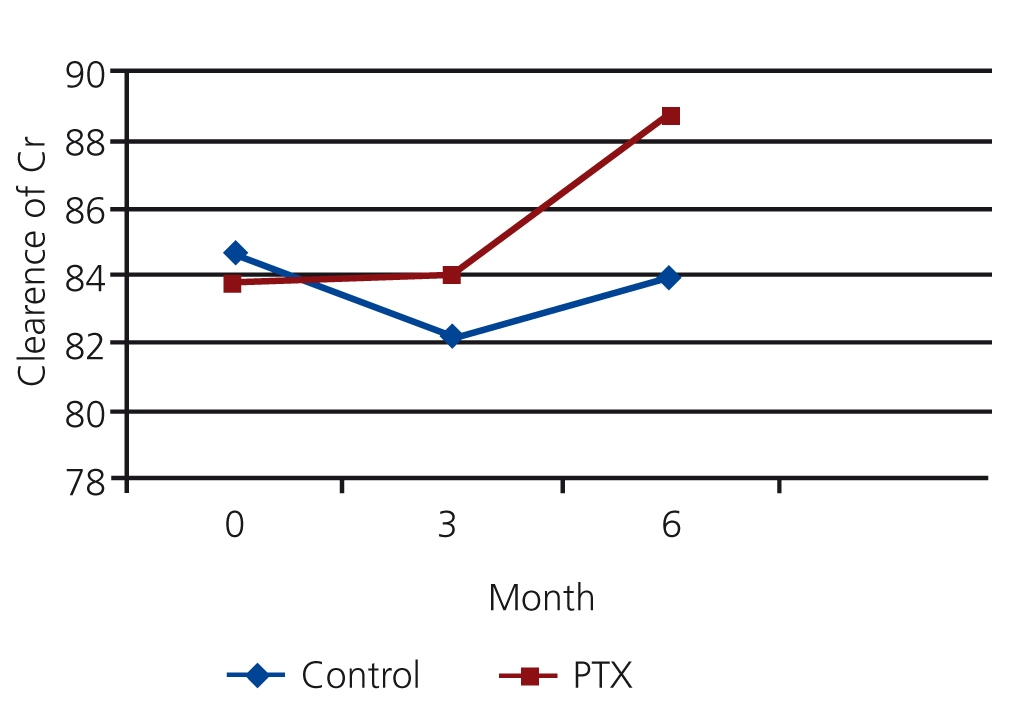
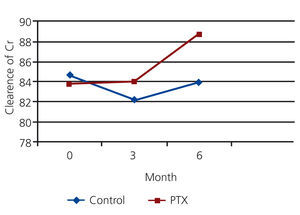
The mean clearance of creatinine in PTX and control group were 83.76 ml/min and 84.48 ml/min at the beginning of the study, 84.05 ml/min and 82.24 ml/min at the 3rd month and 88.68 and 83.88 ml/min at the 6th month. There was no significant difference between mean clearance of creatinine in both groups initially (P=0.11) and after 3month (P=0.08), but it was significantly higher in PTX group after six month (P=0.04) (see figure 3).
DISCUSSION
The earliest clinical manifestation of renal involvement in patients with DM is an increased urinary protein excretion and reduction of this parameter improves renal and cardiovascular outcomes in these patients.13,14
There are different mechanisms by which hyperglycemia might promote the development of DN. Glomerulosclerosis, for example, may result from glomerular hypertension and hyperfiltration induced by renal vasodilatation. Ischemic injuries induced by hyaline narrowing of the vessels supplying the glomeruli also have an important role in glomerulosclerosis in these patients.15-17
Tissue accumulation of irreversible advanced glycation end products (AGEs) increases in diabetic patients, particularly those with renal insufficiency and partly by cross linking with collagen may also contribute to the development of DN and other micro vascular complications.18,19
Some other proposed pathogenesis processes leading to the pathologic mechanisms in DN include activation of protein kinase C, upregulation of heparanase expression, increased plasma prorenin activity, increased vascular growth factors (vascular endothelial growth factor (VEGF), increased expression of TGF-beta in the glomeruli, Activation of cytokines, and increased serum levels of inflammatory markers.20,26
Serum levels of inflammatory markers are higher in diabetic patients with albuminuria in comparison to normoalbuminuric patients and it appears that the activation of cytokines are a potential mechanism in the pathogenesis of DN.27-30 For example, Transforming growth factor beta (TGF-beta) has been related to both the cellular hypertrophy and enhanced collagen synthesis that are seen in DN and according to preliminary human studies and experimental models of diabetes it seems that inhibition of TGF-beta ameliorate nephropathy.31-33 It is also shown that urinary level of tumor necrosis factor-alpha (TNF-alfa) is a predictor of albuminuria in patients with DM type 2.28
PTX that is used clinically to treat peripheral vascular disease, have anti-inflammatory and immunoregulatory properties. It can decrease intraglomerular pressure, renal TNF-α expression, synthesis, and excretion and serum levels of inflammatory markers in diabetic patients. It also has a potent inhibitory effect on cellular proliferation, inflammation and extracellular matrix accumulation.34-36 The hemorrheologic characteristics and its ability to decrease intraglomerular pressure and its anti – inflammatory effect have led to use of it for patients with diabetic nephropathy.37-41
Our study shows that in type 2 diabetic patients with DN and residual proteinuria despite adequate therapy with ARB and ACEI, PTX can significantly provide additive antiproteinuric effect and decrease urinary protein excretion rate. In addition, the beneficial antiproteinuric effect of PTX was not associated to the degree of metabolic control and reduction of blood pressure.
Navarro et al. has also evaluated the antiproteinuric effect of PTX. In this prospective and randomized study, they used PTX in a group of normotensive patients with type 2 diabetes and DN. Their own patients have residual albuminuria despite long-term therapy with different ARBs, including losartan, irbesartan, and candesartan at the recommended dosage; a significant additive antiproteinuric effect of pentoxifylline has been clearly proved in this study. In addition, they also showed that serum and urinary levels of TNF-α decrease in diabetic patients who received PTX. Similar to the results of our trial, the antiproteinuric effect of PTX was also not associated to the decrease of blood pressure or an improvement of metabolic control.38
Harmankaya et al. have also investigated the additive antiproteinuric effect of PTX in case of combination with an ACEI among hypertensivepatients with type 2 DM and persistent microalbuminuria who currently treated with lisinopril. They suggested that the combination of pentoxifylline with an ACEI causes a significant additional reduction in urinary albumin excretion independent of blood pressure and glycemic control.39
The dosage of PTX used in our trial was at a low dosage of 400mg daily and to our knowledge, this is the first study proving the antiproteinuric effect of pentoxifylline in this dosage among patients with DM. In addition, we added PTX to the therapeutic regimen of proteinuric patients under blockade of both an ACEI and an ARB. Previously, this combination had only been used once in a small number of diabetic patients with chronic renal insufficiency.40
There are some other studies that compare the relative efficacy of PTX with ACEI and/or ARB in the treatment of proteinuria among patients with DM. As an example, in a randomized open, crossover, clinical trial, Aminorroaya et al. compared the relative efficacy of PTX and captopril in the treatment of proteinuria among 39 patients with type 2 DM and showed that PTX and captopril both significantly reduce overtproteinuria with equivalent efficacy and safety and postulated that treatment with PTX has a similar benefit with captopril in these patients.41
Rodríguez-Morán et al. have also compared the efficacy of PTX and captopril on the reduction of albuminuria in 130 normotensive diabetic patients with normal renal function and concluded that PTX is an effective alternative agent to ACE inhibitors in reducing albuminuria.42
We also showed in our study that PTX may slow the decrease of GFR in proteinuric patients with DM and although there was no significant difference in mean clearance of creatinine in both groups after 3 month, however it was significantly higher in PTX group after six month. This result is similar to the results of Robert M. Perkins et al. study. They investigated in a pilot randomized double-blind placebo-controlled trial, the effect of PTX on glomerular filtration rate (GFR) decline in patients with chronic kidney disease with different causes including diabetic nephropathy. They evaluated 40 outpatients with decreased GFR (estimated GER of 20 to 40mL/min/1.73m2) and urinary protein excretion greater than 1g/24h who were currently treated with an ACEI or ARB, or the combination at least 2 weeks before enrollment and followed up in a nephrology clinic at a tertiary medical care facility. In the end of the study, the author concluded that PTX may slow the decrease in GFR in hypertensive proteinuric patients. However, the rate of urinary protein excretion was not reduced in PTX group in comparison of placebo group after 6 months and 1 year follow up in this study.43
Our study is limited by short duration and lack of information regarding the antiproteinuric effect of ACEI and ARB before the addition of PTX. It also limited by the lack of knowledge about the time of treatment with blockers of the renin-angiotensin system, specifically double-block. In addition, our study was a single center and we therefore need a multicenter clinical trial with long duration to prove the additive antiproteinuric effect of PTX and its effect on GFR decline among patients with DM.
CONCLUSION
The results of our Randomized and double blinded Clinical Trial Show that in type 2 diabetic patients with DN and residual proteinuria despite adequate therapy with ARB and ACEI, pentoxifylline can significantly provide additive antiproteinuric effect and decreases urinary protein excretion rate independent to the decrease of BP or improvement of metabolic control. We have also showed that pentoxifylline slow the decrease in GFR among these patients. Further clinical investigation with long duration is necessary to prove the additive antiproteinuric effect of pentoxifylline and its effect on GFR decline in patients with DM.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 1. Baseline demographic and clinical characteristics of patients
Table 2. Clinical characteristics of patients at the end of the study
Figure 1. The mean rate of protein excretion in PTX group
Figure 2. The mean rate of protein excretion in control group
Figure 3. The comparison of mean clearance of creatinine in PTX and control group