Cardiovascular diseases are associated with increased morbidity and mortality among CKD (chronic kidney disease) population. Recent studies have found increasing prevalence of PH (pulmonary hypertension) in CKD population. Present study was done to determine prevalence and predictors of LV (left ventricular) systolic dysfunction, LVDD (left ventricular diastolic dysfunction) and PH in CKD 3b-5ND (non-dialysis) patients.
MethodsA cross sectional observational study was done from Jan/2020 to April/2021. CKD 3b-5ND patients aged ≥15 yrs were included. Transthoracic 2D (2 dimensional) echocardiography was done in all patients. PH was defined as if PASP (pulmonary artery systolic pressure) value above 35mm Hg, LV systolic dysfunction was defined as LVEF (left ventricular ejection fraction)≤50% and LVDD as E/e′ ratio >14 respectively. Multivariate logistic regression model was done to determine the predictors.
ResultsA total of 378 patients were included in the study with 103 in stage 3b, 175 in stage 4 and 100 patients in stage 5ND. Prevalence of PH was 12.2%, LV systolic dysfunction was 15.6% and LVDD was 43.65%. Predictors of PH were duration of CKD, haemoglobin, serum 25-OH vitamin D, serum iPTH (intact parathyroid hormone) and serum albumin. Predictors of LVDD were duration of CKD and presence of arterial hypertension. Predictors of LV systolic dysfunction were eGFR (estimated glomerular filtration rate), duration of CKD, serum albumin and urine protein.
ConclusionIn our study of 378 CKD 3b-5ND patients prevalence of PH was 12.2%, LV systolic dysfunction was 15.6% and LVDD was 43.65%.
Las enfermedades cardiovasculares se asocian a un aumento de la morbilidad y la mortalidad entre la población con enfermedad renal crónica (ERC). Estudios recientes han encontrado un aumento de la prevalencia de la hipertensión pulmonar (HP) en la población con ERC. El presente estudio se llevó a cabo para determinar la prevalencia y los predictores de la disfunción sistólica del ventrículo izquierdo (VI), la disfunción diastólica del VI y la hipertensión pulmonar en pacientes con ERC 3b -5ND (sin diálisis).
MétodosSe realizó un estudio observacional transversal desde enero/2020 hasta abril/2021. Se incluyeron pacientes con ERC 3b -5ND de edad ≥15 años. Se realizó una ecocardiografía transtorácica 2D (bidimensional) a todos los pacientes. La HP se definió como un valor de presión sistólica de la arteria pulmonar superior a 35mm Hg, la disfunción sistólica del VI se definió como una fracción de eyección del ventrículo izquierdo ≤ 50% y la DVL como una relación E/e′>14 respectivamente. Se realizó un modelo de regresión logística multivariante para determinar los predictores.
ResultadosUn total de 378 pacientes fueron incluidos en el estudio con 103 en estadio 3b, 175 en estadio 4 y 100 pacientes en estadio 5ND. La prevalencia de la HP fue del 12,2%, la disfunción sistólica del VI fue del 15,6% y la DVL fue del 43,65%. Los predictores de la HP fueron la duración de la ERC, la hemoglobina, la vitamina D 25-OH en suero, la iPTH en suero (hormona paratiroidea intacta) y la albúmina en suero. Los predictores de la EVL fueron la duración de la ERC y la presencia de hipertensión arterial. Los predictores de la disfunción sistólica del VI fueron la tasa de filtración glomerular estimada, la duración de la ERC, la albúmina sérica y las proteínas en orina.
ConclusiónEn nuestro estudio de 378 pacientes con ERC 3b-5ND la prevalencia de la HP fue del 12,2%, la disfunción sistólica del VI fue del 15,6% y la DVL fue del 43,65%.
CKD is a major public health problem and prevalence is increasing in the recent years and is around 16%.1,2 Cardiovascular diseases are the most common cause of death in CKD population.3,4 Widespread utilization of 2D echocardiography helps in early identification of structural and functional abnormalities of heart.
Multiple factors in CKD population including hypervolemia, chronic inflammatory state and uraemia results in cardiac and vascular remodelling causing cardiac structural abnormalities and pulmonary hypertension. Recent studies have found increasing prevalence of PH in CKD population.5 Presence of LV systolic dysfunction, LVDD and PH is associated with increased morbidity and mortality among CKD patients.5–7
HD (Haemodialysis) is associated with high risk for LV systolic dysfunction, LVDD and PH. Most of the published studies on LV function and PH in CKD patients have included patients on HD. Data on prevalence and predictors of LV systolic dysfunction, LVDD and PH in non-dialysis dependent CKD patients in Indian population are sparse. Our study aims to determine prevalence and predictors of LV systolic dysfunction, LVDD and PH in CKD 3b-5ND patients.
Materials and methodsA cross sectional observational study was done from January 2020 to April 2021 in the Department of Nephrology and Cardiology IMS, BHU, Varanasi. CKD patients staged 3b to 5ND aged≥15 years were included in the study. Following patients were excluded from study (1) Prior Cardiac diseases(ischaemic/valvular/congenital/cor-pulmonale), (2) Chronic pulmonary diseases, (3) Patients on RRT (Renal Replacement therapy)-HD/Peritoneal dialysis/Renal transplant, (4) Patients with upper limb AVF (Arterio-venous fistula), (5) HIV patients, (6) Primary pulmonary hypertension, (7) Chronic liver disease-cirrhosis, (8) Thyroid disorders-hyper/hypo thyroidism, (9) Pregnant females and pregnancy related kidney diseases, (10) Patients presented with AKI (Acute Kidney Injury) or underwent haemodialysis/peritoneal dialysis in prior 3 months, (11) Smokers (old/current) & patients not consenting for study. Patients with LVEF <50% were excluded for PH. Institute ethics committee approval and consent from all study participants were taken.
Staging of CKD was done as per KDIGO (Kidney disease Improving Global Outcomes) 2012 guidelines considering eGFR & proteinuria.8 Proteinuria was quantified using 24h urinary volume by Pyrogallol red method. eGFR was calculated using CKD EPI(Chronic Kidney Disease Epidemiology Collaboration) equation using serum creatinine, age, sex & race. Serum haemoglobin, serum electrolytes, serum 25-OH vitamin D, serum iPTH & urine analysis was done in all patients.
Transthoracic 2D and Doppler Echocardiography was done at diagnosis of CKD in all patients
- 1.
PASP was measured using modified Bernoulli equation as follows PASP=4×(TRV)2+RAP, Where TRV is the maximum tricuspid regurgitation jet speed and RAP is the right atrium pressure which is estimated by inferior vena cava diameter. Pulmonary hypertension was defined as if PASP value above 35mm Hg.9
- 2.
LVEF was measured using Modified Simpson method. LV systolic dysfunction was defined as LVEF≤50%. Mild, moderate and severe LV systolic dysfunction was defined if LVEF is 41–50%, LVEF 31–40% and LVEF≤30%.10
- 3.
LVDD was measured by the E/e′ ratio, where E is the early mitral flow velocity on trans-mitral Doppler and e′ is the early mitral annulus velocity obtained from tissue Doppler. An E/e′ ratio of <8 is considered normal, while >14 was considered as LVDD. Grading of LVDD was done using peak E velocity and mitral E/A ratio, where A is late mitral flow velocity. Grade I LVDD was defined if E/A ratio≤0.8 with a peak E velocity of ≤50cm/s, grade 2 LVDD if E/A ratio of 0.9–1.9 and grade 3 LVDD if E/A ratio of ≥2.11
Statistical analysis was done using SPSS software (version 28.0). Continuous variables are expressed in mean±SD and compared using student's t test. Categorical variables are expressed as percentage and compared using Chi-square test. Variables which showed P value <0.1on student's t test and Chi-square test were analyzed using multivariate logistic regression models. Correlation between quantitative variables was done using Pearson's or Spearmann's correlations. P value<0.05 was considered statistical significant.
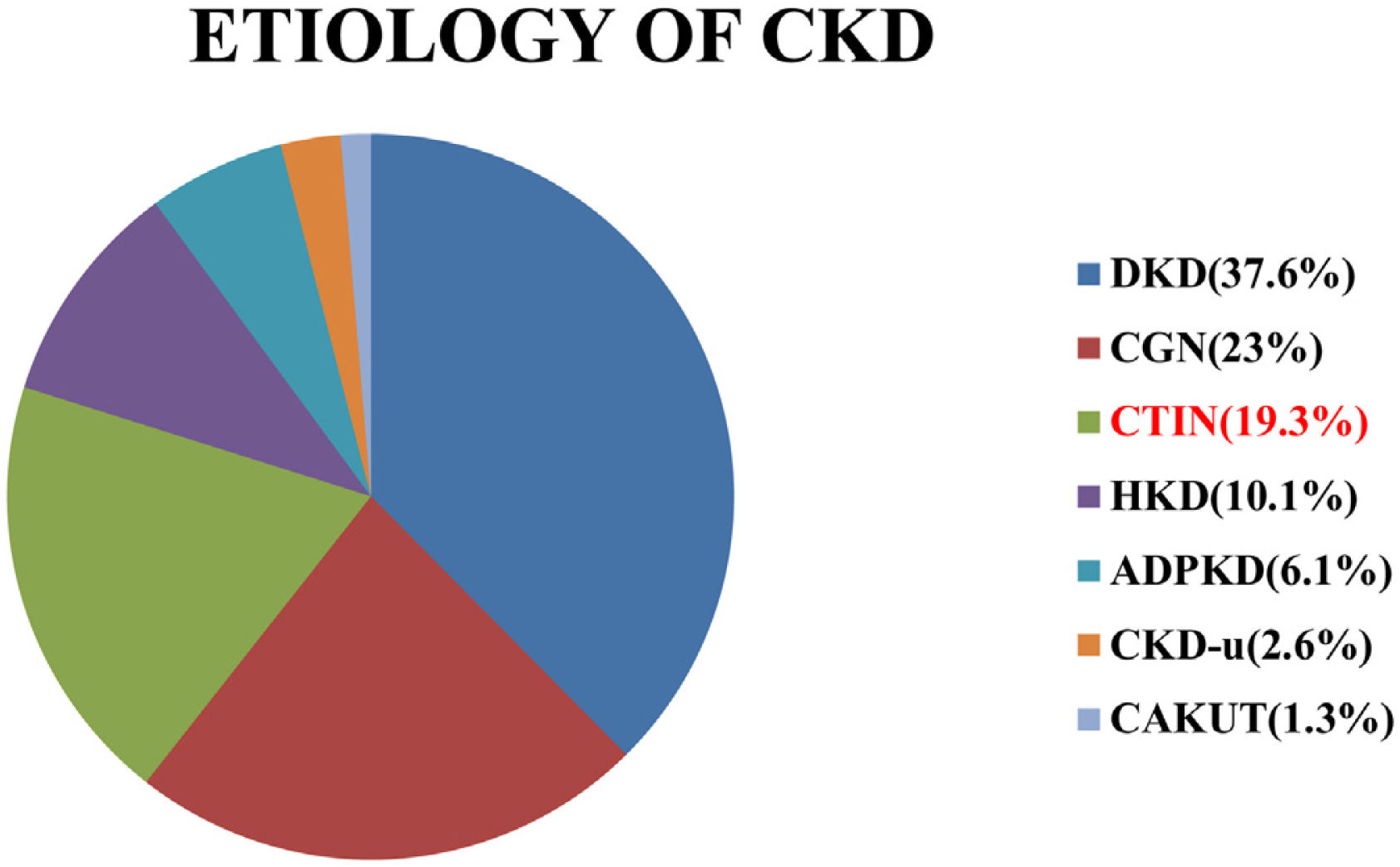
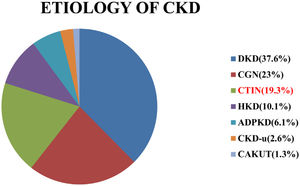
ResultsA total of 378 patients were included in the study. Mean age of the study population was 51.56±13.39 years. Male were 248 (65.6%) and female were 130(34.4%). There were 103 (27.2%) patients in stage 3b, 175 (46.3%) in stage 4 and 100 (26.5%) in stage 5-ND. Aetiology of CKD is shown in Fig. 1.
Frequency of distribution of aetiology of CKD (DKD–diabetic kidney disease, CGN–chronic glomerulonephritis, CTIN–chronic tubule-interstitial nephritis, HKD–hypertensive kidney disease, ADPKD–autosomal dominant polycystic kidney disease, CKD-u–CKD of unknown aetiology, CAKUT–congenital Anomalies of kidney & Urinary Tract).
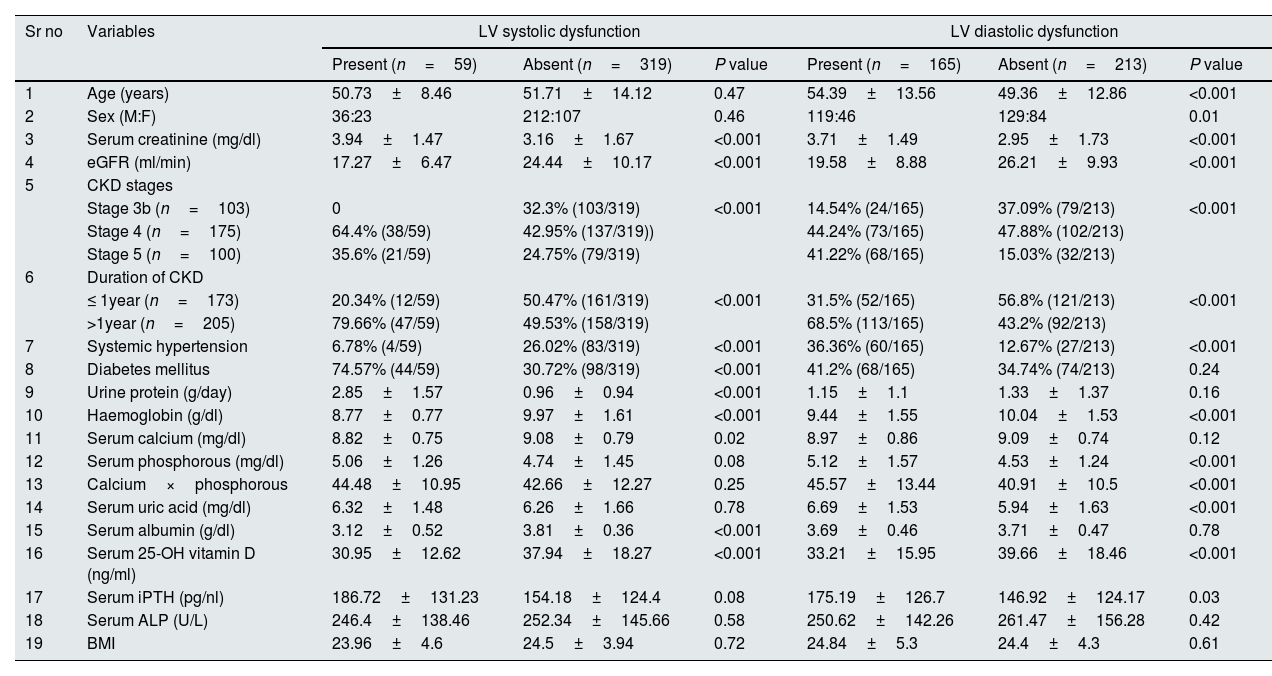
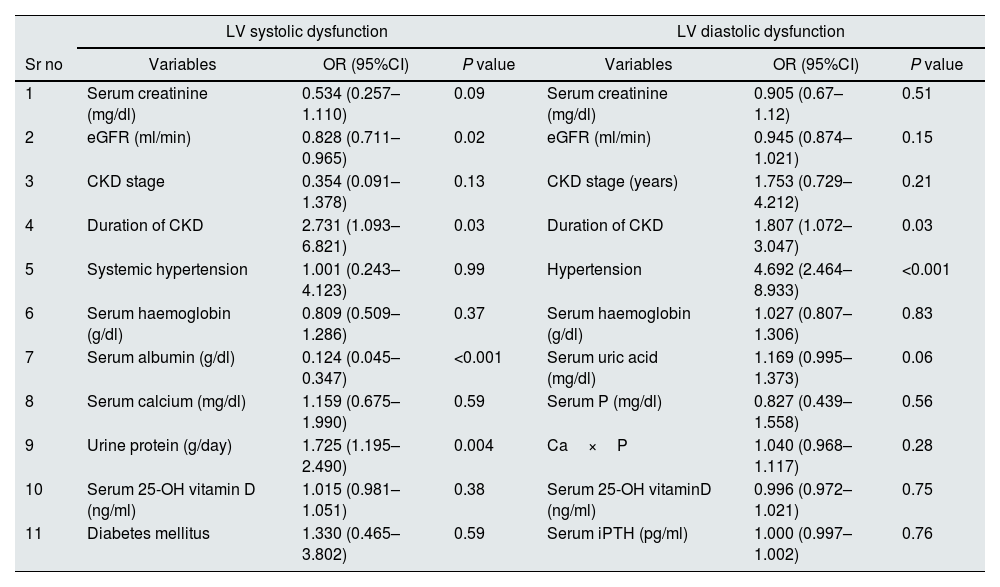
Fifty nine patients had LV systolic dysfunction with prevalence of 15.6%. 17 patients (28.8%) had LVEF 41–50%, 40 patients (67.8%) had LVEF 31% to 40% and 2 patients (3.4%) had LVEF≤30%. Patients were divided into 2 groups depending on presence or absence of LV systolic dysfunction. On comparing patients with and without systolic dysfunction (Table 1), significant variables were serum creatinine (P<0.001), eGFR (P<0.001), CKD stages (P<0.001), duration of CKD (P<0.001), 24h urine protein (P<0.001), presence of arterial hypertension (P<0.001), presence of diabetes mellitus (P<0.001), Haemoglobin (Hb) (P<0.001), serum calcium (P=0.02), serum albumin (P<0.001) and serum 25-OH Vitamin D (P<0.001). On age and sex adjusted multivariate logistic regression analysis significant predictors of systolic dysfunction were eGFR (OR–0.828{0.711–0.965}P=0.02), duration of CKD (OR–2.731{1.093–6.821}P=0.03), serum albumin (OR–0.124{0.045–0.347}P<0.001) and 24h urine protein (OR–1.725{1.195–2.490}P=0.004) (Table 2). In subgroup analysis of 59 patients with systolic dysfunction, severity of LV systolic dysfunction showed statistically significant negative correlation with serum albumin (rho=−0.268, P=0.04) (Table 3).
Comparison of variables in patients with and without, LV systolic dysfunction & LVDD.
| Sr no | Variables | LV systolic dysfunction | LV diastolic dysfunction | ||||
|---|---|---|---|---|---|---|---|
| Present (n=59) | Absent (n=319) | P value | Present (n=165) | Absent (n=213) | P value | ||
| 1 | Age (years) | 50.73±8.46 | 51.71±14.12 | 0.47 | 54.39±13.56 | 49.36±12.86 | <0.001 |
| 2 | Sex (M:F) | 36:23 | 212:107 | 0.46 | 119:46 | 129:84 | 0.01 |
| 3 | Serum creatinine (mg/dl) | 3.94±1.47 | 3.16±1.67 | <0.001 | 3.71±1.49 | 2.95±1.73 | <0.001 |
| 4 | eGFR (ml/min) | 17.27±6.47 | 24.44±10.17 | <0.001 | 19.58±8.88 | 26.21±9.93 | <0.001 |
| 5 | CKD stages | ||||||
| Stage 3b (n=103) | 0 | 32.3% (103/319) | <0.001 | 14.54% (24/165) | 37.09% (79/213) | <0.001 | |
| Stage 4 (n=175) | 64.4% (38/59) | 42.95% (137/319)) | 44.24% (73/165) | 47.88% (102/213) | |||
| Stage 5 (n=100) | 35.6% (21/59) | 24.75% (79/319) | 41.22% (68/165) | 15.03% (32/213) | |||
| 6 | Duration of CKD | ||||||
| ≤ 1year (n=173) | 20.34% (12/59) | 50.47% (161/319) | <0.001 | 31.5% (52/165) | 56.8% (121/213) | <0.001 | |
| >1year (n=205) | 79.66% (47/59) | 49.53% (158/319) | 68.5% (113/165) | 43.2% (92/213) | |||
| 7 | Systemic hypertension | 6.78% (4/59) | 26.02% (83/319) | <0.001 | 36.36% (60/165) | 12.67% (27/213) | <0.001 |
| 8 | Diabetes mellitus | 74.57% (44/59) | 30.72% (98/319) | <0.001 | 41.2% (68/165) | 34.74% (74/213) | 0.24 |
| 9 | Urine protein (g/day) | 2.85±1.57 | 0.96±0.94 | <0.001 | 1.15±1.1 | 1.33±1.37 | 0.16 |
| 10 | Haemoglobin (g/dl) | 8.77±0.77 | 9.97±1.61 | <0.001 | 9.44±1.55 | 10.04±1.53 | <0.001 |
| 11 | Serum calcium (mg/dl) | 8.82±0.75 | 9.08±0.79 | 0.02 | 8.97±0.86 | 9.09±0.74 | 0.12 |
| 12 | Serum phosphorous (mg/dl) | 5.06±1.26 | 4.74±1.45 | 0.08 | 5.12±1.57 | 4.53±1.24 | <0.001 |
| 13 | Calcium×phosphorous | 44.48±10.95 | 42.66±12.27 | 0.25 | 45.57±13.44 | 40.91±10.5 | <0.001 |
| 14 | Serum uric acid (mg/dl) | 6.32±1.48 | 6.26±1.66 | 0.78 | 6.69±1.53 | 5.94±1.63 | <0.001 |
| 15 | Serum albumin (g/dl) | 3.12±0.52 | 3.81±0.36 | <0.001 | 3.69±0.46 | 3.71±0.47 | 0.78 |
| 16 | Serum 25-OH vitamin D (ng/ml) | 30.95±12.62 | 37.94±18.27 | <0.001 | 33.21±15.95 | 39.66±18.46 | <0.001 |
| 17 | Serum iPTH (pg/nl) | 186.72±131.23 | 154.18±124.4 | 0.08 | 175.19±126.7 | 146.92±124.17 | 0.03 |
| 18 | Serum ALP (U/L) | 246.4±138.46 | 252.34±145.66 | 0.58 | 250.62±142.26 | 261.47±156.28 | 0.42 |
| 19 | BMI | 23.96±4.6 | 24.5±3.94 | 0.72 | 24.84±5.3 | 24.4±4.3 | 0.61 |
Age and sex adjusted multivariate logistic regression analysis for predictors of LV systolic dysfunction & LVDD.
| LV systolic dysfunction | LV diastolic dysfunction | |||||
|---|---|---|---|---|---|---|
| Sr no | Variables | OR (95%CI) | P value | Variables | OR (95%CI) | P value |
| 1 | Serum creatinine (mg/dl) | 0.534 (0.257–1.110) | 0.09 | Serum creatinine (mg/dl) | 0.905 (0.67–1.12) | 0.51 |
| 2 | eGFR (ml/min) | 0.828 (0.711–0.965) | 0.02 | eGFR (ml/min) | 0.945 (0.874–1.021) | 0.15 |
| 3 | CKD stage | 0.354 (0.091–1.378) | 0.13 | CKD stage (years) | 1.753 (0.729–4.212) | 0.21 |
| 4 | Duration of CKD | 2.731 (1.093–6.821) | 0.03 | Duration of CKD | 1.807 (1.072–3.047) | 0.03 |
| 5 | Systemic hypertension | 1.001 (0.243–4.123) | 0.99 | Hypertension | 4.692 (2.464–8.933) | <0.001 |
| 6 | Serum haemoglobin (g/dl) | 0.809 (0.509–1.286) | 0.37 | Serum haemoglobin (g/dl) | 1.027 (0.807–1.306) | 0.83 |
| 7 | Serum albumin (g/dl) | 0.124 (0.045–0.347) | <0.001 | Serum uric acid (mg/dl) | 1.169 (0.995–1.373) | 0.06 |
| 8 | Serum calcium (mg/dl) | 1.159 (0.675–1.990) | 0.59 | Serum P (mg/dl) | 0.827 (0.439–1.558) | 0.56 |
| 9 | Urine protein (g/day) | 1.725 (1.195–2.490) | 0.004 | Ca×P | 1.040 (0.968–1.117) | 0.28 |
| 10 | Serum 25-OH vitamin D (ng/ml) | 1.015 (0.981–1.051) | 0.38 | Serum 25-OH vitaminD (ng/ml) | 0.996 (0.972–1.021) | 0.75 |
| 11 | Diabetes mellitus | 1.330 (0.465–3.802) | 0.59 | Serum iPTH (pg/ml) | 1.000 (0.997–1.002) | 0.76 |
OR, Odd's ratio; CI, confidence interval.
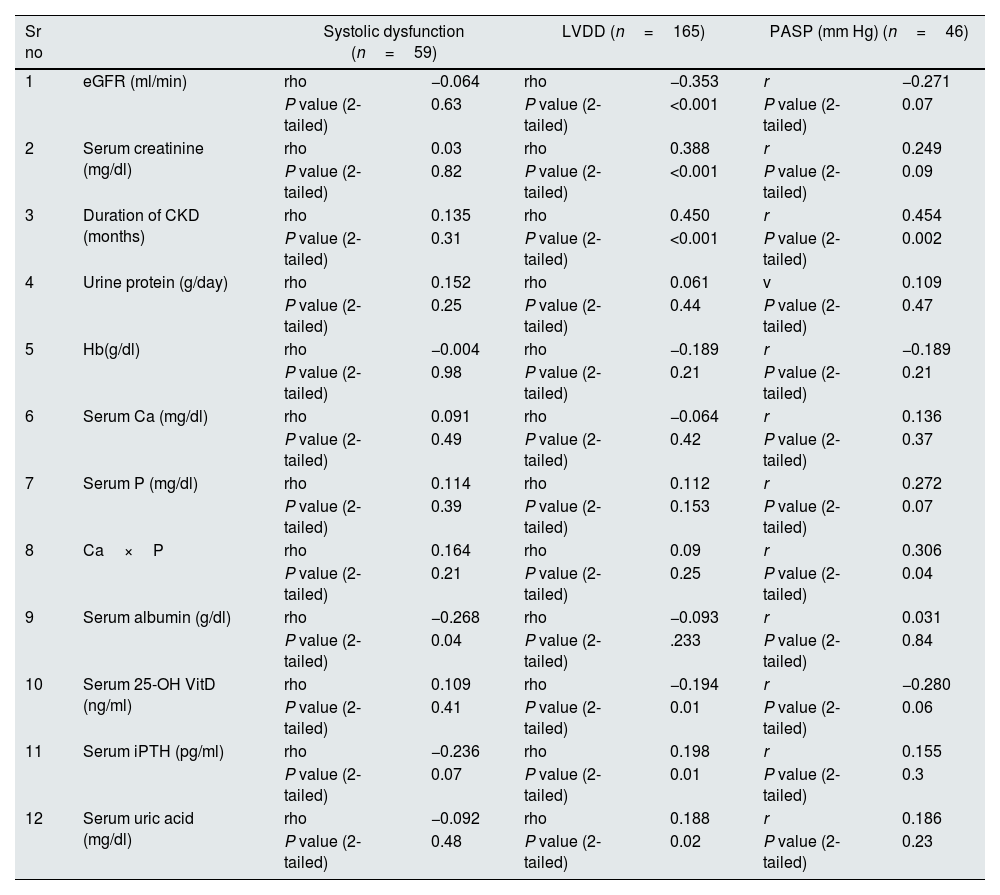
Spearman's 2-tailed correlation between LV systolic dysfunction, LVDD and quantitative variables, Pearson's 2-tailed correlation between PASP and quantitative variables.
| Sr no | Systolic dysfunction (n=59) | LVDD (n=165) | PASP (mm Hg) (n=46) | ||||
|---|---|---|---|---|---|---|---|
| 1 | eGFR (ml/min) | rho | −0.064 | rho | −0.353 | r | −0.271 |
| P value (2-tailed) | 0.63 | P value (2-tailed) | <0.001 | P value (2-tailed) | 0.07 | ||
| 2 | Serum creatinine (mg/dl) | rho | 0.03 | rho | 0.388 | r | 0.249 |
| P value (2-tailed) | 0.82 | P value (2-tailed) | <0.001 | P value (2-tailed) | 0.09 | ||
| 3 | Duration of CKD (months) | rho | 0.135 | rho | 0.450 | r | 0.454 |
| P value (2-tailed) | 0.31 | P value (2-tailed) | <0.001 | P value (2-tailed) | 0.002 | ||
| 4 | Urine protein (g/day) | rho | 0.152 | rho | 0.061 | v | 0.109 |
| P value (2-tailed) | 0.25 | P value (2-tailed) | 0.44 | P value (2-tailed) | 0.47 | ||
| 5 | Hb(g/dl) | rho | −0.004 | rho | −0.189 | r | −0.189 |
| P value (2-tailed) | 0.98 | P value (2-tailed) | 0.21 | P value (2-tailed) | 0.21 | ||
| 6 | Serum Ca (mg/dl) | rho | 0.091 | rho | −0.064 | r | 0.136 |
| P value (2-tailed) | 0.49 | P value (2-tailed) | 0.42 | P value (2-tailed) | 0.37 | ||
| 7 | Serum P (mg/dl) | rho | 0.114 | rho | 0.112 | r | 0.272 |
| P value (2-tailed) | 0.39 | P value (2-tailed) | 0.153 | P value (2-tailed) | 0.07 | ||
| 8 | Ca×P | rho | 0.164 | rho | 0.09 | r | 0.306 |
| P value (2-tailed) | 0.21 | P value (2-tailed) | 0.25 | P value (2-tailed) | 0.04 | ||
| 9 | Serum albumin (g/dl) | rho | −0.268 | rho | −0.093 | r | 0.031 |
| P value (2-tailed) | 0.04 | P value (2-tailed) | .233 | P value (2-tailed) | 0.84 | ||
| 10 | Serum 25-OH VitD (ng/ml) | rho | 0.109 | rho | −0.194 | r | −0.280 |
| P value (2-tailed) | 0.41 | P value (2-tailed) | 0.01 | P value (2-tailed) | 0.06 | ||
| 11 | Serum iPTH (pg/ml) | rho | −0.236 | rho | 0.198 | r | 0.155 |
| P value (2-tailed) | 0.07 | P value (2-tailed) | 0.01 | P value (2-tailed) | 0.3 | ||
| 12 | Serum uric acid (mg/dl) | rho | −0.092 | rho | 0.188 | r | 0.186 |
| P value (2-tailed) | 0.48 | P value (2-tailed) | 0.02 | P value (2-tailed) | 0.23 | ||
rho, Sperman's coefficient; r, Pearson's coefficient.
One hundred and sixty five patients had LVDD with prevalence of 43.65%. 94 patients (56.96%) had grade I, 46 patients (27.88%) had grade II and 25 patients (15.16%) had grade III diastolic dysfunction. Twenty four patients (6.3%) had both LVDD and LV systolic dysfunction. Patients were divided into 2 groups depending on the presence or absence of LVDD. On comparing patients with and without diastolic dysfunction (Table 1), significant variables were age (P<0.001), sex (P=0.01), serum creatinine (P<0.001), eGFR (P<0.001), CKD stages (P<0.001), duration of CKD (P<0.001), presence of arterial hypertension (P<0.001), Hb (P<0.001), serum phosphorous (P<0.001), Ca×P (P<0.001), Serum uric acid (P<0.001), Serum 25-OH VitD (P<0.001) and Serum iPTH (P<0.001). On age and sex adjusted multivariate logistic regression analysis significant predictors of diastolic dysfunction were duration of CKD (OR–1.807{1.072–3.047} P=0.03) and presence of arterial hypertension (OR–4.692{2.464–8.933}P<0.001) (Table 2). In subgroup analysis of 165 patients with diastolic dysfunction, severity of diastolic dysfunction (grade I/II/III) showed statistically significant positive correlation with serum creatinine (rho=0.388, P<0.001), duration of CKD (rho=0.450, P<0.001), serum iPTH (rho=0.198, P=0.01) and serum uric acid (rho=0.188, P=0.02). Severity of diastolic dysfunction (grade I/II/III) showed statistically significant negative correlation with eGFR (rho=−0.353, P<0.001) and serum 25-OH vitamin D (rho=−0.194, P=0.01) (Table 3).
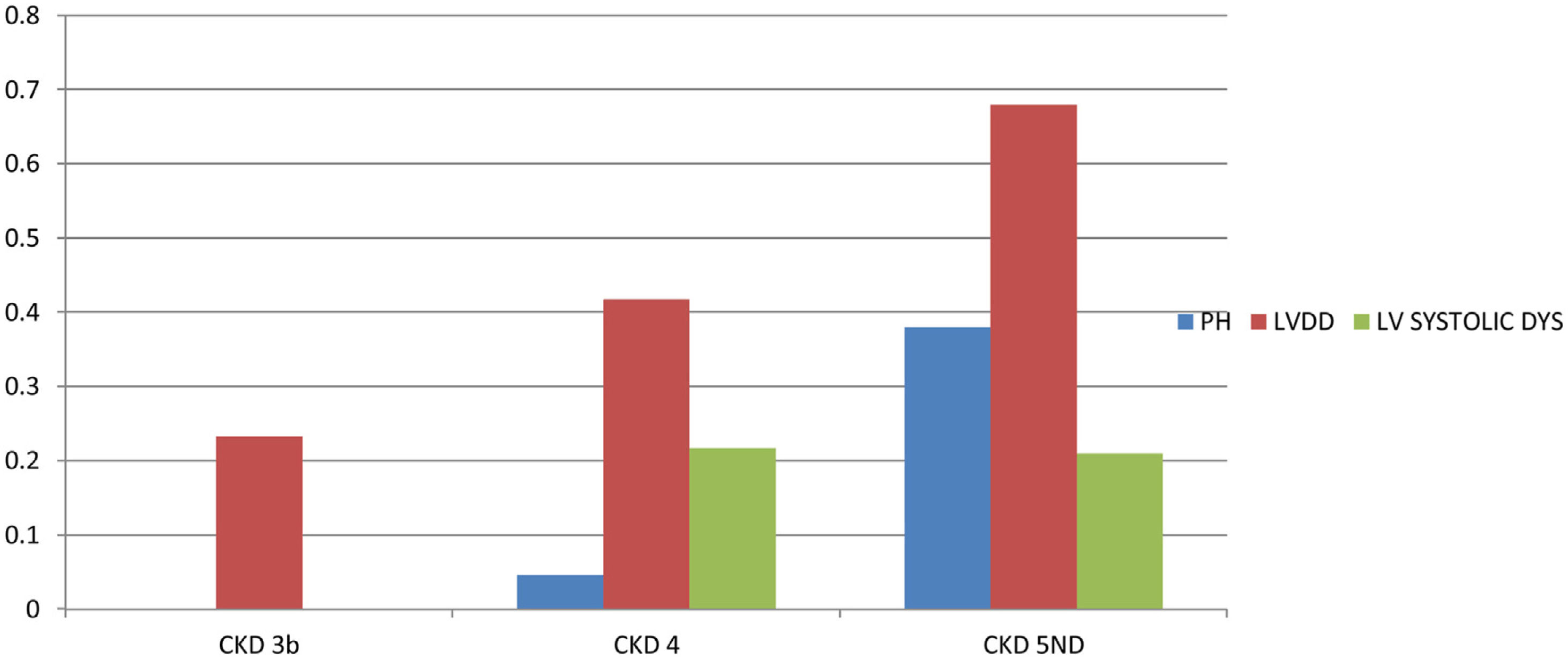
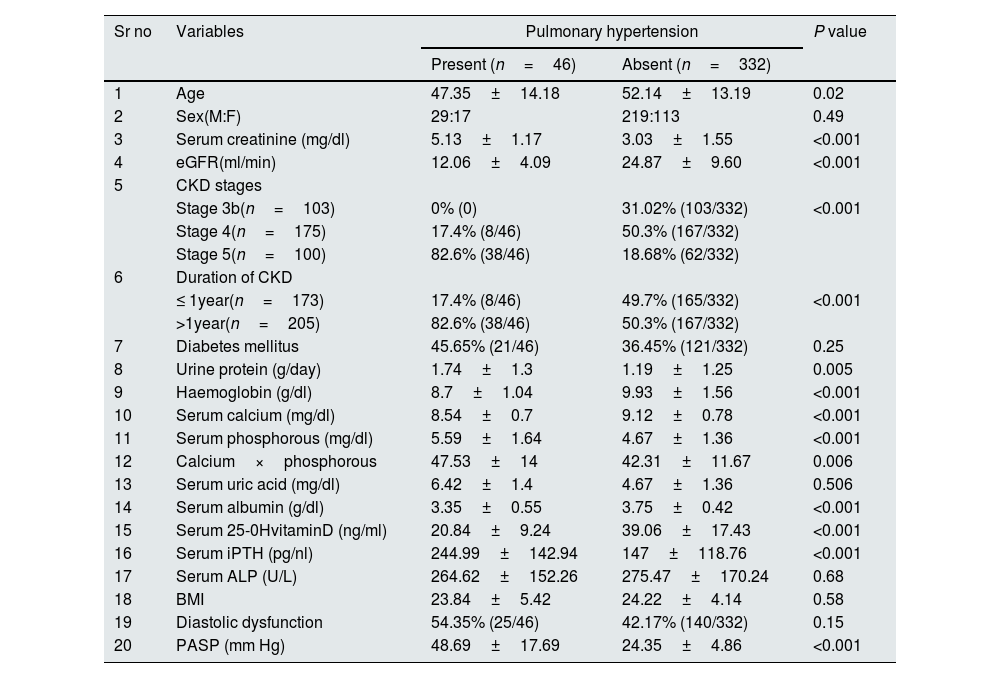
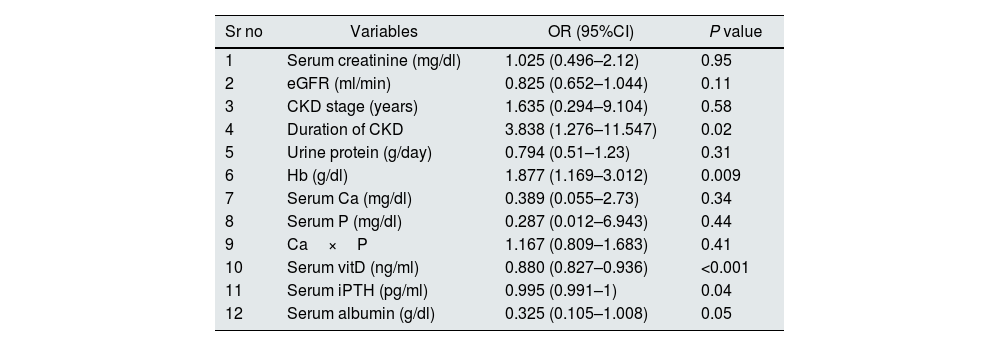
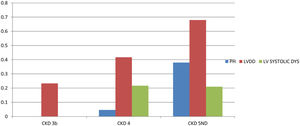
PHForty six patients had PH with prevalence of 12.2%. Twenty five patients (6.6%) had both LVDD and PH. Patients were divided into 2 groups depending on the presence or absence of PH. On comparing patients with and without PH (Table 4), significant variables were age (P=0.023), serum creatinine (P<0.001), eGFR (P<0.001), CKD stages (P<0.001), duration of CKD (P<0.001), 24h urine protein (P=0.005), Hb (P<0.001), serum Calcium (P<0.001), serum phosphorous (P<0.001), Ca×P (P=0.006), Serum albumin (P<0.001), Serum 25-OH VitD (P<0.001) and Serum iPTH (P<0.001). On age and sex adjusted multivariate logistic regression analysis significant predictors of PH were duration of CKD (OR–3.838{1.276–11.547}P=0.02), Hb (OR-1.877{1.169–3.012)}P=0.009), serum 25-OH vitamin D (OR–0.880 {0.827–0.936}P<0.001), serum iPTH (OR–0.995 {0.991–1}P=0.04)and serum albumin (OR–0.325{0.105–1.008}P=0.05 (Table 5). In subgroup analysis of 46 patients with PH, severity of pulmonary hypertension (PASP) showed statistically significant positive correlation with duration of CKD (r=0.454, P=0.002) and Ca×P (r=0.306, P=0.04) (Table 3). Fig. 2 shows prevalence of PH, LVDD and LV systolic dysfunction among different stage of CKD.
Comparison of variables in patients with and without Pulmonary Hypertension.
| Sr no | Variables | Pulmonary hypertension | P value | |
|---|---|---|---|---|
| Present (n=46) | Absent (n=332) | |||
| 1 | Age | 47.35±14.18 | 52.14±13.19 | 0.02 |
| 2 | Sex(M:F) | 29:17 | 219:113 | 0.49 |
| 3 | Serum creatinine (mg/dl) | 5.13±1.17 | 3.03±1.55 | <0.001 |
| 4 | eGFR(ml/min) | 12.06±4.09 | 24.87±9.60 | <0.001 |
| 5 | CKD stages | |||
| Stage 3b(n=103) | 0% (0) | 31.02% (103/332) | <0.001 | |
| Stage 4(n=175) | 17.4% (8/46) | 50.3% (167/332) | ||
| Stage 5(n=100) | 82.6% (38/46) | 18.68% (62/332) | ||
| 6 | Duration of CKD | |||
| ≤ 1year(n=173) | 17.4% (8/46) | 49.7% (165/332) | <0.001 | |
| >1year(n=205) | 82.6% (38/46) | 50.3% (167/332) | ||
| 7 | Diabetes mellitus | 45.65% (21/46) | 36.45% (121/332) | 0.25 |
| 8 | Urine protein (g/day) | 1.74±1.3 | 1.19±1.25 | 0.005 |
| 9 | Haemoglobin (g/dl) | 8.7±1.04 | 9.93±1.56 | <0.001 |
| 10 | Serum calcium (mg/dl) | 8.54±0.7 | 9.12±0.78 | <0.001 |
| 11 | Serum phosphorous (mg/dl) | 5.59±1.64 | 4.67±1.36 | <0.001 |
| 12 | Calcium×phosphorous | 47.53±14 | 42.31±11.67 | 0.006 |
| 13 | Serum uric acid (mg/dl) | 6.42±1.4 | 4.67±1.36 | 0.506 |
| 14 | Serum albumin (g/dl) | 3.35±0.55 | 3.75±0.42 | <0.001 |
| 15 | Serum 25-0HvitaminD (ng/ml) | 20.84±9.24 | 39.06±17.43 | <0.001 |
| 16 | Serum iPTH (pg/nl) | 244.99±142.94 | 147±118.76 | <0.001 |
| 17 | Serum ALP (U/L) | 264.62±152.26 | 275.47±170.24 | 0.68 |
| 18 | BMI | 23.84±5.42 | 24.22±4.14 | 0.58 |
| 19 | Diastolic dysfunction | 54.35% (25/46) | 42.17% (140/332) | 0.15 |
| 20 | PASP (mm Hg) | 48.69±17.69 | 24.35±4.86 | <0.001 |
Age and sex adjusted multivariate logistic regression analysis for predictors of pulmonary hypertension.
| Sr no | Variables | OR (95%CI) | P value |
|---|---|---|---|
| 1 | Serum creatinine (mg/dl) | 1.025 (0.496–2.12) | 0.95 |
| 2 | eGFR (ml/min) | 0.825 (0.652–1.044) | 0.11 |
| 3 | CKD stage (years) | 1.635 (0.294–9.104) | 0.58 |
| 4 | Duration of CKD | 3.838 (1.276–11.547) | 0.02 |
| 5 | Urine protein (g/day) | 0.794 (0.51–1.23) | 0.31 |
| 6 | Hb (g/dl) | 1.877 (1.169–3.012) | 0.009 |
| 7 | Serum Ca (mg/dl) | 0.389 (0.055–2.73) | 0.34 |
| 8 | Serum P (mg/dl) | 0.287 (0.012–6.943) | 0.44 |
| 9 | Ca×P | 1.167 (0.809–1.683) | 0.41 |
| 10 | Serum vitD (ng/ml) | 0.880 (0.827–0.936) | <0.001 |
| 11 | Serum iPTH (pg/ml) | 0.995 (0.991–1) | 0.04 |
| 12 | Serum albumin (g/dl) | 0.325 (0.105–1.008) | 0.05 |
OR, Odd's ratio, CI, confidence interval.
This was a cross-sectional study to determine prevalence and predictors of LV systolic dysfunction, LVDD and PH in CKD stage 3b-5ND patients using 2D M mode and Doppler echocardiography.
LV systolic dysfunctionIn our study prevalence of LV systolic dysfunction (LVEF≤50%) is 15.6%. Among these 28.8% had LVEF 41–50%, 67.8% had LVEF 31–40% and 3.4% had LVEF≤30%. Gupta et al.12 reported prevalence of LV systolic dysfunction of 11.8% among 3939 CRIC study participants. Chillo et al.13 reported a prevalence of LV systolic dysfunction of 16.2% among 191 CKD patients. In comparison with these 2 studies we have got a similar prevalence whereas Rao T et al.14 reported a prevalence of LV systolic dysfunction of 47.8% among 250 CKD patients. Since the latter study included only hospitalized patients could result in higher prevalence rate.
Park et al.15 reported no significant associations between different stages of eGFR and LVEF in 3487 patients as well as Chillo et al.13 Conversely we found eGFR as significant predictor of LV systolic dysfunction but prevalence of LV systolic dysfunction was similar in both stage4 & stage 5 CKD. Probably because of zero prevalence in stage 3 in comparison with stage 4&5 could have resulted in this.
Chen et al.16 in their cross-sectional study of 285 diabetic CKD 3–5 patients reported independent association between low serum albumin levels and decreased LVEF. Matsushita et al.17 reported higher albuminuria/ACR as consistent association with LV systolic dysfunction among 4175 ARIC (Atherosclerosis Risk in Communities) CKD participants. Similar to these findings in our study we found serum albumin and urine protein as significant predictors of LV systolic dysfunction, and negative correlation between severity of systolic dysfunction and serum albumin.
LVDDIn our study prevalence of LVDD is 43.65%. 94 patients (56.96%) had grade-I, 46 patients (27.88%) had grade-II and 25 patients (15.16%) had grade-III diastolic dysfunction. Gupta et al.12 reported prevalence of LVDD of 76.3% among 3939 CRIC study participants. Chillo et al.13 reported a prevalence of LVDD of 68.6% among 191 CKD patients. Rao T et al.14 reported a prevalence of LVDD of 55.2% among 250 CKD patients. Comparing with these studies we have found a lower prevalence of LVDD. Probably different inclusion criteria, method of determining LVDD and different ethnicities may result in varied prevalence among different studies.
We found increased prevalence of LVDD with the stage of CKD. We found positive correlation between severity of LVDD and serum creatinine, and negative correlation between severity of LVDD and eGFR. This is consistent with previously published studies. Qi-Zhe Cai et al.18) reported prevalence of LVDD of 44.4% at baseline and 64.2% at 1 year, with significant change in prevalence and severity of LVDD in lower eGFR groups. Jain et al.7 (N=2056) reported greater degree of diastolic dysfunction and adverse clinical outcomes with worsening EGFR, and presence of LVDD was associated with increased morbidity and mortality.
Arterial hypertension is associated with increased prevalence of LVDD.19,20 Several potential mechanisms including haemodynamic, non-haemodynamic factors and myocardial ischaemia are implicated in development of LVDD in hypertensive patients. Pressure overload and stiffening of larger arteries strongly influence LVDD.21 Similar to these studies we found arterial hypertension as significant predictor of LVDD.
Pandit A et al.22 showed no significant association between vitamin D levels and LVDD (N=1011 patients). Banerjee et al.23 in their RCT and meta-analysis reported that vitamin D supplementation has no beneficial effect on LV mass. We found a negative correlation between LVDD severity and serum 25-OH vitamin D. Since there were more number of grade III LVDD patients in stage 5 and low serum 25-OH vitamina D in stage 5 patients could resulted in this. With cross-sectional nature of our study it is hard to interpret this association.
Kim et al.24 reported serum uric acid as an independent predictor of LVDD (N=1025 pre-dialysis CKD patients with preserved left ventricular systolic function). Pan et al.25 reported that long-term Febuxostat therapy was associated with lower LVDD in patients with hypertensive LVH and asymptomatic hyperuricemia. Consistent with these finding we found serum uric acid as significant predictor of LVDD.
Disturbances of mineral metabolism including elevated PTH levels, hyperphosphatemia, and high values for serum Ca×P are associated with greater risk of cardiovascular disease in CKD patients. Lishmanov et al.26 (N=196 patients with CKD 3&4) reported iPTH level to be associated with increased incidence of cardiovascular events independent of calcium-phosphorous level. Similarly in our study iPTH was found to be significant predictor of LVDD.
Presence of LVDD in CKD is associated with higher mortality.27,28 Factors that have been implicated in the progression of LVDD include haemodynamic overload, alterations in mineral metabolism, circulating uraemic toxins, cardiotonic steroids and increased aortic stiffness.29,30
Pulmonary hypertensionIn our study prevalence of PH is 12.2%, prevalence increased with the stage of CKD with zero prevalence in stage 3b, 4.57% in stage 4 and 38% in stage 5. Navaneethan et al.31 showed prevalence of pulmonary hypertension of 21% among 2959 non-dialysis dependent Chronic Renal Insufficiency Cohort(CRIC) study participants with higher rates in lower EGFR groups. Bolignano et al.32 (N=468 patients with CKD 2–4) found a prevalence of PH of 23%, with higher rates in stage 4. In Jackson heart study by Selvaraj et al.6 of 408 CKD patients with eGFR<60mL/min per 1.73 m2 reported prevalence of PH of 22% and was associated with a higher risk of heart failure admission and mortality. However compared with these studies we found a low prevalence of PH (12.2%). Probably inclusion of patients with lower LVEF or prior cardiac events, higher BMI, COPD, smokers, patients with AVF and different ethnicities among study groups, could have resulted in varied prevalence of PH. Tang et al.5 in their systematic review and meta-analysis on PH in CKD, which included 16 cohort studies with 7112 participants showed prevalence of PH of 26.6% and found dialysis as a risk for PH. Due to the fact we have excluded dialysis dependent CKD patients might resulted in lower prevalence of PH.
In our study significant predictors of PH were duration of CKD, haemoglobin, serum 25-OH vitamin, serum iPTH and serum albumin. Significant positive correlation between the severity of pulmonary hypertension, duration of CKD and Calcium×Phosphorous product was found in our study. Multiple predictors for PH in CKD have been reported in previous studies which includes age, eGFR, haemoglobin, low LVEF, presence of left ventricular hypertrophy, left atrial volume, history of cardiovascular events, diabetes and presence of AVF.31–33 Study by Mehta et al.34 reported positive correlation between PH and duration of CKD, duration of dialysis, BUN, serum creatinine, and calcium×phosphorous product, and negative correlation between haemoglobin and PH. Suresh et al.35 reported left-sided heart failure, anaemia, fluid retention, and increased calcium×phosphorous product as risk factors for developing PH.
Studies have reported higher frequency of iron deficiency and anaemia in PH.36,37 Iron deficiency and anaemia are associated with chronic progressive remodelling of pulmonary vessels with accumulation of pulmonary arterial endothelial cells, pulmonary artery smooth muscle cells, myofibroblasts and pericytes.38 CKD is associated with anaemia and functional iron deficiency which can cause remodelling in pulmonary vasculature and high risk for PH. Serum albumin levels are influenced by nutritional status, inflammatory state, liver function and renal loss in CKD population. Snipelisky et al.39 reported serum albumin as an independent prognostic indicator and non-selective marker for severity in patients with PH. They found that lower serum albumin in patients with PH associated with more systemic comorbidities and represent a progressive clinical course. Thus in our study presence of low serum albumin in PH group may represent severe renal failure and associated co-morbidity.
Studies have reported higher prevalence of vitamin D deficiency in PH.40,41 Tanaka et al.42 in their experimental rat study reported improved right ventricular function after correction of vitamin D in vitamin D deficient rats. Similarly Mirdamadi et al.43 reported improved right ventricular function and PASP in vitamin D deficient PH patients after restoration of serum vitamin D during a follow up period of 3 months. Studies have reported secondary hyperparathyroidism and associated CKD–MBD biochemical abnormalities are associated with increased risk of PH.34,35,44
Multiple pathophysiological changes in CKD have been postulated for the development of PH. The presence of uraemic toxins in CKD impairs vasodilatory effect of nitric oxide and there is elevated endothelin-1 causing pulmonary vascular remodelling.45 As CKD is chronic inflammatory state there is elevated levels of circulating pro-inflammatory cytokines which can modulate downstream signalling pathways that controls vascular structure and tone.45,46 Longer duration of CKD is associated with in increased exposure to uraemic toxins and inflammatory cytokines results in increased risk for PH.
Strength of our study is to evaluate and determine predictors LV systolic dysfunction, LVDD and PH in CKD 3-5ND patients. Most of the studies done on LV systolic function, LVDD and PH in Indian CKD population have included dialysis dependent CKD patients, in contrast we have included non-dialysis dependent CKD 3-5ND patients.
Our study has following limitations. Since it is a cross-sectional observational study it precludes us from concluding causality or association. Furthermore we did not perform 2D echo or measure biochemical parameters at regular intervals to define more accurate association. We did not measure NT–Pro BNP and other inflammatory markers in our study participants. We haven’t done right heart catherization to determine the type of PH. As it is single centre study heterogeneity between different races and ethnic groups could not be evaluated.
ConclusionIn our study the prevalence of PH is 12.2%, LV systolic dysfunction is 15.6% and LVDD is 43.65%. Predictors of PH were duration of CKD, haemoglobin, serum 25-OH vitamin-D, serum iPTH and serum albumin. Predictors of LVDD were duration of CKD and presence of arterial hypertension. Predictors of LV systolic dysfunction were eGFR, duration of CKD, serum albumin and 24h urine protein. Routine screening of CKD population with 2D echocardiography is to be encouraged for early detection of LV dysfunction and PH. Prognostic implications and utility of these predictors requires prospective or randomized control studies for better validation.
FundingNil.
Conflicts of interestNil.