Introducción y objetivo: La definición y clasificación actual de insuficiencia renal aguda se basa en criterios de consenso (sistemas RIFLE y AKIN). De los parámetros recomendados (creatinina, tasa de filtración glomerular y diuresis), la creatinina es el más empleado. En ausencia de valor basal conocido se recomienda su estimación a partir de la ecuación MDRD simplificada, asumiendo en el cálculo una tasa de filtración de 75 ml/min/1,73 m2. El objetivo del presente trabajo fue evaluar la repercusión diagnóstica del empleo de la creatinina basal estimada frente al valor real medido en pacientes operados de cirugía cardíaca. Métodos: Análisis de pacientes operados de cirugía cardíaca mayor incluidos de forma prospectiva en una base de datos. Para cada paciente se calculó el estadio RIFLE máximo alcanzado usando la creatinina basal medida y la estimada. Se analizó la repercusión sobre el diagnóstico mediante coeficientes de correlación intraclase, análisis de concordancia y gráficas de Bland y Altman. Resultados: La incidencia de insuficiencia renal aguda postoperatoria en 2.103 casos operados entre 2002 y 2007 fue del 29,1% al utilizar la creatinina estimada (14,3% con la medida). Esto supone una sobrestimación del 104%, y la correlación intraclase es de 0,12. Excluyendo a los pacientes con insuficiencia renal crónica conocida (tasa de filtrado glomerular [TFG] <60 ml/min/1,73 m2), tanto la sobrestimación (2,4%) como la correlación (0,57) mejoraron. Conclusiones: El cálculo de la creatinina basal a partir de la ecuación MDRD sobrestima la incidencia de insuficiencia renal aguda tras la cirugía cardíaca, y es un método inadecuado para su detección cuando el valor basal se desconoce.
Introduction and objectives: The current definition and classification of acute kidney injury is based on consensus criteria (RIFLE and AKIN systems). Creatinine is the most commonly used of the recommended parameters (creatinine, glomerular filtration rate and diuresis). If the baseline value is not known, it can be calculated based on the simplified MDRD equation, assuming a filtration rate of 75ml/min/1.73m2 for the calculation. The aim of this study was to evaluate the diagnostic impact of using estimated baseline creatinine compared to the actual value measured in patients undergoing cardiac surgery. Methods: Analysis of patients undergoing major cardiac surgery, who were prospectively included in a database. The maximum RIFLE stage reached was calculated for each patient using the measured and estimated baseline creatinine levels. The impact on the diagnosis was analysed using intraclass correlation coefficients, concordance analysis and Bland-Altman plots. Results: The incidence of postoperative acute kidney injury in 2103 cases between 2002 and 2007 was 29.1%, according to estimated creatinine (14.3% with the measure). This represents an overestimation of 104%, with an intraclass correlation of 0.12. By excluding patients with known chronic kidney disease (glomerular filtration rate [<60ml/min/1.73m2), both the overestimation (2.4%) and the correlation (0.57) improved. Conclusions: The calculation of baseline creatinine using the MDRD equation overestimates the incidence of acute kidney injury after cardiac surgery, and is an inadequate method for detection when the baseline value is unknown.
INTRODUCTION
Acute kidney injury (AKI) is a common complication following cardiac surgery. Its appearance leads to a significant increase in both morbid-mortality and hospital stay. AKI frequency varies depending on the definition used. When the most severe forms, those requiring renal replacement therapy (RRT), are considered the range is 2%-5%.1 In recent years there has been a consensus in new definitions for this syndrome and a change in the nomenclature.2,3 The English term acute renal failure, traditionally translated into Spanish as insuficiencia or fracaso renal agudo has changed to acute kidney injury to broaden the spectrum of the syndrome and include milder cases. Liaño et al. suggested that AKI should be coined as alteración renal aguda (ARA) in Spanish.4 Since Lassnig’s study on patients undergoing cardiac surgery, it is known that even slight increases (0.3mg/dl) in serum creatinine (SCr) with respect to baseline have an impact on postoperative morbidity and mortality.5,6 These results have been confirmed in hospitalised patients.7
In 2004, the Acute Dialysis Quality Initiative (ADQI) proposed the use of the RIFLE system (Risk, Injury, Failure, Loss and End stage renal disease) with 3 diagnostic (R, I and F) and 2 prognostic (L and E) categories. The diagnosis is made based on SCr increases compared to baseline, decreases in the glomerular filtration rate (GFR), or absolute decreases in diuresis.2 Patients are diagnosed and classified using the criteria that placed them in the most severe stage. These criteria were reviewed in 2007 and modified by the Acute Kidney Injury Network (AKIN), this latter classification being recognised as the current one.3 However, and perhaps for non-scientific reasons,8 the RIFLE classification is very popular. The number of publications exceeds 150, and a fairly recent systematic review included 24 studies covering more than 70 000 patients.9 Among the problems associated with this classification is the diagnosis and classification of patients whose baseline serum creatinine is unknown. This occurs quite often in patients with an altered SCr value who are admitted to critical care units for acute problems (e.g., septic shock, acute coronary syndrome and heart failure). The ADQI recommendation is to assume that patients with a previously unknown renal function, with no known kidney disease, have a GFR of 75-100ml/min/1.73m2, and then to back-calculate the SCr value using the simplified MDRD equation.10 This method has been used in several epidemiological studies despite its lack of validation.11,12
The aim of this study is twofold: 1) To assess the agreement between estimated baseline SCr (est SCr) against the known baseline SCr in patients undergoing cardiac surgery, and 2) To analyse the impact of differences in baseline SCr on AKI frequency and severity for those episodes observed in cardiac surgery.
MATERIAL AND METHODS
Perioperative data from cardiac surgery patients operated on in our hospital were compiled prospectively in a database kept by the Anaesthesiology Department. We used data from patients operated on between 2002 and 2007. All patients older than 14 years who underwent major cardiac surgery with or without extracorporeal circulation were included. Patients were excluded if they were on dialysis prior to surgery, were kidney transplant recipients, had died within 24 hours of surgery or if they underwent second major surgery during the same hospitalisation period.
The measured baseline SCr (act SCr) was considered as the last SCr measurement before surgery, usually within 24 hours for patients undergoing elective surgery. The kinetic Jaffe method was used. The GFR was estimated from the simplified MDRD formula using four variables (SCr, age, sex and race). To calculate baseline serum creatinine (est SCr) from this equation, considering a GFR of 75ml/min/1.73m2 (the lower normal limit), the ADQI recommendations were followed by using the following formula:
Estimated SCr=(75/[186x(age-0,203)x(0.742 for women)x(1.21 for black people)])-0,887
Patients were assigned the highest stage by the RIFLE classification from SCr determinations during the first week after surgery. The criterion based on percentage GFR decreases was not used, due to its lack of linearity and consistency when compared with increases in SCr.13 The diuresis criterion was also not used due to lack of data.
The agreement between act SCr and est SCr was assessed by the intraclass correlation coefficient14 and Bland and Altman plot.15 The bias, the overall mean of the differences between act SCr and est SCr, was also calculated; as well as precision, defined as the bias standard deviation. The estimated error was calculated as the percentage of patients diagnosed with AKI using est SCr (estAKI) with respect to those diagnosed from act SCr (actAKI), using the formula below:
(estAKI – actAKI)/actAKI
A positive value showed an overestimation while a negative one implied underestimation. An initial analysis of the global sample and a second analysis, excluding patients with stage 3 or higher chronic kidney disease (CKD) according to the National Kidney Foundation K/DOQI classification, were performed.16 The mean and standard deviation or median and interquartile range, depending on the distribution of the quantitative variables studied, were used as measures of central tendency and dispersion. A statistical analysis of the data and plots was performed using SPSS version 18.0.
RESULTS
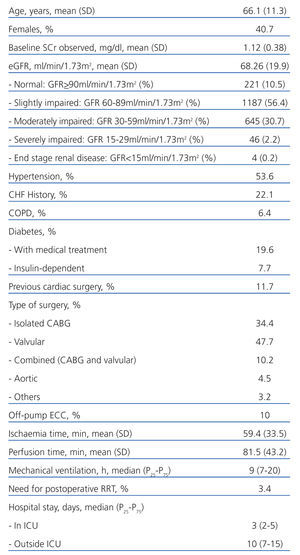
A total 2167 operations were performed on 2131 patients during the study period. Those patients excluded were: 31 patients (32 surgeries) for undergoing preoperative dialysis or being kidney transplant recipients; 27 who died soon after surgery (<24 hours); and 5 for having a second surgery during the same hospitalisation period. The final sample consisted of 2103 operations on 2067 patients, and the main features are shown in Table 1.
Table 2 shows the number and percentage of patients who reached each RIFLE stage during the first week after surgery, according to the type of baseline SCr used, either actual or estimated; the correlation between both; and the bias and precision. The global overestimation rate was 104% (29.1% vs 14.6%). The diagnosis of AKI whith est SCr showed a false positive rate of 14.8%. After excluding patients with a GFR<60ml/min/1.73m2, the correlation was better. The overestimation decreased to 2.4% with a false positive rate of 0.28%.
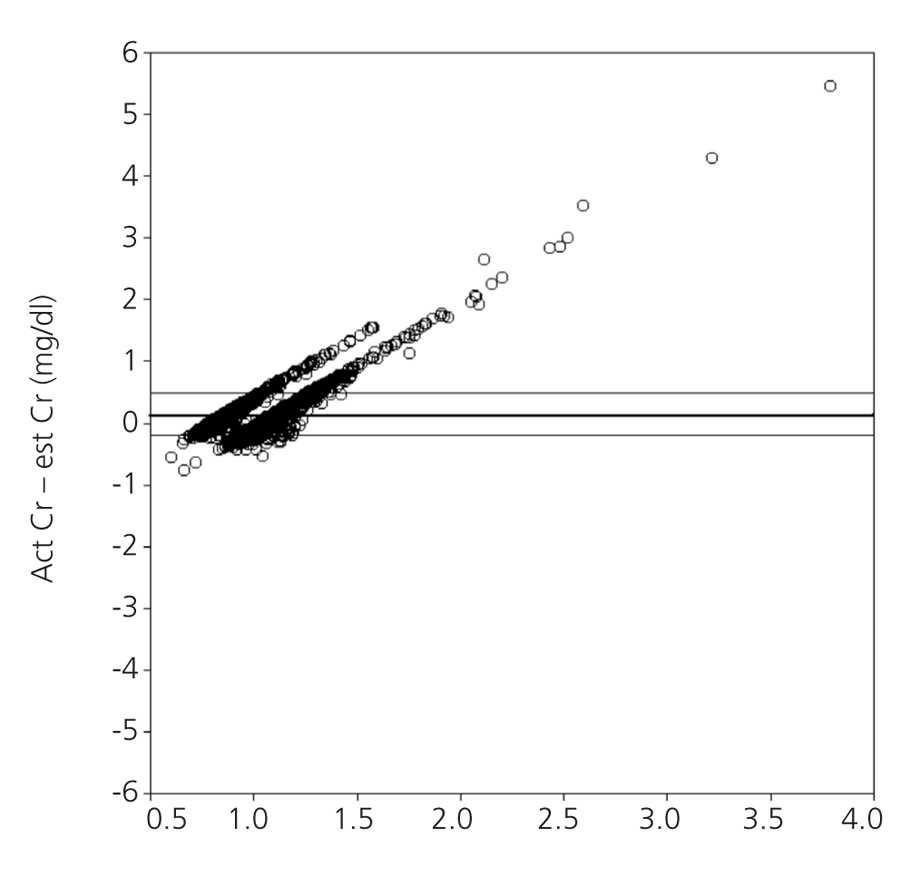
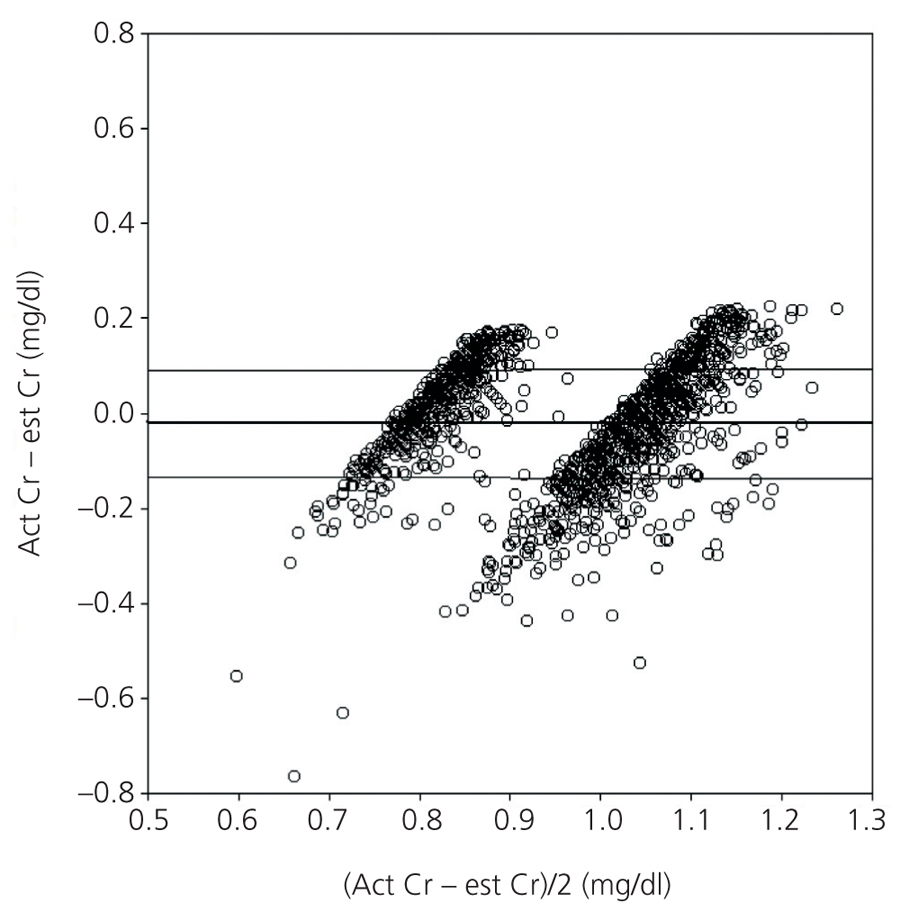
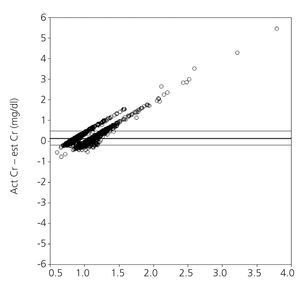
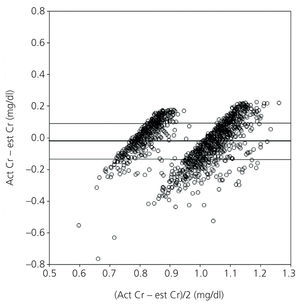
Figure 1 shows the correlation between est SCr and act SCr via Bland and Altman plots. The point cloud arrangement indicates that the degree of agreement decreases when the baseline SCr increases. Graphically, there is greater agreement between both creatinine values after excluding patients with any CKD stage prior to surgery (Figure 2).
DISCUSSION
This study shows that the recommendation to calculate baseline SCr from the MDRD-4 equation assuming a GFR of 75ml/min/1.73m2 overestimates the incidence of AKI in patients undergoing cardiac surgery. A similar conclusion was reached by several authors using more heterogeneous cohorts than ours. In the BEST Kidney study, Bagshaw17 found an overestimation of 42% using the same formula to calculate baseline SCr from a prospective study of over 1300 patients from 54 intensive care units (ICUs) in 23 countries. In a cohort study of almost 5000 patients hospitalised during a year, Siew18 compared 3 different methods to estimate the baseline serum creatinine with values obtained from pre-admission renal function for diagnosing AKI. The methods were SCr calculated from MDRD with a GFR of 75ml/min, the minimum SCr value during admission and the SCr value on admission. The first 2 methods overestimated the incidence by almost 50%, while the use of SCr on admission underestimated the diagnosis by 46%. More recently, Závada19 found similar results when comparing 3 different methods, including the one recommended by ADQI, in non-selected patients from 3 different hospital ICUs. Finally, after prospectively analysing 224 patients from 2 general ICUs used as controls in the EARLYRAF study, Pickering and Endre20 found an overall overestimation of 35.7% with an assumed GFR of 75ml/min, which increased to 92.8% when a baseline GFR of 100ml/min was considered. Unlike our cohort study, only 23% of the patients underwent cardiac surgery, and the rest had thoracic surgery, vascular surgery, sepsis, trauma or cardiac arrest. Our study was the first to focus on postoperative cardiac surgery patients, therefore it is not possible to make direct comparisons with other studies.
Both the prevalence of CKD or preoperative renal dysfunction and the incidence of AKI have a clear impact on the percentage of overestimation. To some extent, the first may be influenced by age (generally higher in surgical patients) and other cardiovascular risk factors (particularly hypertension and diabetes), which were significantly present in our sample. It is worth noting that the best definition of preoperative renal dysfunction for patients undergoing cardiac surgery was established by Wijeysundera,21 as creatinine clearance (CrCl) lower than 60ml/min. The odds ratio for developing severe AKI (with a need for RRT) in a random sample of 2000 patients operated on over a period of 16 months was 5 (confidence interval [CI] 95%; 2-12.6) when presenting this degree of renal dysfunction. The prevalence of preoperative renal dysfunction in this sample was 27%. The prevalence of moderate or severe CKD (GFR<60ml/min/1.73m2) in the Pickering study cited above was 28%, while that of Bagshaw was 46%, which left our study between them (33.1%). Excluding these patients the overestimation decreased in all 3 studies. This effect is easily explained, as a significant number of cases where the patient is given a GFR above the real one are excluded. Another concept worth mentioning, which was also described by Wijeysundera, is that of occult renal insufficiency.22 This is defined as preoperative renal function with a CrCl<60ml/min, but with a preoperative baseline SCr<1.13mg/dl. In a cohort of over 10 000 patients undergoing cardiac surgery, the prevalence of occult renal failure was 9%, and was very similar to our sample (8.5%). This concept stresses the importance of estimating GFR or CrCl in addition to baseline SCr in a preoperative analytical evaluation.
The risk factors for AKI in the context of cardiac surgery have been extensively studied, with several grading systems established to calculate RRT preoperative risk.23-25 Preoperative renal function appears constantly among the factors considered. The incidence of AKI was 14.2% in our sample, lower than both the Pickering (31.2%) and BEST (44.9%) studies. Since the number of diagnosed cases is the denominator in the overestimation calculation, the smaller this number is, the higher the overestimation. Together with the different prevalence of CKD, this situation may explain our findings.
We believe that the main limitation of this study is the use of baseline SCr taken as part of the preoperative evaluation: this value need not necessarily reflect the subject’s SCr at baseline. A significant number of patients admitted to the emergency department with symptoms of ischaemic heart disease or heart failure undergo cardiac surgery several days later, once the patient has been stabilised. However, similar approaches have been used in the above studies. Although the current recommendation is to use a SCr value obtained in the 3 months before admission, there is no known best method for calculating baseline SCr when a baseline measurement is not available. In the study by Pickering and Endre,20 the random assignment of SCr values from a lognormal distribution curve adjusted to the parameters of central tendency and dispersion of the source population was the method that most closely approximated to the actual incidence (with an AKI underestimation of 2.9%). Failing that, and as a more practical method, the authors recommend using the lowest SCr observed during the first 7 days of hospital stay (an AKI overestimation of 5.7%). This recommendation, of course, should only be done for an epidemiological analysis; as such a delay in diagnosis is not acceptable from a healthcare point of view.
A second limitation of this study is the use of SCr criterion only for AKI diagnosis, excluding patients diagnosed from a decrease in diuresis. This is a problem for all retrospective analyses using the RIFLE system, which are the vast majority of those published. This is because this parameter is rarely reflected in databases with sufficient detail to be applied (i.e., on an hourly basis). Therefore, we did not give sensitivity and specificity values, as all criteria must be used to make the diagnosis reliably and to classify those patients with the most severe stage.
CONCLUSIONS
Using baseline creatinine calculated from the MDRD equation in the diagnosis of AKI overestimates the incidence in patients undergoing cardiac surgery. This is common in populations with an increased prevalence of mild CKD, with the intensity depending both on this factor and the incidence of AKI. However, this is acceptable in patients whose premorbid GFR is normal or nearly normal. This observation must be considered, especially in hospital-based epidemiological studies.
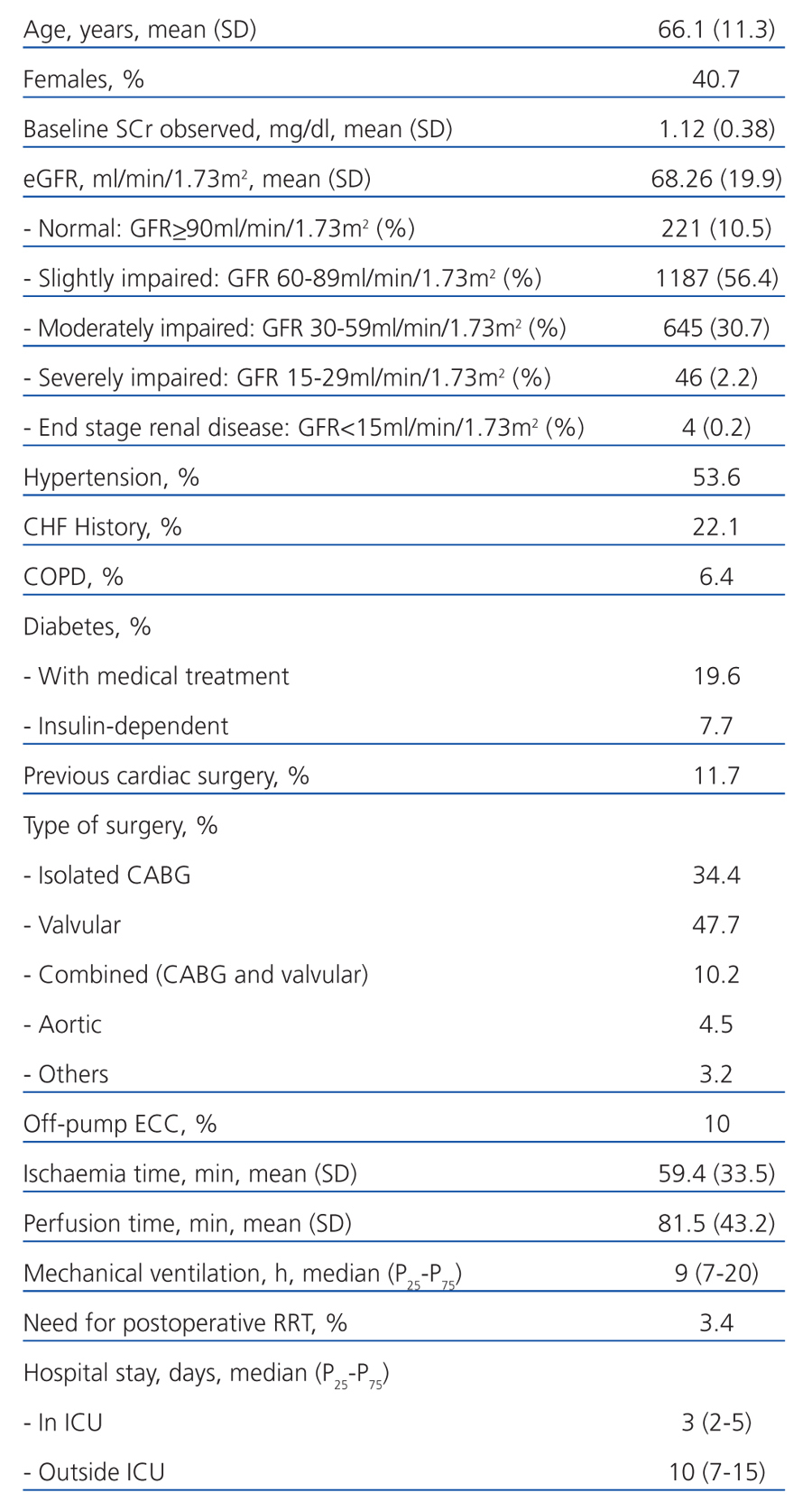
Table 1. Main demographic variables, comorbidity, surgical variables, postoperative variables and hospital stay in the studied cohort (n=2103)
Table 2. Renal function stratified by RIFLE category
Figure 1. Bland and Altman plot for the global cohort (n=2103) showing the degree of agreement between both creatinine values
Figure 2. Bland and Altman plot after excluding patients with impaired renal function, stage 3 CKD (n=1408)