1. To identify the variables that are associated with urinary levels of properdin, MBL, C4d, and C5b-9 in patients with idiopathic IgA nephropathy. 2. To analyse whether urinary levels of MBL and/or C4d are useful for identifying the presence of mesangial deposits of C4d/MBL.
Patients and methodA total of 96 patients with IgA nephropathy were studied. Demographic, clinical and biochemical variables were recorded at the time of diagnosis. Renal lesions were quantified using the Oxford classification. Immunohistochemical staining for MBL, MASP-2, properdin, C4d, and C5b-9 was performed in kidney biopsies, and in urine, the levels of properdin, MBL, C4d and C5b-9 were determined.
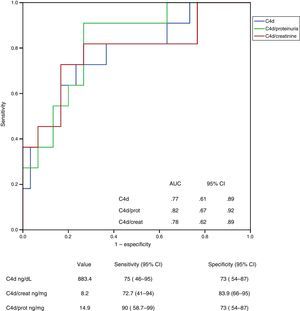
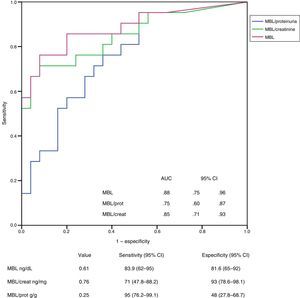
ResultsIn multivariate analysis, the independent predictors of C4d and MBL levels in urine were the mesangial deposits of each protein and, to a lesser extent, the urinary protein excretion. The independent predictors of urinary levels of C5b-9 were MBL properdin and proteinuria. Urinary excretion of C4d had a sensitivity of 90% (95% CI: 58.7–99) and a specificity of 73% (95% CI: 54–87) for detecting mesangial C4d deposits, and the level of MBL had a sensitivity of 83.9% (95% CI: 62–95) and a specificity of 81.6% (95% CI: 65–92) for identifying mesangial deposits of MBL.
ConclusionThe main predictor of urinary concentration of C4d and MBL was the presence of their respective mesangial deposits. Urine MBL may contribute to complement activation in the tubular luz through the lectin pathway. Urinary levels of MBL and C4d could be sensitive and specific biomarkers for the identification of patients with mesangial deposits of MBL and C4d.
1) Identificar las variables que se asocian con los niveles urinarios de MBL, C4d y C5b-9 en enfermos con nefropatía IgA idiopática. 2) Analizar si los niveles urinarios de MBL o C4d son útiles para identificar la presencia de depósitos mesangiales de C4d/MBL.
Pacientes y métodoSe estudió a 96 enfermos con nefropatía IgA primaria. Se registraron las variables demográficas, clínicas y bioquímicas en el momento del diagnóstico. Las lesiones renales se cuantificaron mediante la clasificación de Oxford. En las biopsias, se realizaron tinciones inmunohistoquímicas para MBL, properdina, C4d, y C5b-9. En orina, se determinó el nivel de properdina, MBL, C4d y C5b-9.
ResultadosLos predictores independientes de los niveles de C4d y MBL en orina fueron el depósito mesangial de cada una de ellas y, en menor grado, la proteinuria. Los predictores independientes de los niveles urinarios de C5b-9 fueron los niveles de MBL y properdina, y la proteinuria. La excreción urinaria de C4d tuvo una sensibilidad del 90% (IC 95%: 58,7-99) y una especificidad del 73% (IC 95%: 54-87) para la detección de depósitos mesangiales de C4d y el nivel de MBL tuvo una sensibilidad del 83,9% (IC 95%: 62-95) y una especificidad del 81,6% (IC 95%: 65-92) para identificar depósitos mesangiales de MBL.
ConclusiónEl principal predictor de la concentración urinaria de C4d y MBL es la presencia de depósitos mesangiales de ellas. La MBL podría contribuir a la activación del complemento en la luz tubular a través de la vía de las lectinas. Los niveles urinarios de MBL y C4d podrían ser biomarcadores sensibles y específicos para la identificación de los enfermos que presentan depósitos mesangiales de MBL o C4d.
The IgA mesangial nephropathy is one of the most frequent glomerulopathies. Around 25–30% of patients progress to chronic renal failure.1 The independent predictors renal function deterioration are: presence of renal failure at the moment of diagnosis, proteinuria (>1g/day), sclerosis glomerular or interstitial and hypertension.2–6 The pathogenesis of IgA nephropathy assumes that the galactose-deficient IgA1 is deposited in the mesangium both as isolated protein and forming of IgA/IgG or IgA/IgA complexes, once deposited they interacts with specific mesangial receptors or through the activation of the complement, there is increased activation, proliferation and synthesis of the mesangial matrix, leading to cellular damage.7 Two non-mutually exclusive pathogenic pathways have been proposed whereby deposits of IgA1 could induce complement activation: the alternative pathway and the lectin pathway; the mannose-binding lectin (MBL).8–10
There is data suggesting that the pathway of complement activation may determine long-term prognosis of IgA nephropathy. Separate studies indicate that the presence of MBL and C4d, indicators of complement activation through the MBL pathway, is associated more severe renal lesion and worse long-term prognosis.11–18 Therefore the evidence of activation of lectin pathway in the biopsies could be considered an early sign of poor prognosis independently from other classic predictors of bad outcome. Today the identification of this pathway of complement activation requires a renal biopsy. Since lesions are segmental and focal, a positive result may be valid while to accept a negative result it is necessary to have a sample with abundant glomeruli. In biopsies with only very few glomeruli the results could be false positives. It would be important to have non-invasive methods to identify patients with activation of lectin pathway from the very moment of diagnosis or at an early stage of the disease. Several studies have demonstrated that the urinary excretion of complement proteins and membrane attack complex proteins are elevated in patients with IgA nephropathy and correlate with the severity of renal disease.19–21 Also a recent study22 showed that in patients with IgA nephropathy the levels of urinary MBL are associated with the intensity of lesion observed in the renal biopsy and with the degree of proteinuria.
These data indicate that the determination of urinary levels of protein from the complement cascade and MBL, apart from a possible prognostic value, it could also be useful for the identification of patients with mesangial deposits of MBL and C4d. However the presence of proteins of the complement cascade and C5b-9 in urine has also been described in other proteinuria-associated nephropaties,23–25 and the evidence available today suggests that it could reflect the activation of intratubular complement through alternative pathaway mediated by properdin.26,27 So far none of the studies analysed whether the urinary excretion of C4d and MBL is associated with the presence of mesangial deposits of both proteins.
The goals of the present study are: 1) to identify the clinical, biochemical and anatomopathological variables associated with the urinary levels of properdin, MBL, C4d and C5b-9 in patients with idiopathic IgA nephropathy, and 2) to analyse whether it is possible in these patients to identify the presence of mesangial deposits of C4d or MBL through the urinary levels of MBL or C4d.
Patients and methodThe study included 96 patients with biopsy proven idiopathic IgA nephropathy. At the time of the renal biopsy and before initiating therapy, the demographic and clinical variables were recorded, the serum creatinine levels, the estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI28 equation, the urinary excretion of sodium and the urine protein to creatinine ratio (UP/CR) were determined, and blood and urine samples from the second morning urine were collected to measure the levels of properdin, MBL, C5b-9 and C4d. In all urine samples pyuria was ruled out by analysis of the urine sediment. The urine samples were centrifuged at 1500×g for 10min and stored at −80°C until processing. The creatinine levels were determined using the IDMS traceable compensated method (Hitachi Modular P-800 Roche Diagnostics, Berlin, Germany). The serum and urine levels of properdin, MBL, C4d and C5b-9 were measured using the following available ELISA kits: ELISA properdin (Hycult Biotech, Uden, The Netherlands), ELISA MBL (Antibodyshop, Gentofte, Denmark), C5b-9 ELISA BD Biosciences (San José, California, USA), and ELISA Quidel MicroVue™ C4d Fragment (San Diego, California, USA). The urinary concentrations of each molecule were expressed in absolute values after adjustment for proteinuria (ng/mg of proteinuria) and for urine creatinine (ng/mg of creatinine).
The same determinations were performed in a control group of 30 patients of similar age and gender distribution who had proteinuria with or without an impaired renal function and with a renal biopsy showing glomeruloesclerosis with negative immunofluorescence and with similar proteinuria a glomerular filtration rates.
The study protocol was conducted following the laws and regulations developed by the Declaration of Helsinki and approved by the Hospital Ethics Committee. All patients signed written informed consents. The data were handled following strict rules of confidentiality.
Anatomopathological study of renal biopsiesThe renal tissue was fixed in paraffin, 4μm thick cut sections were stained using haematoxylin-eosin, Masson trichrome, periodic acid-Schiff and Jones methenamine silver. Immunofluorescent studies were conducted in frozen tissue samples using antibodies against immunoglobulins A, G and M, fibrinogen, C3 and C1q. The staining of properdin, MBL, C4d and C5b-9 was performed through immunohistochemistry using the following antibodies: properdin (anti-MRP2 antibody [EPR10997(2)] (ab187644) Cambridge, United Kingdom), MBL (monoclonal antibody to mannose-binding lectin [3E7] Rockford Road, IL, USA) and C5b-9 (anti-C5b-9 antibody [aE11] (ab66768) abcam, Cambridge, United Kingdom). Immunohistochemical staining was performed in deparaffined, rehydrated 4μm thick cut sections after antigen retrieval with 5 minute-microwave pressure-cooking by use of a Tris/EDTA buffer, pH 8.5. As positive controls we used biopsies from patients with humoral rejection, membranose nephropathy, and membranoproliferative glomerulonephritis, and as negative controls we used biopsies from patients with minimal change nephropathy. The extent of interstitial fibrosis and tubular atrophy was assessed through the quantitative morphometric methods using the Olympus WCUE-2 image-analysis system. In addition, renal biopsies were classified following the Oxford criteria.29
Statistical analysisThe results are expressed as mean and standard deviation for variables with normal distribution or as median and quartiles for non-normal variables. The inter-group mean differences were assessed using the Student's t test for independent data or the Mann–Whitney test. Proportion differences were analysed using the chi-square test or Fisher's exact test. For the analysis of quantitative variables correlation the Spearman correlation test was used. To analyse the urinary levels of MBL, C4d and C5b-9 we used the univariate linear regression analysis followed by the stepwise multivariate analysis with manual introduction of variables considering the log of the concentration of each molecule as a dependent variable. Using ROC curves across all the possible combinations of sensitivity versus specificity, we could identify the urinary levels of C4d and MBL which had a higher sensitivity and specificity for the identification of patients with renal deposits of C4d and MBL. Once identified both the sensitivity and specificity of such values were assessed for the identification of mesangial deposits of C4d and MBL. p values <0.05 were considered statistically significant. The statistical analysis was conducted using the SPSS Statistics software, version 20.0.
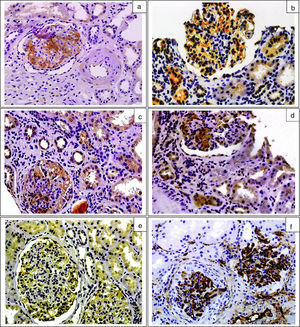
ResultsThe clinical, biochemical and histologic characteristics of patients with IgA nephropathy are shown in Table 1. The data is separated according to the presence or absence of mesangial deposits of C4d or MBL in renal biopsies; the biopsies from control group patients are also presented. There were no inter-group differences in age, sex distribution or the eGFR. Patients with IgA nephropathy positive for C4d showed significantly higher urinary levels of MBL, C4d and C5b-9 than IgA-C4d-negative patients and control group patients. The levels of properdin were significantly higher in patients with IgA nephropathy vs the control group and there were no differences between C4d-positive and C4d-negative IgA patients. Fig. 1 shows representative examples of immunohistochemical staining with mesangial deposits of MBL, C4d, properdin, C3 and C5b-9. All biopsies of patients with IgA nephropathy showed mesangial deposits of C3 and C5b-9. A total of 27 patients (28.1 per cent) showed mesangial deposits of C4d and 25 patients (26 per cent) showed MBL deposits. Among C4d-positive patients (n=27), 25 patients (92.5 per cent) showed mesangial deposits of MBL co-localised with deposits of C4d. As compared with C4d-negative patients, the C4d-positive had a lower prevalence of macrohematuria, a higher prevalence of S1 lesions, and more interstitial fibrosis.
Clinical, biochemical and microscopy findings in patients with IgA nephropathy separated by the presence or absence of mesangial deposits of C4d or MBL and the control group.
| IgA C4d+ N=27 | IgA C4d- N=69 | Controls N=30 | p1 | p2 | p3 | |
|---|---|---|---|---|---|---|
| Age (year) | 35.8 (12.9) | 34.9 (15.5) | 38.4 (18.9) | 0.36 | 0.39 | 0.34 |
| Sex M n (%) | 19 (70.3) | 43 (62.3) | 20 (66.6) | 0.61 | 0.58 | 0.49 |
| Macrohematuria n (%) | 8 (30) | 30 (43.4) | 0 | 0.21 | 0.001 | 0.001 |
| Hypertension n (%) | 5 (18.5) | 13 (19.0) | 7 (23.3) | 0.92 | 0.90 | 0.89 |
| Creatinine (mg/dL) | 1.31 (0.5) | 1.19 (0.4) | 1.21 (0.3) | 0.036 | 0.039 | 0.24 |
| eGFR (ml/min/1.73m2) | 89.6 (15.8) | 93.1 (11.5) | 90 (8.9) | 0.21 | 0.35 | 0.42 |
| Microhematuria (cel/μL) | 96 (27) | 111 (46) | 0 | 0.58 | NA | NA |
| UP/CR (g/g) | 2.3 (0.9) | 1.3 (0.6) | 1.2 (0.5) | 0.039 | 0.024 | 0.31 |
| M1 n (%) | 9 (33.3) | 20 (28.8) | – | 0.96 | NA | NA |
| S1 n (%) | 10 (37) | 12 (17.4) | – | 0.039 | NA | NA |
| E1 n (%) | 4 (14.8) | 10 (14.4) | − | 0.82 | NA | NA |
| Fibrosis n (%) | 0.027 | 0.019 | 0.23 | |||
| T0 | 13 (48) | 47 (68) | 21 (70) | |||
| T1 | 10 (37) | 15 (22) | 7 (23) | |||
| T2 | 4 (15) | 7 (10) | 3 (10) | |||
| Immunohistochemistry | ||||||
| C3 | 27 (100) | 69 (100) | 0 | 0.95 | NA | NA |
| Properdin | 24 (88.8) | 57 (82.6) | 0 | 0.84 | NA | NA |
| C4d+ n (%) | 27 (100) | 0 (0) | 0 | 0.000 | NA | NA |
| MBL+ n (%) | 25 (90) | 0 (0) | 0 | 0.00 | NA | NA |
| C5b-9+ n (%) | 27 (100) | 69 (100) | 0 | 0.8 | NA | NA |
| Serum levels | ||||||
| C4d (μg/mL) | 8.2 [2.1–74.6] | 9.6 [1.9–91.3] | 6.8 [2–66] | 0.42 | 0.45 | 0.39 |
| C5b-9 (ng/mL) | 291 [19.5–457] | 287 [33.2–498] | 229 [17.3–298] | 0.23 | 0.34 | 0.41 |
| MBL (ng/mL) | 709 [276–1723] | 812 [195–1845] | 754 [188–1786] | 0.36 | 0.38 | 0.34 |
| Properdin (μg/mL) | 26 [6–56] | 21.2 [4.8–69] | 20.8 [3.9–54.5] | 0.81 | 0.79 | 0.86 |
| Urinary levels | ||||||
| C4d/creatinine (ng/mg) | 29.5 [2.1–58] | 7.1 [2.2–8.5] | 2.2 [0–4.1] | 0.02 | 0.000 | 0.001 |
| C5b-9/creatinine (ng/mg) | 72.6 [7–296] | 58.3 [6–280] | 5.1 [0.1–23] | 0.04 | 0.000 | 0.001 |
| MBL/creatinine (ng/mg) | 3.82 [0.6–8.4] | 1.02 [0–0.8] | 0.06 [0–0.2] | 0.03 | 0.000 | 0.000 |
| Properdin/creatinine (ng/mg) | 0.61 [0.02–1.7] | 0.57 [0–0.78] | 0.12 [0–0.23] | 0.44 | 0.004 | 0.003 |
Quantitative variables are expressed as mean and standard deviation (SD). The categorical variables are expressed as an absolute value (percentage). Both the serum and urinary levels of C4d, C5b-9, MBL and properdin correspond to the median and [P25–P75].
UP/CR: urine protein to creatinine ratio; eGFR: estimated glomerular filtration rate; NA: non applicable; p1: differences between C4d-positive and C4d-negative patients with IgA nephropathy. p2: differences between C4d-positive patients with IgA nephropathy and controls; p3: differences between C4d-negative patients with IgA nephropathy and controls.
Table 2 shows the correlation matrix between biochemical and histological variables. The urinary levels of properdin, C4d and MBL and C5b-9 were not significantly correlated with their respective serum levels (r: 0.09, p: 0.34; r: 0.09, p: 0.76 and r: 0.02, p: 0.59 respectively) and showed negative correlations with glomerular filtration and positive correlations with the extent of focal glomeruloesclerosis, the infiltrates, and interstitial fibrosis. Furthermore, all of them showed a significant correlation with proteinuria. The deposits of MBL and C4d showed a marked correlation between themselves and with the levels of C5b-9. The levels of properdin were associated with the levels of C5b-9, and also showed a modest correlation with the levels of MBL and C4d. The levels of C5b-9 were significantly associated with the degree of proteinuria and with the urinary levels of properdin, MBL, and C4d.
Matrix of correlations between biochemical and pathological variables.
| eFGR | PIF | SS | PGEP | Int. infil | Prop | C4d | C5b-9 | MBL | |
|---|---|---|---|---|---|---|---|---|---|
| PIF | −0.56** | ||||||||
| SS | 0.21* | 0.25* | |||||||
| PGEP | 0.13 | 0.11 | 0.26* | ||||||
| Int. infil | −0.23* | 0.48** | 0.31* | 0.07 | |||||
| Prop | −0.38** | 0.43** | 0.24* | 0.01 | 0.29* | ||||
| C4d | −0.42** | 0.35** | 0.23* | 0.04 | 0.38** | 0.37** | |||
| C5b-9 | −0.37** | 0.43** | 0.25* | 0.01 | 0.40** | 0.48** | 0.66** | ||
| MBL | −0.49** | 0.38** | 0.30* | 0.13 | 0.41** | 0.24* | 0.75** | 0.59** | |
| UP/CR | −0.37** | 0.38** | 0.26* | 0.03 | 0.19* | 0.46** | 0.39** | 0.54** | 0.43** |
C4d: level of C4d in urine (ng/mg creatinine); C5b-9: level of C5b-9 in urine (ng/mg creatinine); PGEP: percentage of glomeruli exhibiting endothelial proliferation; eGFR: estimated glomerular filtration rate; PIF: percentage of interstitial fibrosis; SS: percentage of glomeruli showing segmental sclerosis; Int. infil: percent of interstitial cell infiltrates; MBL: level of MBL in urine (ng/mg creatinine); Prop: level of properdin in urine (ng/mg creatinine); UP/CR: urine protein to creatinine ratio.
Table 3 shows the independent predictors of the urinary levels of properdin, C4d, MBL and C5b-9 obtained by the multiple regression analysis and the percentage of variability of the predictor variables.
Independent predictors of properdin/creatinine C4d/creatinine, MBL/creatinine and C5b9/creatinine ratios in urine using multiple regression analysis.
| Dependent variables | Predictor variables | B | ET | β | Sig | R2 |
|---|---|---|---|---|---|---|
| Properdin/creatinine ratioa | Urine protein to creatinine ratio | 0.008 | 0.002 | 0.48 | 0.003 | 0.4 |
| Mesangial deposit of properdin | 0.73 | 0.10 | 0.69 | 0.000 | ||
| C4d/creatinine ratioa | Urine protein to creatinine ratio | 0.009 | 0.004 | 0.44 | 0.005 | 0.68 |
| Mesangial deposit of C4d | 0.42 | 0.12 | 0.58 | 0.002 | ||
| MBL/creatinine ratioa | Urine protein to creatinine ratio | 0.006 | 0.003 | 0.40 | 0.007 | 0.71 |
| Mesangial deposit of MBL | 0.61 | 0.14 | 0.65 | 0.000 | ||
| C5b-9/creatinine ratioa | MBL/creatinine ratio | 0.59 | 0.37 | 0.81 | 0.000 | 0.70 |
| Urine protein to creatinine ratio | 0.22 | 0.28 | 0.46 | 0.002 | ||
| Urine protein to creatinine ratio | 0.09 | 0.01 | 0.19 | 0.046 |
β: standardised regression coefficient; R2: proportion of variance explained by the set of independent variables.
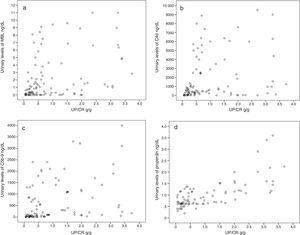
The UP/CR was identified as an independent predictor of all of them (urinary levels of properdin, C4d, MBL and C5b-9) but its influence was different in each case. In the case of properdin, the UP/CR was the only predictor identified. When it comes to the levels of C4d and MBL, the main predictor was the presence of mesangial deposits of MBL, and UP/CR was not that important. The main independent predictors of the levels of C5b-9 were the urinary levels of properdin and MBL, and to a lesser extent the UP/CR. Fig. 2 shows the scatter plot the urinary excretion of MBL, C4d, C5b-9 and properdin vs the UP/CR. Taken as a whole, the urinary excretion of the four molecules tends to increase with proteinuria (see Table 2). However patients with low levels of proteinuria may present with elevated excretion of MBL, C4d, C5b-9 and properdin, while patients with elevated levels of proteinuria may present with low excretion levels of all of them. Figs. 3 and 4 show the ROC curves that evaluate the value of the urinary levels of C4d and MBL in predicting the presence of their respective mesangial deposits. Included are the cut off values of higher sensitivity and specificity in absolute values and after adjustment for creatinine and proteinuria. To predict the presence of mesangial deposits of C4d, the urinary levels of C4d showed a similar sensitivity and specificity in absolute terms and after adjusted values for creatinine and proteinuria. To predict mesangial deposits of MBL, sensitivity was also similar, regardless of how urinary excretion of MBL was expressed, but specificity was higher (p: 0.027) if expressed in absolute terms or corrected by creatinine than when expressed as MBL/proteinuria ratio.
ROC curve showing the value of analysis of urinary C4d levels to predict the presence of mesangial deposits of C4d. AUC: area under the curve; SD: standard deviation; 95% IC: 95 per cent confidence interval. No significant differences were observed between the curve of C4d in absolute values and curves corresponding to C4d/creatinine or C4d/proteinuria ratios.
Value analysis of MBL levels in urine in order to identify the presence of mesangial deposits of MBL using ROC curves. No significant differences could be seen between curves corresponding to absolute values of MBL and curves corresponding to MBL/creatinine or MBL/proteinuria ratios. AUC: area under the curve; DT: deviation type; 95% IC: 95 per cent confidence interval.
The results of our study show than in patients with idiopathic IgA nephropathy, the urinary levels of properdin, MBL, C4d and C5b-9 are high, and have a strong correlation with glomerular filtration, proteinuria, and the extent and severity of tubulointerstitial fibrotic lesions. Also our results indicate that the urinary levels of C4d and MBL may be useful to identify patients with mesangial deposits of these two proteins.
The immunohistochemical analysis of renal biopsies was similar to the one described in former studies.11–18 The presence of mesangial deposits of C3 was a constant and was associated with the mesangial deposits of properdin and C5b-9 which indicates activation of the complement through the alternative pathway, common to patients with IgA nephropathy. The presence of mesangial C4d could only be confirmed in some patients and was associated with MBL deposits – suggesting complement activation through the lectin pathway. The presence of C4d does not exclude the possibility of simultaneous activation through the alternative pathway. Complement activation through different pathways could explain, at least partially, why patients with mesangial deposits of C4d have more extensive glomerular and turbointerstitial lesions and a worse renal function.
The presence of properdin, MBL, C4d and C5b-9 in urine may be due to a loss of integrity of glomerular barrier. However this explanation is not totally satisfactory since the urinary levels of properdin were significantly higher in patients with IgA nephropathy than in the control group. Also the mesangial deposits of MBL and C4d were significantly higher in IgA C4d-positive patients than in IgA C4d-negative patients and patients with non-complement mediated nephropaties after being adjusted for proteinuria. In the multivariate models obtained, proteinuria only explained a very small fraction of the variation in the urinary levels of properdin, MBL, C4d and C5b-9; these variations mostly depended of the presence of the respective mesangial deposits. These results indicate that the pattern of urinary proteins associated with complement activation could provide information on the intensity and the predominant complement activation pathway in any given moment of clinical course.
The evidence that the urinary excretion of MBL and C4d were significantly increased in patients with mesangial deposits of these both molecules and the demonstration of a strong association between the urinary levels of MBL and C4d, (in absolute values and after being adjusted for proteinuria or creatinine) and higher sensitivity and specificity for the identification of patients with mesangial deposits of C4d and MBL, could be considered a proof of concept supporting its clinical utility and warrant further investigation to define pre-analytical procedures and an adequate standardisation of the urine protein measurement for clinical practice.
In addition to providing information on the complement activation, our data shows that the amount of urinary excretion of MBL and C4d may also be correlated with the extent of turbointerstitial lesions. Several studies revealed that in different proteinuria-associated nephropathies including IgA nephropathy, the loss filtration barrier integrity would allow the passage to the tubular lumen of molecules able to activate the complement cascade on the surface of tubular cell membrane. This complement activation at the level of the tubular lumen has been proposed as one of the possible mechanisms whereby which proteinuria could induce turbointerstitial lesions.
Available evidence shows that the complement activation in the tubular lumen occurs basically through the alternative pathway and starts after the specific and pH-dependent binding of properdin (present in urine) to the heparan-suphate of tubular cell membranes.26,27,30,31 Our results are consistent with this hypothesis and also suggest that the presence of MBL in urine could also contribute to the intraluminal activation of the complement through the lectin pathway; note that in the multiple regression models the levels of MBL explained, in a large proportion, the variation of C4d levels and were independent predictors of the excretion of C5b-9 after adjusted values for creatinine and proteinuria. The combination of complement activation at the tubular level through both, properdin and MBL, could also explain why mesangial deposits of MBL and C4d and the urinary excretion of both proteins, corrected for proteinuria, present extensive turbointerstitial lesions at the moment of diagnosis11–18 and, in recent studies the elevated levels of MBL in urine have been identified as a sign of poor prognosis.22
In summary, the data from our study indicate that in patients with idiopathic IgA nephropathy, the urinary excretion of MBL is correlated with the presence of mesangial deposits of MBL and C4d, and is strongly associated with the urinary excretion levels of C4d and C5b-9 and with the extent of turbointerstitial lesions, regardless of proteinuria and the glomerular filtration rate. The urinary levels of MBL and C4d could be sensitive and specific biomarkers of mesangial deposits of such proteins. These findings warrant further investigation aimed at defining the technical aspect in relation with the measurement of these proteins so prospective clinical studies can be performed to evaluate the utility of measuring such molecules in clinical practice.
SponsorsThis study has been financed by the Fondo de Investigaciones Sanitarias FISS (Sanitary Research Fund), file number: PI14/01831.
Conflicts of interestsThe authors declare no conflicts of interests linked to this article whatsoever.
Please cite this article as: Segarra-Medrano A, Carnicer-Caceres C, Valtierra-Carmeno N, Agraz-Pamplona I, Ramos-Terrades N, Jatem Escalante E, et al. Estudio de las variables asociadas a la activación local del complemento en la nefropatía IgA idiopática. Nefrologia. 2017;37:320–329.