La eritropoietina es una hormona glicoproteica esencial para la progenie eritrocitica como factor de crecimiento y de viabilidad.La senalizacion bioquimica de la EPO implica un proceso de fosforilacion de la tirosina en el receptor homodimerico de la EPO,con una subsecuente activacion intracellular de proteinas kinasas, y factores de transcripcion.El tratamiento con las agentes estimulantes de la eritropoietina(ASE) se consideraba efectivo y falto de efectos deleterious mejorando asi el manejo de las anemias asociadas al enfermedad renal cronica,permitiendo la disminucion del requerimiento de transfusiones sanguineas.Sin embargo, recientes estudios aleatorizados han mostrado un incremento de la mortalidad, lo que ha hecho que se replantee su uso.Esta revision quiere mostrar lo que se sabe acerca de la fisiologia de este factor plasmatico,que representa mas que un factor de produccion de eritrocitos., y de su efecto pleitropico con su uso clinico en la incidencia de maliginidades,trombosis,hypertension y retinopatia diabetica
The glycoprotein hormone erythropoietin is an essential viability and growth factor for the erythrocytic progenitors. EPO signalling involves tyrosine phosphorylation of the homodimeric EPO receptor and subsequent activation on intracellular proteins, kinases and transcription factors. Treatment with erythropoietic stimulating agents (ESA) or ecombinant human EPO (rHuEPO) is efficient and safe in improving the management of the anaemia associated with chronic kidney disease, and allowing avoidance of transfusions with blood products. However, the unanticipated increase in mortality found in recent randomized studies is prompting a reassessment of this view. The present review will show what is known about the physiology of this plasma factor that, it is now clear, is more than just an erythrocyte production factor, and its pleitropic effects influencing the incidence of malignancy, thrombosis, hypertension and retinopathy.
INTRODUCTION
EPO was isolated for the first time by Carnot and Deflandre in experiments carried out in 1905 and 1906.1
The term erythropoiesis was not used until 1948 to describe the presence of plasma factors that had been unidentified until then and were generated during anoxia to stimulate the production of erythropoietin.2 Over the next 40 years, EPO was isolated and purified,3 enabling the development of a sufficiently sensitive test to understand its physiology.4 Since rHuEPO was cloned, not only has it been used successfully in the treatment of anaemia caused by chronic kidney disease (CKD) but it has also become the most effective tool for improving our understanding of this glycoprotein.5 Fifteen years ago, before rHuEPO was available, 25% of renal replacement therapy patients required transfusions of blood derivatives. rHuEPO has improved the quality of life of anaemic patients and is one of the most widely sold treatments in the world. While epoetin and darbepoetin alpha (modified to prolong its halflife) adequately stimulate erythrocyte production, there is increasing concern regarding its use. This concern emerged after the publication of two randomized studies involving patients with stage 3 and 4 CKD.6,7 These studies indicated a lack of benefits and significant complications when rHuEPO was used for obtaining a target haemoglobin of 13-15g/dl compared with patients who obtained a target haemoglobin between 10.5 and 11.0g/dl. The warnings published by the FDA (Food and Drug Administration) suggest that there are still many unanswered questions regarding its use.8 In order to be able to understand the use of EPO as a treatment for anaemia beyond obtaining target haemoglobin levels, it is important to understand its physiology and its receptor (EpoR) and how they intervene as growth factors and cytokines. This perspective could help clinicians understand the link between normal target haemoglobin levels and the adverse effects. The aim of this review is to describe the association between the physiology of EPO and its apparent oncogenic potential, the tendency to develop thrombosis, the increased incidence of hypertension and the mechanisms of the pleiotropic effects associated with diabetic retinopathy. It has to be taken into consideration the differences between patients, specifically the oncology patients as their response cannot always be extrapolated to renal patients, especially with regard to doses and EPO administration intervals.
THE STRUCTURE AND PHYSIOLOGY OF HUMAN ERYTHROPOIETIN
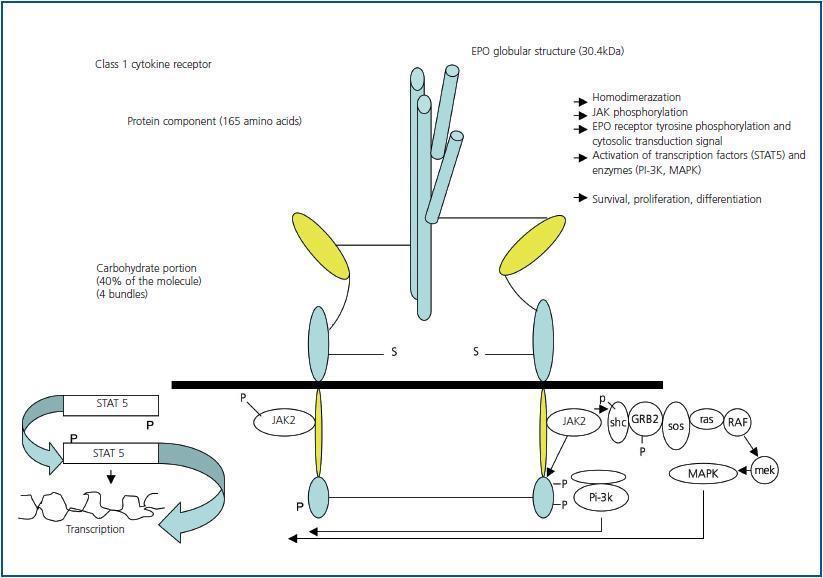
EPO is a pleiotropic and pro-angiogenic cytokine which also plays a protective role in other non-haematopoietic tissues It is also a glycoprotein hormone that acts as a primary regulator of erythropoiesis. As previously mentioned, it belongs to the family of class I cytokines which have a globular and compact four alpha helix bundle structure.9 The molecular mass is 30.4 kilo Daltons10, and it consists of 165 amino acids large enough to act as a binding receptor and erythropoiesis stimulating agent in vitro. The carbohydrates (40% of the molecule) are necessary to ensure the stability of the hormone in vivo.11 EPO is produced in the hepatocytes during the foetal period. After birth, the source of the circulating EPO is the cortical peritubular cells.12 The primary stimulus for its production is tissue hypoxia. EPO is present in the serum of patients with CKD, however the levels do not correlate well with levels of serum haemoglobin. In contrast, levels that increase exponentially with a decrease in serum haemoglobin concentration have been observed in patients with preserved renal function. Values may increase to 10,000U/l compared with normal values of 15U/l.13 Like other plasma glycoproteins, EPO exist in various circulating isoforms that differ in glycosylation, molecular mass, biological activity and immunoreactivity. Daytime serum concentrations can vary and nocturnal values can be up to 40% higher than daytime values.14 A transcription factor called a hypoxiainducible factor (HIF) regulates blood levels using oxygen sensors. EPO is regulated by the distribution of oxygen rather than by haemoglobin levels. Serum oxygen is crucial (but difficult to detect and measure) when managing anaemia which means that target haemoglobin is inadequate as a therapeutic parameter. The mechanism of circulating EPO degradation is yet to be clearly defined. There is evidence that EPO is eliminated via receptor-mediated endocytosis by erythrocytes and other cells that possess EPO receptors.15 New rHuEPO formulations have been developed like CERA (continuous erythropoiesis receptor activator), which contain methoxypolyethylene glycol to prevent the internalization of the drug and prolong its biological half life.
EPO binds two identical receptors (EpoR) that are homodimers.16 Two janus kinase 2 molecules (tyrosine kinases), that are in contact with the cytoplasmic region of the receptor are activated,17 stimulating signal transduction. The effect of EPO is delayed by the action of haematopoietic cellular phosphate (HCP) which catalyzes JAK218 (figure 1). At first, researchers maintained that EPO acted exclusively in the haematopoietic system. Recent studies have shown that it is a pleiotropic hormone. The first experiments that described EPO action outside the haematopoietic system were carried out by Anagnostou et al. which showed a mitogenic and chemotactic effect of EPO on endothelial cells, as well as the presence of binding sites or receptors for EPO (EpoR).19 Additionally, an antiapoptotic effect of EPO-induced neuronal tissue protection has been described.20 These observations have been confirmed in the tissues of other organs by researchers who have suggested that this protective effect takes place not via the interaction of the classic EpoR homodimer which stimulates erythropoiesis but via a heteroreceptor made up of an EpoR that interacts with a non-EPO receptor producing dimerization or trimerization.21 A messenger RNA for EPO receptors in the endothelium, epicardium, pericardium, mesangium, epithelial cells, pancreatic islets, placenta and certain defined areas of the brain has been isolated. Based on these findings, it has been suggested that EPO may have angiogenic and neurotropic functions.22 However, its physiological role and its binding to the EPO receptor in non-erythrocyte tissue is yet to be clearly defined.
MALIGNANCY AND ANAEMIA ASSOCIATED WITH RECOMBINANT HUMAN EPO TREATMENT (rHuEPO)
Anaemia is an independent prognostic factor in the survival of cancer patients.23 The European study on anaemic cancer patients indicated that 72% of haematologic cancer patients and 66% of patients with solid tumours presented anaemic symptoms, although prevalence varied depending on the stage of the tumour. The pathophysiology is multifactorial. There is evidence to suggest that anaemia can cause a lack of therapeutic response to radiotherapy, chemotherapy and even surgery.24 Different clinical studies have suggested an improvement in the survival outcome of cancer patients that received rHuEPO for anaemia. However, other studies describe the adverse effects of using rHuEPO in the treatment of different cancers. A safety assessment carried out in a randomized study on the use of alpha EPO in patients with large cell lung cancer resulted in the termination of the study.25 The assessment suggested a lower survival outcome for patients treated with rHuEPO. There have been similar results recorded in studies involving patients with head and neck cancer, as well as breast cancer.26,27 Previous survival data showed an increase in premature deaths of cancer patients, suggesting that the prescribed doses of rHuEPO were causing increased tumour growth. In addition, some researchers have speculated that through an increase in serum haemoglobin, rHuEPO decreases tumour growth factors by reducing HIF (hypoxia inducible factor), a protein made up of two subunits (a and b) which bind and become HIF-1 when tissue oxygen is low, thereby regulating the expression of up to twelve genes involved in transporting oxygen, increasing capilarization, anaerobic metabolism and cell proliferation through increased oxygen transport as proposed by Semenza and Wang in 1992.28 It is worth highlighting that before the introduction of rHuEPO, cancer incidence in renal replacement therapy patients was lower than expected.29 An analysis involving 28,049 patients with CKD who underwent
renal replacement therapy before using rHuEPO revealed a standard incidence ratio of 0.9. A later analysis carried out by ESRDS (United States renal data system) until 1994 indicated a standard incidence rate of 1.2 with the greatest increase affecting patients under the age of 35.30 A better understanding of this incidence may be obtained by studying the chemical structure of the rHuEPO receptor. The EPO receptor has an extracellular N terminus associated with binding capacity, whereas the intracellular C terminus is associated with transduction signals. EPO binds to two identical receptors that are homodimers as mentioned previously. EPO receptor activation occurs via the phosphorylation of the tyrosine in a protein complex which contains the receptor. This phosphorylation occurs via the interaction of the tyrosine-kinase enzyme. This chemical reaction produces a pleitropic cell response.31 The activity of the tyrosine-kinase enzyme is important not only for the growth factor receptors but also for oncogens given its mitogenic stimulation potential; the latent oncogenic capability of these molecules has been established.32 Several researchers have documented the presence and expression of EPO receptors in numerous tumour cell lines (liver cancer cells among others). Prostate cancer cells and kidney cancer cells also expressed receptors and it seems to serve as a growth factor for these cells.33 Ovarian cancer cells exposed to rHuEPO presented increased EPO signal transduction and resistance to chemotherapy through a reduction in the proteins Bcl-2 and Bcl10 which are associated with cellular apoptosis.34
rHuEPO may influence tumour growth in other ways. EPO may encourage tumour angiogenesis through the proliferation of endothelial cells. The presence of EPO receptors in the tissue of large cell undifferentiated lung carcinoma may reduce patient survival, suggesting a possible paracrinal role of endogenous EPO in determining the aggressiveness of tumour growth.35 Another possible link between EPO and cancer can be found in cystic renal diseases. Patients with Von Hyppel-Lindau (VHL) disease often present kidney tumours and the morphology of the kidney is similar to that of renal polycystosis. Patients also present high levels of serum haemoglobin and EPO.36 Evidence of this in various studies which used EPO to treat the anaemia of cancer patients is worrying and on the basis of the aforementioned studies and the data taken from other clinical studies, the ODAC (FDA’s Oncology Drug Advisory Committee), met on two occasions (May 2004 and 2007), and issued recommendations on the use of ESA in patients with tumours. If we assume that therapeutic doses of epoetin and darbopoetin (and other agents that are being developed) may stimulate the growth of certain tumours, how can new EPO molecules being synthesized exert haematopoietic action without causing deleterious effects on tumour tissue? The notion that heteroreceptors act as EPO receptor complexes in tumour cells could be used as the domain that binds non-EPO receptors that would be different from the other two domain receptors. In this way, a “mutant” EPO molecule could be designed.37
THROMBOSIS AND ESA
Before rHuEPO was used in the treatment of anaemia, the incidence of deep vein thrombosis and pulmonary embolism was rare38,39 in patients with CKD. Nephrologists then noticed an alarming increase in thrombotic events.40 Other data showed an increased risk of pulmonary embolism among renal replacement therapy patients, with an incidence ratio adjusted for age of 2.11 after excluding dialysis patients with risk factors for thrombotic events.41 Recent retrospective studies showed that the use of rHuEPO resulted in a higher number of thrombotic events (hazard ratio 1.4, CI 95% 1.06-1.96) affecting critically ill patients and patients with breast cancer.42 We know that rHuEPO increases platelet aggregation43 and in addition can reduce levels of proteins C and S. This aggregation can be reversed by taking aspirin. However, vascular access thrombosis affecting haemodialysis patients cannot be prevented with aspirin and it is clear that the physiopathological mechanisms of this type of thrombosis are complex. Blood viscosity directly related to haemoglobin levels and it was thought that this increase was linked to a higher incidence of thrombosis in cases of polycythaemia. By treating anaemia with rHuEPO, one would expect an increase in cases of vascular access thrombosis. This however does not seem to be associated with haemoglobin levels.44 Vascular access thrombosis is caused by intimal hyperplasia which begins with the proliferation of smooth muscle cells, which does not respond to aspirin but can be stabilised by using angiotensin converting enzyme inhibitors.45 Recently, it has been shown that angiotensinogen II stimulates a growth factor similar to insulin by inducing tyrosinekinase receptors in vascular smooth muscle cells, which in turn produces an increase in vascular intimal hyperplasia.46 Therefore, it seems that both EPO and angiotensinogen II are growth factors that are similar to other cytokines that activate tyrosine-kinase receptors, and that may explain the not uncommon harmful effects of both, such as hypertension and vascular disease. From a clinical perspective, another possible explanation for the high incidence of thrombosis affecting this population could be thrombocytosis due to the presence of iron deficiency caused by ESA.47 In a study involving cancer patients receiving chemotherapy that took ESA, intravenous iron treatment reduced the increase in platelets by more than 350,000. In fact, patients with a platelet count above 350,000 had a higher incidence of thromboembolic events. The use of intravenous iron reduced the risk of thromboembolic events by 40%.48 It is however worth mentioning that in relation to this hypothesis that intravenous iron may have protective effects of intravenous iron, in some studies involving haemodialysis patients with cardiovascular problems, the use of iron did not exert any protective action and even caused deleterious effects,49 although these results should be interpreted with a degree of scepticism given that the data regarding treatment was the result of post-hoc analysis. It is therefore necessary to confirm these findings in future studies.
HYPERTENSION AS A SIDE EFFECT OF EPO AND ESA
The initial data collected in the literature indicating the effectiveness of rHuEPO stated that the most common side effect was hypertension (HT).50 These results are not surprising especially since HT can be associated with conditions like policythaemia.51 However, the link between HT and an increase in haematocrit has not been proven with absolute certainty.52 The use of rHuEPO increases peripheral vascular resistance and decreases cardiac output. This is due to an increase in endothelins, angiotensin, impaired vascular entothelial relaxation, altered calcium levels in vascular smooth muscle cells and the release of serotonin by the platelets. It is well known that ESA treatment can affect arterial blood pressure in 30% of cases.53 A recent metaanalysis carried out in a review of the literature on haemoglobin normalization showed a consistent increase in arterial blood pressure in high haemoglobin target values compared with low target values.54 In the CREATE6 study, a group of patients with normalized target haemoglobin presented systolic blood pressure above 160mmHg in 50% of cases. A possible explanation for such high arterial blood pressure values in these studies could be the lack of effective blood pressure monitoring for these patients, which means that the relationship between hypertension and the use of ESA cannot be proved with absolute certainty. Hampl et al.,55 in Germany, used an intensive anti-hypertensive treatment regime that included maximum doses of beta blockers, angiotensin II blockers (ARA II) and ACE-inhibitors, obtaining normalized blood pressure readings before the beginning of renal replacement therapy sessions, making this study different from recent randomized studies on ESA and conventional practise in haemodialysis units. In fact, the results of this group of researchers indicated that the normalization of serum haemoglobin levels was not only associated with no increase in arterial blood pressure but also with a reduction in blood pressure. Nevertheless, the use of these anti-hypertensive treatment regimes in our population resulted in an increased incidence of hypotension during dialysis. Basic research has shown that the anti-hypertensive effect of EPO may be separate from the haematopoietic effect. There are specific sites on the EPO molecule that determine the hypertensive effect and this has led to the creation of genetic animal models in which the haematopoietic effect is preserved without the hypertensive element.56
DIABETIC RETINOPATHY AND ESA
EPO is a growth factor that is common with other cytokines activating tyrosine-kinase receptors. A direct relationship has been established between the incidence of proliferative retinopathy in renal replacement therapy patients and the use of recombinant erythropoietin.57 It seems that erythropoietin itself is an angiogenic growth factor,58 and levels of EPO in the vitreous and aqueous humour are more accurate predictors of retinopathy and macular oedema than endothelial growth factors. In a recent study which used an animal model of retinopathy, the use of EPO protected from the neuronal apoptosis induced by tissue hypoxia and, at the same time, increased neovascularisation over time.59 Therefore, the stage of diabetic nephropathy may determine whether EPO causes beneficial or deleterious effects.60
CONCLUSION
It is clear that the glycoprotein that was originally isolated as a hormone because of its haematopoietic effects is in fact a cytokine with growth factor characteristics that have evolved to the point of acquiring different cell properties that are independent from erythropoiesis. These nonhaematopoietic effects may be deleterious. However, a greater understanding of the physiology of this molecule and its receptors is necessary to develop a new molecule with a minimum number of these effects. This knowledge is essential for today’s clinicians and those of the future. As previously mentioned, it is clear that a hypothesis needs to be formulated on the basis of randomized studies of EPO to minimise possible side effects of ESA therapy. Perhaps through intensive anti-hypertensive therapy, aggressive intravenous iron treatment, optimisation of oxygen consumption with the correction of metabolic acidosis and the use of supplements for energy metabolism such as l-carnitine, vitamins B6 and B12 and folic acid, as well as the avoidance of high doses of ESA, the deleterious effects associated with HT and thrombosis can be prevented and the rising trend in mortality highlighted by the CREATE, CHOIR and NHCT (Normal Haematocrit Cardiac Trial) studies, among others, can be reversed.
Figure 1.