Vascular access for haemodialysis is key in renal patients both due to its associated morbidity and mortality and due to its impact on quality of life. The process, from the creation and maintenance of vascular access to the treatment of its complications, represents a challenge to decision-making, because of the complexity of the existing disease and the diversity of the specialities involved. With a view to finding a common approach, the Spanish Multidisciplinary Group on Vascular Access (GEMAV), which includes experts from the five scientific societies involved (nephrology [S.E.N.], vascular surgery [SEACV], vascular and interventional radiology [SERAM-SERVEI], infectious diseases [SEIMC] and nephrology nursing [SEDEN]), along with the methodological support of the Cochrane Center, has updated the Guidelines on Vascular Access for Haemodialysis, published in 2005. These guidelines maintain a similar structure, in that they review the evidence without compromising the educational aspects. However, on the one hand, they provide an update to methodology development following the guidelines of the GRADE system in order to translate this systematic review of evidence into recommendations that facilitate decision-making in routine clinical practice, and, on the other hand, the guidelines establish quality indicators which make it possible to monitor the quality of healthcare.
© 2017 Sociedad Española de Nefrología. Published by Elsevier España, S.L.U.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0).
Guía Clínica Española del Acceso Vascular para Hemodiálisis
El acceso vascular para hemodiálisis es esencial para el enfermo renal tanto por su morbimortalidad asociada como por su repercusión en la calidad de vida. El proceso que va desde la creación y mantenimiento del acceso vascular hasta el tratamiento de sus complicaciones constituye un reto para la toma de decisiones debido a la complejidad de la patología existente y a la diversidad de especialidades involucradas. Con el fin de conseguir un abordaje consensuado, el Grupo Español Multidisciplinar del Acceso Vascular (GEMAV), que incluye expertos de las cinco sociedades científicas implicadas (nefrología [S.E.N.], cirugía vascular [SEACV], radiología vascular e intervencionista [SERAM-SERVEI], enfermedades infecciosas [SEIMC] y enfermería nefrológica [SEDEN]), con el soporte metodológico del Centro Cochrane Iberoamericano, ha realizado una actualización de la Guía del Acceso Vascular para Hemodiálisis publicada en 2005. Esta guía mantiene una estructura similar, revisando la evidencia sin renunciar a la vertiente docente, pero se aportan como novedades, por un lado, la metodología en su elaboración, siguiendo las directrices del sistema GRADE con el objetivo de traducir esta revisión sistemática de la evidencia en recomendaciones que faciliten la toma de decisiones en la práctica clínica habitual y, por otro, el establecimiento de indicadores de calidad que permitan monitorizar la calidad asistencial.
© 2017 Sociedad Española de Nefrología. Publicado por Elsevier España, S.L.U.
Este es un artículo Open Access bajo la licencia CC BY-NC-ND (http://creativecommons.org/licenses/by-nc-nd/4.0).
Vascular access (VA) used to perform haemodialysis (HD) is fundamental in the case of patients with kidney disease and, currently, its influence on morbidity and mortality is no longer questioned. Therefore, due to the great significance it holds for these patients, a Guide on vascular access is needed for use in decision-making during routine clinical practice. This guide should not only collect all the available evidence, but also convey it to professionals in a way which allows daily clinical application.
The first edition of the Sociedad Española de Nefrología (Spanish Society of Nephrology) Vascular Access Guide was published in 2005 with the collaboration of the other societies involved in the current guide. This Guide has been a reference point for professionals working in HD since then. It has become a key document to be consulted in dialysis units and has had a considerable impact on the literature. The current edition aims to renew this Guide, updating all the subjects included in it and adding new concepts that have been raised since its publication.
The format of the current Guide maintains a similar structure, and thus has the same Sections. It is worth mentioning that the topic of “Quality indicators” has now grown to become a section in its own right (Section 7) with 29 indicators, rather than an appendix with only 5 indicators as it was in the previous version. With regard to content, a mixed approach has been preserved, that is to say, on the one hand, recommendations have been derived from the analysis of the current scientific evidence and, on the other, the teaching bent of the previous edition has not been discarded.
COMPOSITION OF THE GROUP DEVELOPING THE GUIDEAfter a meeting in Madrid on 29 June, 2012 representatives of the Sociedad Española de Nefrología (Spanish Society of Nephrology [S.E.N.]), Sociedad Española de Angiología y Cirugía Vascular (Society of Angiology and Vascular Surgery [SEACV]), Sociedad Española de Radiología Vascular e Intervencionista-Sociedad Española de Radiología Médica (Spanish Society of Vascular and Interventional Radiology-Spanish Society of Medical Radiology [SERVEI-SERAM]), Sociedad Española de Enfermería Nefrológica (Spanish Society of Nephrology Nursing [SEDEN]) and in an subsequent meeting of the Grupo de Estudio de la Infección Relacionada con la Asistencia Sanitaria/Grupo de Estudio de la Infeccion Hospitalaria-Sociedad Española de Enfermedades Infecciosas y Microbiologia Clinica (Study Group of Healthcare-related Infection/Study Group of Hospital Infection-Spanish Society of Infectious Diseases and Clinical Microbiology [GEIRAS/GEIH-SEIMC]) took the decision to update the Spanish Clinical Guideline on Vascular Access for Haemodialysis. The multidisciplinary working group was composed of members of the 5 scientific societies involved and the members were chosen for both clinical and research experience in the area of vascular access. During the meeting of the 6 October, 2014, the group took the name Grupo Español Multidisciplinar del Acceso Vascular (Spanish Multidisciplinary Group on Vascular Access [GEMAV]), the name by which the group was to be known thereafter. It was also decided to use the methodological support of the Centro Cochrane Iberoamericano (Iberoamerican Cochrane Center) to systematically review the literature pertaining to the Guide’s clinical questions prioritised by GEMAV. All authors have been involved in the edition of the Guide in a strictly professional way, and have no type of conflict of interest. Some of the authors also carry out some representative tasks for their respective scientific societies. Below are the names of the coordinators of the Guideline, the editors, the members of GEMAV (in representation of the five societies), the external reviewers and the representatives of kidney patient associations.
Coordinators of the Guide
- •
Jose Ibeas. Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Barcelona
- •
Ramón Roca-Tey. Hospital de Mollet, Fundació Sanitària Mollet, Mollet del Vallès, Barcelona
Editors
- •
Jose Ibeas. Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Barcelona
- •
Ramón Roca-Tey. Hospital de Mollet, Fundació Sanitària Mollet, Mollet del Vallès, Barcelona
- •
Joaquin Vallespín Aguado. Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Barcelona
- •
Carlos Quereda Rodríguez-Navarro. Editor of the journal Nefrología for Clinical Practice Guidelines
On behalf of the five societies
S.E.N.
- •
Dolores Arenas. Vithas Hospital Internacional Perpetuo, Alicante.
- •
Pilar Caro. Hospital Ruber Juan Bravo, Madrid
- •
Milagros Fernández Lucas. Hospital Universitario Ramón y Cajal, Universidad de Alcalá, Madrid
- •
Néstor Fontseré. Hospital Clínic, Universitat de Barcelona, Barcelona
- •
Enrique Gruss. Hospital Universitario Fundación Alcorcón, Alcorcón, Madrid
- •
José Ibeas. Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Barcelona Secretary of Vascular Access Working Group of the Spanish Society of Nephrology
- •
José Luis Merino. Hospital Universitario del Henares, Coslada, Madrid
- •
Manel Ramírez de Arellano. Hospital de Terrassa, Consorci Sanitari de Terrassa, Barcelona
- •
Ramon Roca-Tey. Hospital de Mollet, Fundació Sanitària Mollet, Mollet del Vallès, Barcelona Official Representative of the Spanish Society of Nephrology. Coordinator of the Vascular Access Working Group of the Spanish Society of Nephrology and of the Spanish Multidisciplinary Group on Vascular Access (GEMAV)
- •
María Dolores Sánchez de la Nieta, Hospital General Universitario de Ciudad Real, Ciudad Real
SEACV
- •
Angel Barba. Hospital Galdakao-Usansolo, Bizkaia
- •
Natalia de la Fuente. Hospital Galdakao-Usansolo, Bizkaia
- •
Fidel Fernández. Complejo Hospitalario Universitario de Granada, Granada
- •
Antonio Giménez. Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Barcelona
- •
Cristina López. Complejo Hospitalario Universitario de Granada, Granada
- •
Guillermo Moñux. Hospital Clínico Universitario San Carlos, Madrid Official Representative of the Sociedad Española de Angiología y Cirugía Vascular (SEACV), Coordinator of the Chapter of Vascular Access of the Sociedad Española de Angiología y Cirugía Vascular
- •
Joaquin Vallespín. Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Barcelona Secretary of the Chapter of Vascular Access of the Sociedad Española de Angiología y Cirugía Vascular
SERVEI
- •
José García-Revillo García. Hospital Universitario Reina Sofía, Córdoba
- •
Teresa Moreno. Hospital Juan Ramón Jiménez, Complejo Hospitalario Universitario de Huelva, Huelva Official Representative of the Sociedad Española de Radiología Vascular e Intervencionista (SERVEI), Chapter of the Sociedad Española de Radiología Médica (SERAM)
- •
Pablo Valdés Solís. Hospital de Marbella, Málaga
SEIMC
- •
José Luis del Pozo. Clínica Universidad de Navarra, Pamplona Official Representative of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (Grupo de Estudio de la Infeccion Relacionada con la Asistencia Sanitaria/Grupo de Estudio de la Infección Hospitalaria [GEIRAS/GEIH])
SEDEN
- •
Patricia Arribas. Hospital Infanta Leonor, Madrid
- •
David Hernán. Fundación Renal Íñigo Álvarez de Toledo, Madrid
- •
Anna Martí. Consorcio Hospital General Universitario, Valencia Official Representative of the Sociedad Española de Enfermería Nefrológica (SEDEN)
- •
María Teresa Martínez. Hospital General Universitario Gregorio Marañón, Madrid
External reviewers
S.E.N.
- •
Fernando Álvarez Ude. Hospital de Segovia, Segovia
- •
Jose Antonio Herrero. Hospital Clínico Universitario San Carlos, Madrid
- •
Fernando García López. Centro Nacional de Epidemiología. Instituto de Salud Carlos III
SEACV
- •
Sergi Bellmunt. Hospital Vall d’Hebron, Barcelona
- •
Melina Vega. Hospital Galdakao-Usansolo, Bizkaia
SERVEI
- •
Jose Luis del Cura. Hospital de Basurto, Vizcaya.
- •
Antonio Segarra. Hospital Vall d’Hebron, Barcelona
SEIMC
- •
Jesús Fortún Abete. Hospital Universitario Ramón y Cajal, Madrid
SEDEN
- •
Isabel Crehuet. Hospital Universitario Río Hortega, Valladolid
- •
Fernando González. Hospital General Universitario Gregorio Marañón, Madrid
Kidney patient associations
ADER
- •
Antonio Tombas. President
ALCER
- •
Daniel Gallego Zurro. Council member
Rationale of the Guide edition
The aim of this Guide is to provide orientation in the comprehensive handling of vascular access for patients undergoing haemodialysis. It has been developed in order to provide information and assistance when making decisions in clinical practice. This Guide has been developed as a joint project of the five Scientific Societies referred to above, represented by experienced specialists in this field. The five Societies agreed on the need to update the first edition of the Vascular Access Guide, which was edited by the Spanish Society of Nephrology (S.E.N.) with the collaboration of the other four Societies and published in 2005.
Who is the Guide aimed at
The Guide provides decision-making support for any professional involved in vascular access for haemodialysis. This includes nephrologists, vascular surgeons, interventional radiologists, infectious disease specialists and nephrological nursing. In addition, due to the Guide’s teaching bent, it is also directed at professionals undergoing training in these fields. It has therefore been considered of great interest to synthesise the necessary information for the user to build up the knowledge essential to understand the different aspects included in the Guide. Thus, sections are included with the additional explanations considered appropriate. And finally, it aims to provide a tool for healthcare managers responsible for administration and for health policy. To this end, the indicators section aims not only to provide professionals with the tools necessary to help improve the quality of care, but it also aims to support those responsible for resource management to be able to optimise resources as well as healthcare quality.
Scope of the Guide
The Guide deals with patients with advanced chronic disease either in pre-dialysis or on dialysis who need a VA or treatment of its complications, as well as knowledge related to maintenance and care. The Guide does not include the paediatric population as it understands this group as patients who require a specific approach.
METHODOLOGY FOR THE DEVELOPMENT OF THE GUIDEEstablishment of the Guide development group
The board of the five participating societies, S.E.N., SEACV, SERVEI, SEDEN and SEIMC, approved the selection of the respective experts who were to represent these societies. The coordinators of the Guide consensually selected those responsible for each section, who coordinated the group of experts in these sections, who in turn were members of all the Societies involved. The group consisted of experts in vascular access creation, in the treatment of complications, both surgically and endovascularly, in catheter placement and the treatment of associated complications, in prevention and treatment of infections, in the preparation, monitoring, care and maintenance of the vascular access, in quality indicators and with knowledge of methodology of systematic reviews and evidence-based medicine. The Ibero-American Cochrane Center was asked to provide methodological support to develop the systematic review of the evidence in relation to clinical questions prioritised by GEMAV, and in some other stages in the development of the Guide.
Selection of clinical questions
Firstly, the most relevant clinical questions in routine clinical practice were prioritised, and secondly, recommendations were formulated by applying a systematic and rigorous methodology. For this update, GEMAV selected the most relevant questions from the original guide regarding clinical practice and new questions were added if deemed necessary for the new Guide.
Considering the scope of this Guide, specific clinical questions were identified and a systematic review performed:
- I.
Does the preservation of the venous network prevent complications/facilitate the creation of the arteriovenous fistula?
- II.
In patients with chronic kidney disease, what are the demographic, clinical and analytical parameters in order to determine when the arteriovenous fistula (either native or prosthetic) should be created?
- III.
What criteria are required for arteriovenous fistula planning (based on different types of fistula)?
- IV.
What risk factors have been shown to influence the development of limb ischaemia after arteriovenous fistula creation?
- V.
Can an order of preference be recommended when performing the arteriovenous fistula?
- VI.
Are exercises useful for developing arteriovenous fistulae?
- VII.
What is the minimum maturation time required for a native or prosthetic arteriovenous fistula to be mature enough for needling?
- VIII.
What is the needling technique of choice for the different types of arteriovenous fistula: the three classical ones and self-cannulation?
- IXa.
In which situations is it necessary to indicate antithrombotic prophylaxis after creating/repairing the arteriovenous fistula?
- IXb.
Does the use of antiplatelet agents prior to arteriovenous fistula creation have an impact on patency and reduce the risk of thrombosis?
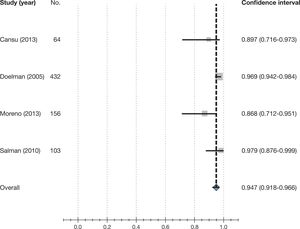
- X.
How reliable is Doppler ultrasound in determining blood flow in the arteriovenous fistula in comparison to dilution screening methods?
- XI.
Can regulated Doppler ultrasound performed by an experienced examiner replace angiography as the gold standard to confirm significant arteriovenous fistula stenosis?
- XII.
Which non-invasive monitoring or surveillance screening method for haemodialysis arteriovenous fistula presents predictive power of stenosis and thrombosis and increased patency of the prosthetic arteriovenous fistula in the prevalent patient and what is the frequency?
- XIII.
Which non-invasive monitoring or surveillance screening method for haemodialysis arteriovenous fistula presents predictive power of stenosis and thrombosis and increased patency of the native arteriovenous fistula in the prevalent patient and what is the frequency?
- XIV.
What are the demographic, clinical and haemodynamic factors and variables with predictive power of thrombosis in an arteriovenous fistula that presents stenosis?
- XV.
Is there a treatment with better outcomes (percutaneous transluminal angioplasty versus surgery) in juxta-anastomotic stenosis, assessed in terms of patency and/or thrombosis and cost/benefit?
- XVI.
Are there any criteria that indicate in which cases, when and how to treat central vein stenosis, assessed in terms of usable arteriovenous fistula patency and/or thrombosis?
- XVII.
In native arteriovenous fistula thrombosis, what would be the initial indication (percutaneous transluminal angioplasty versus surgery) assessed in terms of patency of the native arteriovenous fistula and/or thrombosis? Does it depend on location?
- XVIII.
In prosthetic arteriovenous fistula thrombosis, what would be the initial indication (percutaneous transluminal angioplasty versus surgery versus fibrinolysis) assessed in terms of patency of the arteriovenous fistula and/or thrombosis? Does it depend on location?
- XIX.
In the presence of stenosis in the native arteriovenous fistula, is there a significant difference between elective intervention and performing treatment after thrombosis?
- XX.
Is there a treatment with better outcomes (percutaneous transluminal angioplasty versus surgery or prosthesis interposition) in non-matured arteriovenous fistula management, evaluated on arteriovenous fistula, which enables it to be used in dialysis, patency and/or thrombosis?
- XXI.
What is the approach to native or prosthetic arteriovenous fistula diagnosed with steal syndrome?
- XXII.
In native and prosthetic arteriovenous fistula pseudoaneurysm, when is surgery versus percutaneous versus conservative management indicated, assessed in terms of severe bleeding complications or death?
- XXIII.
In the high-flow arteriovenous fistula, what therapeutic approach should be taken and what are the criteria (risk factors)?
- XXIV.
In patients who cannot undergo native arteriovenous fistula creation, is the central venous catheter the vascular access of choice versus prosthetic arteriovenous fistula?
- XXV.
Are there differences in the indication to use non-tunnelled catheters versus tunnelled catheters?
- XXVI.
What is the best material and design for a tunnelled central venous catheter?
- XXVII.
Should ultrasound be used as a reference standard for the placement of central venous catheters?
- XXVIII.
What is the best treatment for the persistent dysfunction of the tunnelled central venous catheter (stripping, fibrin sheath angioplasty, fibrinolytics or catheter replacement)?
- XXIX.
What influence do the different types of central venous catheter lumen lock have on its dysfunction and infection?
- XXX.
Is the use of antibiotic prophylaxis justified to lock a tunnelled central venous catheter for haemodialysis?
- XXXI.
Does catheter-related bacteraemia secondary to infection with Staphylococcus aureus, Pseudomonas sp. and Candida spp. force catheter withdrawal and therefore contraindicate antibiotic lock treatment to attempt to preserve the catheter?
- XXXII.
Should empirical antibiotic treatment to cover gram-positive bacteraemia in haemodialysis patients who are tunnelled central venous catheter carriers initially be started with cefazolin (vancomycin if MRSA level > 15%) or daptomycin, associated with the treatment for gram-negatives, when the catheter is preserved?
- XXXIII.
Does the detection and eradication of Staphylococcus aureus in nasal carriers reduce episodes of catheter-related bacteraemia? Is it cost-effective?
The previous recommendations of the former Guideline which have not been substantially updated can be consulted in each section of the Guide and which, therefore, GEMAV has made their own.
Finally, GEMAV identified a series of questions with less impact on clinical practice, but for which the members of GEMAV themselves produced an update based on a narrative review of the literature. These sections can generate recommendations approved by consensus in GEMAV.
Development of clinical questions
These questions have a structured format in order to identify the type of patient, the intervention or diagnostic test to be assessed, the comparisons, where necessary, and the outcomes of interest (PICO format). As detailed in the methodology section, recommendations for these clinical questions have been elaborated in accordance with the GRADE system guidelines.
The working group collaborated in the development of these questions and formatted them to allow the systematic search of the evidence following the routine established by the PICO methodology. That is to say, the initial specification of the type of patient (P), the type of intervention (I), the comparator (C) and the outcome (O) for the questions related to interventions and diagnostic tests. For each question, the group agreed on some systematic review criteria including specific characteristics depending on the design of the sought-after studies.
Classification of the relative importance of the outcomes
For each intervention question, the group compiled a list of possible outcomes, reflecting both the benefits and harm, and alternative strategies. These outcomes were categorised as critical, important or less important in relation to the decision-making process. For example, outcomes associated with important health variables such as mortality in the patient or thrombosis in vascular access were considered critical, and outcomes such as blood flow were pondered less important.
Identification of the clinical questions, recommendations from the previous version of the Guide and narrative updates of the literature
Throughout the document, recommendations relating to clinical questions and updates are marked with the label “new”. Likewise, recommendations corresponding to clinical questions, which were elaborated on the basis of a systematic and rigorous process of formulating recommendations, are identified with the symbol (•). The contents expressed in the rest of the recommendations come from the previous version of the Guide.
Structure of the sections of the Guide
The contents of the guide have been structured in areas of knowledge set out below. In order to coordinate the work in each of them, one or two area coordinators were selected along with some experts, depending on the volume and characteristics of the matter to be analysed. The areas studied, along with the respective coordinators and experts, are listed below.
The current professional activity of the authors of this Guide and a brief summary of their trajectory, which accredits them as experts, are shown in Annex 1.
- 1.
PROCEDURES PRIOR TO VASCULAR ACCESS CREATION Joaquín Vallespín, Fidel Fernández (coordinators), José Ibeas, Teresa Moreno.
- 2.
ARTERIOVENOUS FISTULA CREATION Guillermo Moñux (coordinator), Joaquín Vallespín, Natalia de la Fuente, Fidel Fernández, Dolores Arenas.
- 3.
ARTERIOVENOUS FISTULA CARE Néstor Fontseré (coordinator), Pilar Caro, Anna Martí, Ramon Roca-Tey, José Ibeas, José Luis del Pozo, Patricia Arribas, María Teresa Martínez.
- 4.
MONITORING AND SURVEILLANCE OF ARTERIOVENOUS FISTULA Ramon Roca-Tey (coordinator), José Ibeas, Teresa Moreno, Enrique Gruss, José Luis Merino, Joaquín Vallespín, David Hernán, Patricia Arribas.
- 5.
COMPLICATIONS OF ARTERIOVENOUS FISTULA José Ibeas, Joaquín Vallespín (coordinators), Teresa Moreno, José García-Revillo, Milagros Fernández Lucas, José Luis del Pozo, Antonio Giménez, Fidel Fernández, María Teresa Martínez, Ángel Barba.
- 6.
CENTRAL VENOUS CATHETERS Manel Ramírez de Arellano, Teresa Moreno (coordinators), José Ibeas, María Dolores Sánchez de la Nieta, José Luis del Pozo, Anna Martí, Ramon Roca-Tey, Patricia Arribas.
- 7.
QUALITY INDICATORS Dolores Arenas (coordinator), Enrique Gruss, Ramon Roca-Tey, Cristina López, Pablo Valdés.
The contents of the sections and their importance have been justified in a “preamble”. Subsequently, the “clinical aspects” develop the clinical contents of each section and bring together the recommendations, in the following sections:
- •
Recommendations: each section begins with the compilation of the recommendations, accompanied by a correlative numbering to facilitate identification. As mentioned, the new recommendations are identified with the label “new” and those corresponding to the clinical questions with the symbol (•).
- •
Rationale: discussion on the relevance and rationale of each clinical section.
- •
The clinical questions are identified in a correlative manner with Roman numerals (I, II, III, etc.). For these questions a formal review process of the scientific literature was followed and recommendations were formulated following the GRADE methodology, as detailed below. The section shows a summary of the results collected in the literature review assessed for each clinical question, with an electronic link to the original versions of the reviews. Then in a section called ‘From evidence to recommendation’, a rationale is laid out for the aspects assessed when formulating recommendations and grading their strength, and how agreement was reached among members of GEMAV, which in some situations was achieved through a formal process of voting. Finally, each clinical question is closed with recommendations derived from the assessment of the literature and the rationale process described.
- •
In the case of the updates, a section has been developed where the clinical content of every aspect of interest is described, followed by a table with the recommendations derived from consensus within GEMAV.
Methodology to elaborate recommendations of the clinical questions
As described in the previous section, the update of this Guide was initiated with a process of prioritisation in which the following were identified: a) sections of the original version that would be assumed as its own; b) aspects which GEMAV would update from a narrative review of the literature, and c) clinical questions that would follow a systematic and rigorous process of analysis of the scientific literature. For the development of the different phases, standardised methodological guidelines have been followed, taking as reference the Methodological Manual for elaborating National Health System Guides for Clinical Practice.1
At an initial working meeting two methodologists introduced the clinical members of GEMAV to the theoretical principles used to formulate answerable questions.2 The scope of the contents addressed in the initial version of the Guide was then assessed and these contents were transformed into clinical questions, adding those aspects that GEMAV members considered appropriate. During the meeting and in a subsequent electronic exchange of comments using the Google Drive platform, the most relevant clinical questions that needed to be developed were systematically prioritised, and outcomes of interest for each question were identified.
The clinical questions identify the type of patient, the intervention or diagnostic test to be assessed, the comparisons when necessary and the outcomes of interest (PICO format). Outcomes of interest were defined in order to assess the benefit and unwanted effects of the different procedures and were categorised according to their importance in decision-making.2
Thereafter, exhaustive searches on the clinical questions were made, terminology related to the scope of each question defined, and controlled and natural language identified to recover adequate results from relevant studies in the bibliographic databases. In the case of updates, one methodologist with expertise in the design of exhaustive literature searches designed a search strategy on MEDLINE (accessed through PubMed) and gave the search results to the GEMAV members responsible for each of the sections.
For the prioritised clinical questions an initial search of other Guides, literature reviews and clinical trials was designed to identify those questions with fewer studies to support them and require more exhaustive searches. Subsequently a search strategy on MEDLINE (accessed through PubMed) and The Cochrane Library was designed for each clinical question. In the event that the mentioned study designs were not identified, observational studies were assessed, and if no studies were identified, searches were refined based on networks of citations from relevant studies in ISI Web of Science (Thomson Reuters). The bibliographic search algorithms used in this work can be consulted in the following electronic link. No relevant limits were applied to these algorithms, which were implemented between October 2013 and October 2014. From then up to the date of the edition, the Guide coordinators have carried out a sentinel search task to identify studies that could have a major impact on the recommendations, identifying the last relevant study in April 2016 (clinical question VI).
A structured summary of the results of the most relevant studies was carried out within the scope of each clinical question. For each outcome of interest the quality of evidence was classified according to the standardised criteria defined in the GRADE system. This allows for the establishment of the confidence of the estimators of the effect available in the scientific literature to support the recommendations.3 The quality of evidence can be classified as high, moderate, low and very low. The following factors, which may modify the confidence in the outcomes available in the scientific literature, were considered: risk of bias, consistency between the results of the available studies, the availability of direct evidence, and the precision of the estimators of the effect.3 In the case of observational studies, the following were also taken into account: magnitude of the effect, dose – response relationship, and the potential impact on the results of confounding factors. Each clinical question is accompanied by a summary of findings obtained from the literature review, synthesised at the end of each question in a section called “Summary of evidence”. The summary of findings is accompanied in each case by the classification of the quality of evidence. This process is also contained in summary tables of the results, available for each clinical question in the electronic appendices.
Based on the outcomes of the literature reviews, recommendations were formulated for each clinical question. These may be in favour of or against a particular intervention, and are graded as strong or weak. The strength of recommendations accompanying the questions is reflected by how they are expressed. Hence, strong recommendations are formulated using the expression “we recommend...” or “we recommend not...”, and weak recommendations, or ones where there is more uncertainty, use the expression ‘we suggest...” or “we suggest not...”.
To grade the strength of recommendations, a number of aspects is evaluated. These determine the confidence with which the implementation of the recommendations results in more desirable than unwanted effects for patients.4 The strength of the recommendations is based on a balance between the benefits and risks of interventions, the costs, the quality of evidence, and the values and preferences of patients. Grading the strength of recommendations depends on the more or less favourable and relevant balance among these factors. The recommendations derived from the clinical questions are accompanied by a section called “From evidence to recommendation” in which GEMAV justifies the reasons for supporting a recommendation in a particular way. In exceptional circumstances, where there was insufficient agreement on the clinical questions and the rationale behind the strength of a specific recommendation, a method of consensus by voting was used.5
The recommendations arising from the update sections did not follow a structured process like that previously described. The recommendations corresponding to these sections were formulated by consensus within GEMAV. The contents of the Guide should be updated within a maximum of five years, or sooner if new scientific literature provides relevant data for the current recommendations. In the upgrading process the guidelines of the corresponding methodological handbook will be followed.1
Perspective for users of this Guide. External review
A draft of the Guide underwent external review by 1 to 3 experts selected by each of the scientific societies. A draft was also submitted to the 2 main renal patient societies in the country, ALCER and ADER. Finally, the final text was posted on the websites of the societies for evaluation by members. All comments and suggestions were answered. Both reviewers’ comments and responses are available via the following electronic link.
CONFLICTS OF INTERESTThe expert members of each group were independently proposed by each of the societies without receiving any financial compensation.
All experts from GEMAV signed a form declaring any external relationships of a personal, professional, teaching or work-related nature that could have generated conflicts of interest in relation to the contents of this Guide. A summary of these can be found in Annex 2.
All professional societies participated directly in the financing of this Guide. The Spanish Society of Nephrology (S.E.N.), through the Foundation for Assistance to Research and Training in Nephrology (SENEFRO Foundation), received partial and unconditional assistance for the final edition of this Guide from AMGEN, BARD, BAXTER, COVIDIEN, FRESENIUS, HOSPAL, IZASA, MEDCOMP, NOVARTIS and RUBIO. The Spanish Society of Vascular and Interventional Radiology (SERVEI), in addition to its direct financing, also received financial support from BARD. The Spanish Nursing Society of Nephrology (SEDEN) received unconditional assistance from the non-profit Foundation Íñigo Álvarez de Toledo (FRIAT). The other professional societies: Spanish Society of Vascular Surgery (SEACV) and Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) participated directly in the financing of this work.
DEVELOPMENT OF THE GUIDELINE SECTIONS1Procedures prior to vascular access creationCONTENTS
- 1.1.
Clinical history
- 1.2.
When to create the arteriovenous fistula
- 1.3.
Pre-operative assessment
Preamble
Nephrology departments must have a clinical care programme for patients with advanced chronic kidney disease (ACKD). This programme should include the provision of detailed information about integrated renal replacement therapy (RRT) systems for patients and family members, and offer the appropriate treatment based on the patient’s clinical characteristics. RRT mode must be finally agreed upon in accordance with the preferences and specific circumstances of each patient.6
The morbidity and mortality of patients on haemodialysis (HD), both before and during RRT, is directly related to vascular access (VA) type. The risk of infectious complications at the start of HD is multiplied by four with central venous catheter (CVC) as compared with native arteriovenous fistula (nAVF) and prosthetic arteriovenous fistula (pAVF). Infections increase sevenfold if CVC is the prevalent VA. Likewise, there is a significant increase in the risk of mortality associated with the use of CVC, especially in the first year of HD.7
Handling the HD patient’s VA is a multidisciplinary task which involves different specialities: nephrology, vascular surgery, interventional radiology, nursing and infectious diseases. The goal is to maintain the highest incidence and prevalence of nAVF.8 But coordination is as important as the work of this multidisciplinary team: it has been shown that efficient management of the team can decrease the prevalence of CVC.9
This first phase, prior to VA creation, is of particular importance, as the patient’s prognosis and illness are, to a great extent, determined by management and the measures undertaken. It is when patients must be informed of the types of RRT available so the most appropriate choice for their circumstances can be made, and strategies to preserve the venous network of upper limbs must be implemented. Likewise, the factors involved in the choice of the ideal access must be determined using oriented medical records and correct pre-operative assessment, and the risk of developing access-associated complications must also be assessed. Finally, the optimal timing for VA creation has to be decided so the need for CVC placement to start HD is minimised, and performing premature interventions should also be avoided.
1.1Clinical historyRecommendations
R 1.1.1) We recommend that all nephrology centres which generate patients for renal replacement therapy have educational programmes, in which a multidisciplinary team participates. The aim of these programmes should be to instruct patients and their families on the different aspects relating to advanced chronic kidney disease, modes of treatment and importance of having an arteriovenous fistula to start haemodialysis
R 1.1.2) We recommend that, in order to select the appropriate type of vascular access, a medical history must be built up, associated comorbidity ascertained and it must be possible to assess the risk factors of failure related to vascular access development, as well as the possible morbidity caused after its creation
- (•)
NEW R 1.1.3) We recommend that extreme care should be taken to preserve the superficial venous network of both upper limbs, which should remain free of needling and cannulations in order to facilitate the creation of an arteriovenous fistula in patients with advanced chronic kidney disease. To this end, it is necessary to instruct healthcare staff and inform the patient
Rationale
There are numerous circumstances associated with ACKD patient comorbidity that can influence the correct development of the VA, which requires prior awareness of all factors involved. During the review of the medical record, all the pathological antecedents that may increase the risk of AVF failure in some way or predispose to the appearance of morbidity related to its creation must be considered.10
Regarding the antecedents related to the risk of VA failure, firstly, there are the comorbidities associated with a bad prognosis of the VA in general (Table 1): advanced age, diabetes mellitus, peripheral arterial disease, smoking or obesity; and secondly, it is important to consider factors that will determine the optimal location of the arteriovenous fistula (Table 2): previous history of CVC or pacemaker (PM), previous VA, trauma or previous surgery in the arm, shoulder girdle or chest, and previous venous cannulations.10
Local factors to be assessed previously in the indication of the arteriovenous fistula
| History | Associated pathology |
|---|---|
| History of CVC | Presence of central venous stenosis |
| History of PM | Presence of central venous stenosis |
| History of previous VA | Vascular anatomy disorders |
| History of cardiac/thoracic surgery | Presence of central venous stenosis |
| Trauma in arm, shoulder girdle or chest | Presence of central venous stenosis |
| Vascular anatomy disorders | |
| Breast surgery | Existence of secondary lymphoedema |
CVC, central venous catheter; PM, pacemaker; VA, vascular access.
Likewise, a particular underlying pathology, which may be aggravated by the presence of the new AVF, such as heart failure, or prosthetic valves, which may be infected if CVC is used, must be taken into consideration. Moreover, it is important to bear in mind the dominance of the upper limbs to minimise the impact on daily activity, as well as factors like anticoagulant therapy.
Finally, other factors which may affect the election of a given type of AVF should be considered (Table 3). These include life expectancy associated with the patient’s comorbidity, which may advise a more conservative approach by using a CVC, or patients eligible for transplant from a living donor, where a CVC may also be highly recommended.
Other factors determining the choice of vascular access type
| History | Associated pathology |
|---|---|
| Congestive heart failure | Worsening of cardiac function |
| Prosthetic valves | Risk of infection |
| Limited life expectancy | Assess CVC placement |
| Candidate for living donor transplant | Assess CVC placement |
CVC, central venous catheter.
The high prevalence of ischaemic heart disease in HD patients in our setting11 means bearing in mind that both the entire systemic situation and vascular tree of patients undergoing HD is significantly worse than the general population’s. Therefore, strategies must be established to choose the best territory in which to create the VA, taking into consideration the future of the VA and, of course, the patient.
→ Clinical question I Does the preservation of the venous network prevent complications/facilitate the creation of the arteriovenous fistula?
(See fact sheet for Clinical question I in electronic appendices)
| Summary of evidence | |
| No scientific evidence has been found in observational studies or randomised controlled trials in answer to the question on whether venous tree preservation prevents complications or aids VA creation | Very low quality |
| The evidence currently available is based on a review of the bibliography,12 which outlines pre-operative care prior to AVF creation, including vein preservation, as well as group recommendations made in the different published clinical guidelines6,10,13-15 | |
Evidence synthesis development
In order to create an AVF, a suitable vascular bed must be available, both arterial and venous, and the anatomical and functional integrity of both beds is required. Given its deeper location, the arterial bed mainly depends on the patient’s comorbidity and is less exposed to external forms of aggression than the venous bed. As the superficial venous bed may deteriorate and this may have repercussions on the success of the future AVF, the need for measures of protection has to be addressed. The absence of these measures explains why many patients do not have a mature nAVF when they need it to start HD.
The superficial veins of the upper limbs are the most common venous access point in the hospital setting, given the ease of access and safety of the technique. In patients with multiple hospital admissions, this is precisely what causes the venous network to become impaired, as repeated and multiple cannulations produce trauma and the administration of medication provokes an inflammatory response at the vein level (chemical phlebitis).
Despite this, there is no available evidence in the form of observational studies or randomised controlled clinical trials which answers the question of whether the preservation of the venous network prevents complications or facilitates the creation of the VA. Thus, the recommendations made by both clinical practice guidelines (CPG) and the literature are based on the opinions of different groups of experts.12
Most of the CPG in use today,10,13-15 and the literature,16 recommend an aggressive policy aimed at preserving the venous network in HD candidates, through a series of measures prepared to this end (Table 4) and summarised in 2 directives:
Recommendations for preserving the venous network in patients who are candidates for haemodialysis10,13-16
|
CVC, central venous catheter; VA, vascular access.
- 1.
Patient education related to the importance and the measures required to preserve veins in the upper limb.
- 2.
Information and commitment on the importance of vein preservation among healthcare professionals.
From evidence to recommendation
There is no quality scientific evidence to back up an evidence-based recommendation. Therefore, based on good clinical practice criteria, after voting on the recommendation, GEMAV unanimously agreed to formulate a strong recommendation in favour of a strict preservation strategy to preserve the vascular bed, given the clear relationship between its preservation and the viability of the future VA.
Clinical question I. Recommendation
R 1.1.3) We recommend that extreme care should be taken to preserve the superficial venous network of both upper limbs, which should remain free of needling and cannulations in order to facilitate the creation of an arteriovenous fistula in patients with advanced chronic kidney disease. To this end, it is necessary to instruct healthcare staff and inform the patient
Informing the patient about vascular access: when and how should it begin?
Information on HD should include details relating to the VA, the need for its creation, its importance, care and complications. This information must be reinforced in subsequent ACKD check-ups and should be continued when the VA has been created and during the HD programme.17 If a patient has to start HD urgently, he must be informed that a VA is required when this situation is detected. This information will be completed in accordance with their evolution and needs.
Time to start giving information about renal replacement therapy
The optimal time to start RRT requires adequate planning. There is an increased risk of mortality associated with inadequate nephrological care in pre-dialysis and to the use of a CVC as the first VA.18 A lack of organisation at this stage causes greater incidence of starting HD through a CVC with its associated morbidity. If the patient is referred to the nephrologist with enough time, he will receive adequate treatment and preparation from the pre-dialysis phase, as well as information on different RRT techniques: HD, peritoneal dialysis (PD) and kidney transplant (KT). In the Sociedad Española de Nefrología (Spanish Society of Nephrology) agreement document for managing ACKD, the preparation of patients for RRT is based on estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, if applicable. At this time, in addition to the information about different RRT techniques, the patient should receive VA-related information.19 This appropriate referral implies a lower incidence of complications, especially infections and cardiovascular complications.
RRT should be considered when GFR is < 15 mL/min/1.73 m2 and, in general, dialysis is initiated with an eGFR between 8 and 10 mL/min/1.73 m2, the limit being around 6 mL/min/1.73 m2.19 Expectation of entry into HD is related to the time when detailed information about VA preparation has to be given to the patient. However, the nephrologist’s ability to predict the dialysis commencement is not totally precise and there is a tendency to underestimate the patient’s renal function, which means that HD may begin later than the time estimated.20 This delay is more common in elderly patients, primarily over 85. A number of unnecessary surgical procedures has been shown in this patient group, as they may die before starting dialysis.21 Thus, eGFR on its own may not be sufficient to decide on timing for VA creation.
Informing the patient about ACKD and the various RRT options needs to be coordinated with VA creation. The very creation of the VA may determine the decision to choose HD to the detriment of other techniques, like PD or even conservative management. A systematic review of the timing of the decision-making process of patients and caregivers on RRT22 shows that, from the patient’s perspective, waiting until the final phase of ACKD may not be appropriate. This has important implications, as an unwanted surgical procedure may be performed or the patients perceive that a decision has been taken for them regarding RRT, without their participation. Once the AVF has been created, the patient tends to reject another type of RRT, due to the preference for maintaining the status quo, so this type of treatment is not changed. Therefore, information related to VA creation should be given after the patient has been informed of the various options for RRT and has specifically chosen HD.22
Content and manner of providing the information
At the time of providing information to the patient about the VA, it should be borne in mind that from their perspective, in addition to renal disease and RRT options, the VA is among the main concerns.23 For the patient who needs one, living with an AVF is an important issue as they depend on it and have to provide the necessary care to maintain the access viable. This may generate the feeling of vulnerability, dependence, distrust and it may even become a stigma. Therefore, informing the patient about the need for a VA may generate a pronounced emotional response that should be taken into account.24 Indeed, one of the largest barriers to the creation of the AVF is the actual patient’s refusal.25
Because of all of this, the nephrologist must show particular sensitivity when informing the patient. There are studies which highlight that rejection of AVF creation may be explained by a previous negative experience and the information they receive from other patients and carers may not be well expressed. In addition, it is possible that the information has not been adequately assimilated as it is given at the same time as all the other details relating to entry into HD. Likewise, it is worth pointing out that patients are not usually aware that the use of CVC for HD carries a risk of mortality with it.26 Programmes aimed at helping patients decide which RRT technique to choose mean that patients opting for HD have a significantly higher likelihood of beginning treatment with an AVF.27 This is accomplished by motivating both patient and family from the creation phase to subsequent care. Moreover, the participation of other patients in the orientation of new patients can be of benefit. It has been shown that more patients with AVF recommend this VA than those who use a tunnelled CVC.28
Finally, when providing the patient with all the necessary information on VA, as well as the different forms of RRT, the nephrologist should give information about the different types of definitive VA and their characteristics (nAVF, pAVF and tunnelled CVC). The advantages and drawbacks of each one should be explained, highlighting the fact that the tunnelled CVC is not an acceptable alternative to AVF, if the latter is possible, given its high association with morbidity and mortality (section 6). The risks of tunnelled CVC should be systematically explained, making it clear that CVC is only indicated as a temporary measure pending AVF creation or when it is impossible to create one.
Ethical-legal considerations
Some literature reviews propose that there may be legal implications, in addition to ethical issues, if severe complications of tunnelled CVC arise in a patient who may be eligible for an AVF. In this context, in the same way as the patient signs a consent form before the surgical procedure, some groups suggest that this should be done before inserting the tunnelled CVC, and all the risks agreed to.29
1.2When to create the arteriovenous fistulaRecommendations
- (•)
NEW R 1.2.1) We recommend that the creation of vascular access be considered in patients with progressive chronic kidney disease when eGFR is less than 15 mL/min/1.73 m2 and/or when estimating that dialysis will be needed in 6 months
- (•)
NEW R.1.2.2) We recommend that a native arteriovenous fistula be created 6 months before the start of haemodialysis
- (•)
NEW R. 1.2.3) We suggest that prosthetic arteriovenous fistula be created 3 to 6 weeks prior to the initiation of haemodialysis
- (•)
NEW R.1.2.4) We recommend an arteriovenous fistula be created as a priority in patients with rapid chronic kidney disease progression, lack of arteriovenous fistula maturation and non-tunnelled central venous catheter carriers
→ Clinical question II In patients with chronic kidney disease, what are the demographic, clinical and analytical parameters in order to determine when the arteriovenous fistula (either native or prosthetic) should be created?
(See fact sheet for Clinical QUESTION II in electronic appendices)
| Summary of evidence | |
| Early referral to the nephrologist of patients with ACKD to prepare the AVF improves the success rates of initiation of HD using a mature AVF | Very low quality |
| Planning for AVF creation should be determined by the rate of reduction of renal function in the patient from ACKD stage 4: eGFR < 30 mL/min/1.73 m2, after being adjusted for age, gender and body surface area and possible comorbidities | |
| Indication to start dialysis is at level of GFR < 10 mL/min/1.73 m2 or higher if there are other factors which recommend an earlier commencement | |
Evidence synthesis development
For the patient with ACKD it is very important to have a functional AVF when starting HD to avoid the use of CVC and its associated comorbidity. This requires careful planning of its creation.
There are no clinical trials, only observational studies designed to determine when a definitive VA should be created. Recommendations in clinical guidelines are based on this type of studies and experts’ opinions and the proximity of HD is established according to levels in the decline in renal function. They highlight the importance of using these parameters adjusted for age, gender and body surface area. There are 6 CPG that assess the appropriate timing for VA creation in the literature review.6,10,13-15,30
Clinical practice guidelines recommendations
The previous edition of this guide, as well as the Japanese and Canadian guidelines, consider that the VA should be created when eGFR is less than 20 mL/min/1.73 m2. KDOQI (Kidney Disease Outcomes Quality Initiative), European and British guidelines recommend VA planning when eGFR falls below 30 mL/min/1.73 m2. The recommended minimum time elapsed between the creation of VA and the beginning of dialysis varies. The Spanish guidelines recommend 4 to 6 months; KDOQI and European guidelines, between 2 and 3 months; British guidelines, between 3 months to one year and Japanese guidelines, between 2 and 4 weeks. They all recommend assessing the earliest possible VA creation when ACKD evolves rapidly, there is VA failure and in patients with a CVC. They all agree that as the pAVF takes around 3 weeks to mature, it has to be created at least 3 weeks before initiation of HD. Finally, CVC does not require specific preparation, except that needed for the placement procedure itself, as it is for immediate use.
Available evidence
The latest review of clinical guidelines still fails to bring to light clinical trials, only observational studies. These publications emphasise the need for early referral to the nephrologist to guarantee adequate information on the various aspects of RRT and the possibility of starting HD with an AVF. Different observational studies made in recent years present data on how influential and important the time taken to refer the patient to the nephrologist and surgeon is regarding the appropriate moment to construct the VA.10,13-15,30
These observational studies show there is a direct relationship between the length of time in the care of a nephrologist and the significantly higher number of AVF created prior to the initiation of HD. This time period ranges from 431,32 to 12 months33,34, passing through 6 months.35,36
However, there have been changes in the latest recommendations for starting RRT. In recent years these criteria have evolved from higher levels of eGFR, > 15 mL/min, to much lower values approaching 5 mL/min. After the publication of clinical trials showing not only the lack of benefit, but even a higher morbidity with the early initiation of dialysis,37-39 the KDIGO guidelines40 (Kidney Disease Improving Global Outcomes) suggest that HD should be started when clinical symptoms of terminal CRF (chronic renal failure) are visible, seen with eGFR ranges between 5 and 10 mL/min/1.73 m2. In the agreement document for managing ACKD produced by the Sociedad Española de Nefrología (Spanish Society of Nephrology), RRT is considered when GFR is < 15 mL/min/1.73 m2, although an earlier start may be considered if there are symptoms of uraemia, hyperhydration, hypertension difficult to control, or a worsening nutritional status. In general, dialysis is initiated when GFR is between 8 and 10 mL/min/1.73 m2 and would be mandatory if eGFR was < 6 mL/min/1.73 m2, even in the absence of uraemic symptoms.19 However, in patients at risk, an early start for HD should be considered on a case-by-case basis.
From evidence to recommendation
In the absence of clinical trials or observational studies addressing criteria related to timing for VA creation and clinical trials addressed to HD commencement, GEMAV put this recommendation to the vote. It was thought appropriate to consider 2 options. For the first option—when eGFR drops below 15 mL/min and/or estimation of entry at 6 months—there were 15 votes, and for the second option < 20 mL/min and/or estimation of entry at 6 months there were 2 in favour and 3 abstentions. Therefore, considering that three quarters were clearly in favour of one of the options, the working group decided to formulate as a strong recommendation that the creation of the definitive VA should be requested when eGFR ≤ 15 mL/min, or with an estimation of entry into dialysis lower than 6 months.
Patients with rapid CKD progression, with a non-matured AVF or a non-tunnelled CVC should be given priority.
As pAVF take between 3 to 6 weeks to mature (except in those with immediate needling), we suggest this be the time period required for creation prior to the planned HD commencement (section 3).
Clinical question II. Recommendations
R 1.2.1) We recommend that the creation of vascular access be considered in patients with progressive chronic kidney disease when eGFR is less than 15 mL/min/1.73 m2 and/or when estimating that dialysis will be needed in 6 months
R.1.2.2) We recommend that a native arteriovenous fistula be created 6 months before the start of haemodialysis
R. 1.2.3) We suggest that prosthetic arteriovenous fistula be created 3 to 6 weeks prior to the initiation of haemodialysis
R.1.2.4) We recommend an arteriovenous fistula be created as a priority in patients with rapid chronic kidney disease progression, lack of arteriovenous fistula maturation and non-tunnelled central venous catheter carriers
Recommendations
- (•)
NEW R 1.3.1) When planning the vascular access, we suggest decisions not only be based on isolated clinical characteristics, socio-demographic factors, or any risk prediction model. We recommend that the decision be based on a global assessment of clinical history, physical examination of the vasculature, pre-operative ultrasound and patients’ individual preferences
R 1.3.2) We recommend that, during arterial physical examination, peripheral pulses be assessed, the Allen test performed and brachial arterial pressure be taken. During venous physical examination, we recommend the presence of a visible candidate vein be identified after tourniquet placement, with a superficial trajectory in the subcutaneous tissue and absence of significant tortuosity
- (•)
NEW R 1.3.3) We recommend vascular mapping with ultrasound be routinely performed prior to vascular access creation. The ultrasound must evaluate the diameter and the quality of the arterial wall as well as the anatomy and patency of the deep and superficial venous system of the limb
- (•)
NEW R 1.3.4) In patients at high risk for ischaemia (diabetics, age>60 years, presence of peripheral arterial disease, female gender), we suggest the prioritisation for distal arteriovenous fistulae and end-to-side anastomosis, avoiding large anastomoses (> 7 mm). We recommend close clinical monitoring of these patients to detect early signs of ischaemia
Rationale
An important factor to consider in choosing the ideal VA location is the influence that this location will have on subsequent accesses. The surgeon must plan a long-term strategy for possible future access locations. Despite the absence of randomised clinical trials (RCTs) on the order to be followed in access creation, there is general consensus among different groups10,23,24 that access location should be as distal as possible, thereby allowing the creation of further VA in the same limb in the future. The VA should preferably be created in the non-dominant limb to maintain patient’s comfort. Furthermore, the creation of nAVF with preference to pAVF is recommended, although individual conditions may suggest a different approach.
Patient assessment must include a detailed clinical history to identify risk factors for early failure and lack of maturation of the nAVF. It is also necessary to perform a physical examination to detect limitations in joints, motor or sensory deficits, thickness of the skin and subcutaneous fat, limb oedema, presence of collateral circulation in the arm or shoulder, scars or indurated veins.
Physical examination must include pulse palpation to assess presence and quality, including the Allen test, measurement of blood pressure in both upper limbs and the examination of the venous system by palpation with and without tourniquet41 (Table 5).
Clinical criteria required in physical examination for AVF creation1
| Venous examination |
| Cephalic vein visible after tourniquet placement |
| Superficial venous pathway visible and/or palpable in subcutaneous tissue |
| No significant tortuosity |
| Arterial examination |
| Radial pulse easily palpable |
| Permeability of the palmar arch (Allen test) |
| No difference in SBP > 15 mmHg between both upper limbs |
SBP, systolic blood pressure.
Complementary examinations should be performed as a necessary and indispensable aid to define what strategy to follow when deciding which order to choose for VA creation.
→ Clinical question III What criteria are required for arteriovenous fistula planning (based on different types of fistula)?
(See fact sheet for Clinical question III in electronic appendices)
| Summary of evidence | |
| A meta-analysis of three RCTs including 402 patients, as shown later, finds that there was a non-statistically significant difference in achieving AVF success in patients studied with ultrasound mapping in addition to physical examination | Low quality |
| In VA planning, evidence from clinical series is not conclusive enough to make a recommendation about the use of isolated clinical or socio-demographic factors, nor about the validity of specific multivariate models to predict the probability of VA success | Low quality |
Evidence synthesis development
1.3.1 The role of Doppler ultrasound in arteriovenous fistula planning
Since its incorporation into daily clinical practice, different publications have attempted to determine the usefulness of Doppler ultrasound (DU) in the pre-operative assessment of VA candidates.
According to a review by Ferring et al.,42 in order to assess a suitable place for AVF surgery, physical examination must initially be performed in all patients, reserving pre-operative (DU) for certain cases: patients with poor physical exam (obese, no pulses, multiple previous surgeries on the limb), patients with possible arterial disease (elderly, diabetes, cardiovascular diseases) or in patients with possible venous disease (previous cannulation).
Later, Wong et al.43 published a systematic review of the literature, based on the three RCTs published to date on the systematic use of pre-operative ultrasound mapping.44-46 Two of the articles showed the systematic use of pre-operative DU was significantly beneficial, while in the third no benefit in terms of effective access use was shown to carry out HD. The authors conclude that the review suggests positive results in patients who underwent ultrasound mapping prior to VA creation, which may improve long-term patency rates.
Besides the reviews assessed while preparing the recommendations in this section, other more recently published systematic reviews have shown non-uniform results. Although a meta-analysis of five clinical trials suggests that the routine preoperative use of DU is beneficial47 in line with the publication by Wong et al.,43 a systematic Cochrane review48 emphasises that using preoperative imaging does not improve AVF outcome and that new studies with a better design are needed to confirm the result.
1.3.2 Vessel diameter as a criterion for arteriovenous fistula planning
Several studies have tried to determine the ultrasound parameters that may predict AVF outcome.16,42,49-51 Some degree of correlation has been found between the following ultrasound parameters and AVF function: diameter of the artery, presence of arteriosclerosis (measurement of intima/media thickness), flow characteristics at artery level (resistance index after reactive hyperaemia, peak systolic velocity), vein diameter and venous compliance.52
Among these, the parameter most widely documented and in which a higher level of evidence has been found as predictor of AVF function is the inner diameter of artery and vein measured by DU.53-59
Several articles have published series trying to document the minimum diameter of the artery and the vein associated with good AVF prognosis (Tables 6 and 7).49,53-58,60-63
Minimum arterial diameter and prognosis of arteriovenous fistula
| Author | Year | Location | Number of cases | Parameter assessed | Diameter (mm) |
|---|---|---|---|---|---|
| Lauvao et al.53 | 2009 | Wrist and elbow | 185 | Functional primary patency | Without predictive value |
| Glass et al.54 | 2009 | Wrist | 433 (meta-analysis) | Functional primary patency | 2.0 |
| Khavanin Zadeh et al.55 | 2012 | Wrist and elbow | 96 | Maturation | — |
| Parmar et al.56 | 2007 | Wrist | 21 | Immediate success | 1.5 |
| Korten et al.57 | 2007 | Wrist | 148 | Primary patency | 2.1-2.5 |
| Malovrh60 | 1998 | Wrist | 35 | Early failure | 1.5 |
| Wong et al.58 | 1996 | Wrist | 60 | Early failure | 1.6 |
| Silva et al.61 | 1998 | Wrist | 172 | Primary failure | 2.0 |
Minimum venous diameter and prognosis of arteriovenous fistula
| Author | Year | Venous diameter (mm) | Location |
|---|---|---|---|
| Glass et al.54 | 2009 | 2.0 | Wrist |
| Lauvao et al.53 | 2009 | 4.0 | Wrist and arm |
| Hamish et al.62 | 2008 | 2.0 | Wrist and arm |
| Smith et al.49 | 2012 | 2.0 | Wrist and arm |
| Wong et al.58 | 1996 | 1.6 | Wrist |
| Malovrh60 | 1998 | 1.6 | Wrist |
| Silva et al.61 | 1998 | 2.5 | Wrist |
| Ascher et al.63 | 2000 | 2.5 | Wrist |
1.3.3 Patient comorbidity as a criterion for arteriovenous fistula planning
There is considerable evidence of the influence of underlying pathology, comorbidities and the patient’s own parameters on the prognosis of the VA to be created.42,49,59
Advanced age
The available evidence suggests VA prognosis is considerably worse in older patients.64 The authors suggest that distal AVF should be avoided in the elderly.
Female gender
Contrary to general opinion and to some authors,65 the best available evidence does not demonstrate that female gender is a risk factor for AVF prognosis66; this is attributed to the small diameter of vessels found in female patients.
Diabetes
Different prospective series show the negative effect of diabetes on AVF prognosis, having less impact in proximal AVF.67,68
Hypotension
Evidence from prospective series suggests a negative effect of sustained hypotension in the prognosis of AVF due to an increased risk of access thrombosis.69,70
Smoking
Smoking has been associated with a worse AVF prognosis in published prospective studies.71-73
Obesity
While a worse prognosis in obese patients with Body Mass Index (BMI) > 30 has not been proved, the evidence available suggests that obesity with a BMI > 35 is a risk factor in AVF prognosis.74
Other factors
Several studies have tried to determine the influence of other factors in access prognosis. These factors are considered to have a minor impact, either due to the lack of clinical evidence (use of systemic heparin during surgery, type of anastomosis, suture technique), or because despite the importance they have shown in limited studies, there is a need for further studies to demonstrate their usefulness in clinical practice (intra-operative heparin dose, use of transdermal nitrates, range of distribution of red blood cells).42,49,59,75-77
1.3.4 Models/rules to predict arteriovenous fistula failure
Using data from 422 patients, Lok et al.78 developed a rule for predicting the risk of AVF failure. They found that poor prognosis factors include age ≥ 65 years, peripheral vascular disease and coronary heart disease, while being Caucasian was a good prognostic factor. These data were used to elaborate a classification of risk of AVF failure.
Despite the acceptable predictive capability shown in this study, there have been no subsequent studies to confirm its clinical usefulness; in fact, it has been questioned by other studies.79
1.3.5 Determining factors for the success of a prosthetic arteriovenous fistula
Rosas et al.80 found some factors of poor prognosis: presence of vascular claudication, number of previously implanted grafts, dialysis dependence at the time of surgery and the use of vascular clamps during the procedure. On the other hand, the use of the brachial artery and the axillary vein, acute-angle anastomosis and grafts of a specific brand (Gore-Tex®) were factors suggesting favourable prognosis.
From evidence to recommendation
The introduction of portable DU in the pre-operative examination of AVF candidates has undoubtedly helped professionals to decide when to create an AVF.
DU has proved to be an essential tool in those units where it is available because it provides a reliable image plus haemodynamic information on the vessels in the pre-operative evaluation.
The progressive increase in the age of patients candidates to arteriovenous fistula creation, with the resulting increase in associated comorbidities, as well as the high prevalence of obesity, often make it difficult to carry out a complete physical examination in these patients, so essential information required to create the AVF is missing (Table 5). In these cases, both clinical practice and the available evidence unanimously recommend the use of DU as the imaging test of choice, before requesting other radiological examinations (phlebography, magnetic resonance imaging).16,42,81,82
However, there is no unanimity in the available literature regarding DU use in patients with a favourable physical examination. There are studies documenting that routine pre-operative ultrasound increases VA patency.10,44,46,47,49,65,83 However, in most of these reports the benefit does not reach the level of significance needed to make a recommendation about the generalised use of DU.42,43,45 Thus, in fact, these authors do not recommend the routine use of DU because it has no proven benefits, both because of the delay other tests may cause and because of the possibility of ruling out AVF creation in vessels with borderline diameter. In contrast, the reasons given in favour of its routine use include the reduction in the number of unnecessary surgical interventions due to low vessel size, no creation of AVF with poor venous drainage, the detection of subclinical arterial disease and the better use of the available vascular bed.
This last point, together with the trend described in the literature, was the main argument which led GEMAV to unanimously decide to recommend the systematic use of DU in the pre-operative examination of all candidates for AVF. It allows physicians to non-invasively obtain a map of a patient’s entire venous capital during the pre-operative evaluation, thus allowing them to decide on the location of the VA bearing in mind the real options for future accesses.
During this examination, the diameter and quality of the arterial wall, as well as the anatomy and patency of the limb’s superficial and deep venous system, must be assessed by creating a map of the aforementioned venous capital of the patient.16,42,52,81,82,84
Current evidence does not allow for a recommendation regarding the minimum diameter of vessels to be used for the AVF; the decision whether the vein or artery should be considered apt for AVF creation must be taken in accordance with diameter – basically, the bigger the diameter, the better the prognosis – and with the available VA alternatives. In any case, in accordance with published articles, arteries < 1.5 mm and veins < 1.6 mm in diameter, following placement of a proximal tourniquet, are considered of dubious feasibility.
Finally, although the prognostic factors in each case should be taken into consideration, it is suggested that the VA location not be decided by taking into account any isolated clinical or socio-demographic factor, or any specific multivariate risk prediction model. It is recommended that the decision be based on a global assessment of each patient’s medical history, physical vascular examination, pre-operative DU and on their individual preferences.
Clinical question III. Recommendations
R 1.3.1) When planning the vascular access, we suggest decisions not only be based on isolated clinical characteristics, socio-demographic factors, or any risk prediction model. We recommend that the decision be based on a global assessment of clinical history, physical examination, pre-operative ultrasound and patients’ individual preferences
R 1.3.3) We recommend vascular mapping with ultrasound be routinely performed prior to vascular access creation. The ultrasound must evaluate the diameter and the quality of the arterial wall as well as the anatomy and patency of the deep and superficial venous system of the limb
→ Clinical question IV What risk factors have been shown to influence the development of limb ischaemia after arteriovenous fistula creation?
(See fact sheet for Clinical question IV in electronic appendices)
| Summary of evidence | |
| No systematic reviews or RCT have been found in the evidence review. The evidence is based on CPG and prospective and retrospective observational studies | |
| In the study by Rocha et al.85 on a population of largely elderly patients, the relationship between steal syndrome and coronary artery disease and peripheral vascular disease was not evident. Female gender was associated with increased risk of ischaemia. However, these are two comorbidities significantly associated with diabetes, which was an independent predictor of steal syndrome. Diabetes is the most important risk factor for the development of VA-associated ischaemic syndrome. Age, AVF type, duration of renal replacement therapy and factors involved in endothelial damage were not significantly associated with steal syndrome. The results highlight the need for careful AVF monitoring, particularly among women and diabetics. The preferential use of end-to-side anastomosis is recommended in the surgical approach | Low quality |
Evidence synthesis development
AVF creation in an upper limb determines significant changes in the limb’s haemodynamics. The direct communication created between the arterial and venous system, which avoids passing through the capillary bed, causes a shunt with a large flow rate that may compromise the vascularisation of the arterial bed distal to the access. In many cases of ischaemia, the situation is aggravated by the presence of previous arterial disease in the proximal or distal territories.
This can lead to the development of distal hypoperfusion ischaemic syndrome in the limb, known as “fistula steal”. This is an uncommon complication after the access creation, with an incidence ranging between 1% and 20%,85-87b but it may have serious consequences and could lead to important tissue loss and amputation.
That is why various authors have tried to identify epidemiological and clinical factors which may be associated with the development of this syndrome so that patients at risk of ischaemia following VA creation can be detected.
Type of arteriovenous fistula
The main prognostic factor, accepted by all authors, is the type of VA.10,85-89 Accesses with increased risk of ischaemia are nAVF created in the brachial artery (brachiocephalic, brachiobasilic and brachioperforating); 10-25% of patients with these VA present clinically relevant ischaemia. This percentage drops to 4.3-6% in pAVF, while nAVF created in the forearm and wrist are those with the lowest risk of ischaemia (1-1.8%).87 These authors associate this difference with the greater flow present in proximal AVF and the presence of collateral circulation through the ulnar artery, which decreases the severity of ischaemia in AVF in the wrist and forearm.
Peripheral arterial disease
The great prevalence of cardiovascular risk factors in the HD population implies a high incidence of patients with symptomatic peripheral arterial disease. Although this disease usually affects the upper limbs less than other territories, the presence of haemodynamically significant lesions has been reported in up to 62-100% of patients with distal hypoperfusion syndrome.86 The prior existence of these lesions, both in the proximal artery and distal trunk territories, is an important predisposing factor to the onset of ischaemic symptoms in the limb upon creation of the VA.86-88,90
Diabetes mellitus
The presence of DM is, for all authors, one of the main risk factors for developing ischaemia.86-88,90,91 Observational studies show that the presence of diabetes is a predictor for developing ischaemia.85,92 The effect on the distal arterial bed determines the lack of vasodilation capacity in that territory and the appearance of distal tissue hypoperfusion.
Advanced age
Advanced age, which is considered to be > 60 years, is widely accepted as a predisposing factor in the onset of ischaemia, due to a mechanism similar to the one in diabetic patients.87,88,90,91
Female gender
Female gender is unanimously considered in the literature to be an isolated risk factor for presenting ischaemia.85,87,88,90,91 The authors do not deter mine the mechanisms involved, although hormonal and vessel size factors are suggested.
Other factors
Different publications describe the influence of other factors on the development of ischaemia, such as time on dialysis,73 end-to-side anastomoses,85 previous VA in the same limb87,88,90 and racial factors.92 However, the potential influence they have in these cases is not unanimously accepted.
Regarding the drainage vein used in elbow nAVF, a direct comparison between brachiocephalic and brachiobasilic nAVF has not shown a difference in terms of incidence of ischaemia.85
Finally, despite the evident relationship between the blood flow in the AVF and the development of ischaemia, no direct relationship has been shown, probably due to the intervention of other factors in the physiopathology of the disease. Nevertheless, some authors recommend anastomosis creation < 7 mm to limit excessive flow rate to the VA.87
From evidence to recommendation
There are no systematic reviews or clinical trials on this subject. The level of evidence is limited to published observational articles and to experts’ opinion stated in the various clinical guidelines.
While there are well defined ischaemia risk factors, whose influence is unanimously taken into consideration (type of access, peripheral arterial disease, diabetes mellitus, advanced age and female gender), there are other factors whose role has not been well defined.
Furthermore, there are no published recommendations regarding the strategy to follow in daily clinical practice in the presence of these risk factors, which are highly prevalent in HD patients.
There is, however, widespread agreement among authors regarding the need for close post-operative monitoring of those patients considered high risk (diabetics, aged > 60, presence of peripheral arterial disease, female), so distal ischaemia can be detected and treated as soon as possible to avoid serious consequences. Authors also agree on the need to prioritise the creation of distal accesses in these patients over an access on the brachial artery.10,85-88,92
For all these reasons, although there are no systematic reviews or clinical trials on the subject, based on the published studies, the opinion of experts and good clinical practice, GEMAV suggests that firstly, surgical techniques aimed at minimising the risk of ischaemia be promoted; and secondly, that there must be close clinical monitoring of patients considered high risk after VA creation, in order to prevent the appearance of irreversible complications.
Clinical question IV. Recommendation
R 1.3.4) In patients at high risk for ischaemia (diabetics, age>60 years, presence of peripheral arterial disease, female gender), we suggest the prioritisation for distal arteriovenous fistulae and end-to-side anastomosis, avoiding large anastomoses (> 7 mm). We recommend close clinical monitoring of these patients to detect early signs of ischaemia
CONTENTS
- 2.1.
Types of arteriovenous fistula
- 2.2.
Native arteriovenous fistula
- 2.3.
Prosthetic arteriovenous fistula
- 2.4.
Fall-back techniques
- 2.5.
Sequence for vascular access creation
- 2.6.
Antibiotic prophylaxis for arteriovenous fistula creation
Preamble
The mission of the multidisciplinary team treating a patient in a haemodialysis (HD) programme must be to create an arteriovenous fistula (AVF), preferably native, which has a high patency and as few complications as possible. To this end the strategies needed to ensure that the patient with advanced chronic kidney disease (ACKD) starts dialysis with a mature AVF must be set up. In addition, subsequent AVF, if required, should be done in a timely manner and all the professionals involved and the patient must take an active role.
2.1Types of arteriovenous fistulaRecommendations
R 2.1.1) We recommend that the native arteriovenous fistula be considered the vascular access of first choice
R 2.1.2) In the event that there are no appropriate veins to create a native arteriovenous fistula, we recommend creating a prosthetic arteriovenous fistula
R 2.1.3) We recommend that a tunnelled central venous catheter be placed when a native or prosthetic arteriovenous fistula is not viable, or when haemodialysis therapy must be initiated without a definitive mature vascular access
R 2.1.4) Although the native arteriovenous fistula is the vascular access of first choice, the appropriate vascular access and its location must be decided on a case-by-case basis in accordance with the clinical characteristics of the patient and the findings of the vascular mapping
Rationale
Prioritising nAVF over pAVF is a basic recommendation in numerous clinical guidelines and among experts, given the low rate of complications and excellent long-term patency it presents once the nAVF has matured.
Arteriovenous fistula patency
Primary patency rates for nAVF at 6 and 18 months is 72% and 51%, while secondary patency is 86% and 77% respectively. In pAVF, however, primary patency at 6 and 18 months is 58% and 33% and secondary is 76% and 55%, respectively.93 The main disadvantage of nAVF versus pAVF lies in the high risk of primary failure, due to the high rate of immediate thrombosis (5-30% for radiocephalic nAVF) and in maturation failure (28-53%), compared to only 0-13% primary failure for pAVF in the forearm and 0-3% for pAVF in the arm.8
In addition, there has been a demographic change in incident patients starting renal replacement therapy (RRT) in recent years. This means that there has been a progressive trend towards a decrease in patency rates reported in the literature.94 Thus, the analysis of results from 46 articles between 2000 and 2012 provided by Al-Jaishi et al.94 estimate a primary failure rate for nAVF of 23%—significantly higher in distal nAVF (28%) than in proximal (20%). They found a primary patency (including primary failures) of 60% after 1 year and 51% after 2 years, with a significant difference depending on the location of the nAVF (distal or proximal) after 1 year, but not after 2 years. These same authors found a secondary patency rate of 71% after 1 year and 64% after 2 years, with no difference in the location of the nAVF.94 It has also been reported that the routine use of a preoperative ultrasound study may reduce immediate nAVF failures.46
Rate of complications
nAVF are associated with a decreased morbidity and mortality compared to pAVF and catheters (CVC).95 According to Ravani et al.,96 the use of pAVF and CVC versus nAVF is associated with an increased mortality of 18% and 53%, respectively. In addition, nAVF have a lower rate of infections than pAVF, which is lower than CVC.96,97
As a result of all this, when compared with nAVF, the risk of hospitalisation increases by 26% with pAVF and by 68% with CVC.96
Another advantage of nAVF is that they have a lower rate of re-intervention than pAVF, which implies a lower maintenance cost.98
Thus, in any patient who requires RRT using HD, the ideal VA must be created, one which allows appropriate dialysis, has greater patency and a lower rate of complications. The VA that brings all of these characteristics together is nAVF10,99-102 and, therefore, this must be the first VA to be considered. When nAVF cannot be constructed because there is no venous capital or venous capital is damaged, pAVF should be used99-101, while CVC placement must only be considered when neither of the above are possible or when HD treatment must be initiated without a mature VA.103,104
Permanent VA should be indicated on a case-by-case basis, depending on vascular examination, the patient’s previous VA, as well as other factors such as age, comorbidity and the urgency for VA use.10,82,99-101,105-108
2.2Native arteriovenous fistulaIn the case of nAVF, the most distal location possible should be chosen as first option when planning VA creation, in order to preserve the maximum peripheral venous network for future VA. All things being equal, the non-dominant limb should be prioritised to help facilitate patient comfort both during HD sessions and in their daily activities.109
2.2.1 Native arteriovenous fistula in wrist and forearm
Radiocephalic native arteriovenous fistula at the wrist (Brescia-Cimino arteriovenous fistula)
The radiocephalic AVF at the wrist, described by Brescia-Cimino in 1966, is still the VA reference for HD.10,110,111 It preserves proximal venous capital for future VA, has a low rate of complications, especially VA-induced ischaemia and infections, and those that mature correctly have an excellent patency rate.99-101,109 The main limitation of this technique is the relatively high immediate failure rate, which ranges between 10% and 30%, but reaches almost 50% in some groups, especially in diabetic, elderly and female gender.100,112,113 A further disadvantage of the radiocephalic AVF is its high incidence of maturation failure, so that approximately 30% of these AVF have not matured enough for use at 3 months.100,102,105 Primary patency at 6 months ranges from 65% to 81%; this is lower than the 79-89% found in pAVF, although they equal out after the first year, with fewer complications.100,101
Arteriovenous fistula in the anatomical snuffbox
The anatomical snuffbox AVF, using the posterior branch of the radial artery located between the tendons of extensor pollicis brevis and extensor pollicis longus as a donor, is used less frequently due to its greater surgical complexity. Despite this, results in units where it is normally performed are good114: 11% immediate thrombosis, 80% maturation at 6 weeks and cumulative patency at one and five years of 65% and 45%, respectively. In this case, its greatest benefit is that it does not exclude the possibility of performing a radiocephalic nAVF in the same limb if the access fails. Both sites allow proximal reconstructions in the forearm when either juxta-anastomotic stenosis or thrombosis appears.
Radiocephalic arteriovenous fistula in forearm
This technique differs from the previous one in that it is performed in a more proximal area; it is indicated as surgical treatment in AVF juxta-anastomotic stenosis in the wrist, and in cases of non-viability of the cephalic vein in the wrist, usually due to early bifurcations.
Radiobasilic transposition
When the cephalic vein in the forearm is not adequate for a radiocephalic AVF, a possible option before using more proximal veins is radiobasilic transposition.115,116 The basilic vein must be transposed from the wrist proximally towards the antecubital fossa and subcutaneously tunnelled as far as the radial artery to create anastomosis. The antebrachial basilic vein is usually free of previous vein cannulations. However, its lower consistency makes it more vulnerable to possible lesions during the transposition process, with a greater tendency to torsion, so its use in clinical practice is limited by the vein’s development and by the experience of the surgical team.
Other venous transpositions
When the radial artery is not suitable for radiocephalic AVF, other possible venous transpositions in the forearm include the cephalic or basilic vein, placed in the shape of a loop in the palmar face of the forearm, towards the proximal radial artery in the antecubital fossa.117 This is how different ulnar-basilic transposition options in the forearm, as well as brachiobasilic in the shape of a loop, and different configurations using the great saphenous vein have been reported.111 Its use is limited in practice to specific anatomical situations in certain patients.
2.2.2 Native arteriovenous fistula in the antecubital fossa (elbow)
According to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines10 radiocephalic and brachiocephalic AVF are the first and second options for creating a VA, respectively.10 The antecubital fossa contains larger vessels, which usually provide higher flow and have lower rates of primary failure and alterations in maturation, while their main drawback is the shorter needling segment available and the fact that they limit subsequent use of a more distal access.
Brachio-cephalic arteriovenous fistula
The brachiocephalic AVF is the vascular access of choice for this location.6,10,109 It has the advantage over radiocephalic AVF of achieving higher flow and the cephalic vein in the arm is usually more accessible for needling and aesthetically more discrete than in the forearm. However, it may cause greater oedema in the limb and it has an increased risk of causing VA-induced distal ischaemia.
Brachioperforating arteriovenous fistula (Gracz arteriovenous fistula)
A widely used variant of the previous technique consists of creating the AVF between the brachial artery and the perforating vein in the antecubital fossa (brachioperforating AVF), using the technique described by Gracz118 and subsequently modified by Konner et al.67,119 in order to obtain arterialisation of both the cephalic and the basilic vein.120
Arteriovenous fistula using the proximal radial artery
As an alternative to the brachial artery, the proximal radial artery in the antecubital fossa can be used as a donor.113,119,121-123 This technique has certain functional advantages. The risk of VA-related distal ischaemia is lower when the donor artery is the radial, compared to procedures performed with the brachial artery. As this anastomosis is constructed on the radial artery, its smaller size favours the appropriate resistance of the new VA and minimises the risk of distal ischaemia. Likewise, as a lower flow in the AVF is obtained, it limits the cardiological impact in patients at risk. Moreover, venous confluence in this location allows the possibility of setting up a two-way flow in the venous drain.
In cases where few veins are available for needling, technical variations have been proposed to promote two-way flow in the veins distal to the AVF, mainly in the elbow, in order to increase the segment available for cannulation,124 by retrograde valvotomy of the drainage veins.125
The antecubital fossa presents multiple anastomoses between veins that may allow the intervention to be performed. Although short case studies are described in the literature, there is not enough documented evidence to determine its usefulness in practice and to assess the clinical significance of venous hypertension arising from this technique. As a result, its use is limited to cases with short needling trajectory in which this is anatomically feasible.111
Brachiobasilic arteriovenous fistula
Those patients who cannot have a radiocephalic or brachiocephalic AVF created may opt for a brachiobasilic AVF with venous superficialisation or transposition as an alternative to vascular prosthetic implants.126
The depth of the basilic vein protects it against repeated venipunctures so it tends to be preserved in HD candidates; however, this greater depth may cause difficulty when needling, requiring superficialisation. In addition, the trajectory of the basilic vein is adjacent to the neurovascular bundle in the limb, which leaves these structures vulnerable to potential cannulation lesions during dialysis. Therefore, as well as being superficialised, it can be transposed to an anterior and lateral location in the arm to move away from these structures and to improve patient comfort during dialysis.
Given that two surgical procedures are usually required, in clinical practice they can either be carried out in the same intervention, or in two procedures separated in time.
When the surgery is performed, the basilic vein is dissected and transposed; thus the new trajectory is created and finally the arteriovenous anastomosis constructed. The advantage of performing the two procedures in one session is that it shortens the time required before being able to cannulate the VA; the disadvantage is that it increases the likelihood of mechanical complications during surgery, as the mobilisation and/or transposition of the vein has to be performed with a vein without the necessary prior arterialisation.
When performed in two procedures, the anastomosis is firstly constructed between the basilic vein and the brachial artery, and from day 30-90, after using a Doppler ultrasound to check the correct maturation of the AVF and the absence of stenosis, the second procedure is performed and the vein is superficialised and/or transposed.127
Three superficialisation techniques are described in order to allow cannulation in this type of access128: a) anterior transposition in the arm, by creating a new subcutaneous tunnel; b) anterior transposition in the arm, by creating a lateral flap of skin and subcutaneous tissue, and c) simple superficialisation without transposition.
Brachiobrachial fistula
When there are no available superficial veins, a suggested alternative is to create an arteriovenous fistula between the brachial artery and the brachial vein.129-131
The brachial vein lies next to the artery and can be single or double. As this vein corresponds to the deep venous system, it is well preserved from prior needling. As a result, despite being a complex operation, if the vein develops well, it may be technically feasible.
The surgical procedure is the same as brachiobasilic AVF: the anastomosis is created in the antecubital fossa, mobilising the brachial vein with collaterals ligation, and performing superficialisation and/or transposition.129
Once the VA has matured, the results in terms of patency and complications are similar to those of the brachiobasilic AVF. However, the severe limitation of this technique is the high rate of primary failure, which may reach 53%, and its low primary patency after one year (35-40%),129,131 probably related to the increased technical complexity of mobilising and transposing the brachial vein. Therefore, given the lack of broader studies, this technique is not usually considered as a valid alternative option to using synthetic prostheses in the arm.128
Other venous transpositions
As in the forearm, there are also various possibilities for venous transpositions; their use is limited to certain clinical situations with particular anatomical layouts.111
2.3Prosthetic arteriovenous fistulaRecommendations
Rationale
2.3.1 Rationale for prosthetic arteriovenous fistulae
The use of prosthetic material to create VA for HD has been shown to be a viable and effective solution to achieve a permanent VA.93,99,132,133
However, the high economic cost involved and the associated morbidity and deterioration in the patient’s quality of life, due to the need for procedures to maintain VA usefulness, mean that it is not considered a first choice technique when planning the VA, a role reserved for nAVF.10,93,98,109,132-135
As it is technically less complex to perform, this may help surgeons who have little expertise in creating VA lean towards this procedure from the onset.99
Despite not being the first-choice VA, nowadays this access plays a highly relevant role because an ever increasing number of patients require HD for very long periods of their lives, consequently putting the vascular bed at risk, and there is also a progressive increase in the average age of the patients on a HD programme. In addition, pAVF offer some theoretical advantages, such as a shorter maturation time and greater ease of needling in certain cases, as in the case of obese patients. They may also make it easier to create a secondary native VA, by helping to dilate previously unsuitable veins in the arms to create an AVF.132
2.3.2 Prosthetic arteriovenous fistula planning and creation
Arteries or veins with a diameter suitable for pAVF placement should first be identified (not less than 4 mm).63,116,133 In most cases, with an already exhausted distal venous bed, arterial anastomosis should be as distal as possible; venous anastomosis should also be as distal as possible, whenever correct drainage towards central venous trunks can be ensured.
It should be noted that the use of antecubital fossa veins for anastomosis involves the integrity of that territory and its respective drainage veins. Therefore, in these cases, the priority indication would be nAVF creation using these veins. For this reason, there are authors136 who recommend avoiding prosthetic loops in the forearm, since they consider that in these cases a native AVF can be created.
Expanded polytetrafluoroethylene
The material recommended for the prosthesis is ePTFE, given that it offers better rates of infection and integration than Dacron.10 Apart from this standard material, there are other prostheses which may be used in special circumstances.137,138
Immediate puncture prosthesis
Immediate needling prostheses are bilayer ePTFE prostheses reinforced with a third elastomer layer between the two, which means it does not need to be integrated in the tissues for needling. It provides similar results to conventional prostheses with the advantage of allowing cannulation after 24 hours, if necessary.139
Biosynthetic prostheses
Good results have also been published in relation to bioengineered prostheses. This is a prosthesis manufactured from a polyester matrix in which collagen from sheep is cultivated. It has a potential benefit in terms of presenting a lower incidence of infections when it is not possible to create an nAVF.140 The main evidence regarding the use of this type of prosthesis is that published by Morosetti et al., comparing the prosthetic access with the brachiobasilic AVF access in patients without other alternatives; although results with autologous access were more favourable, patency results and complication rates were similar to those of other studies with ePTFE.135
Surgical technique
The prosthesis can be implanted in a straight line or in the shape of a loop, the latter being the preferred layout in the forearm.141 These layouts are determined by the characteristics of the patient.
The order of preference for arterial anastomosis location is the brachial artery in antecubital fossa, brachial artery in arm, brachial artery close to axilla and axillary artery. However, because a pAVF is usually created after several failed nAVF, the location will depend on the well preserved vascular bed. Venous anastomosis can be performed on the veins in the antecubital fossa or above the elbow, as well as in the cephalic, basilic, axillary, subclavian and jugular vein.
Arterial anastomosis of the prosthesis should preferably be end to side. There are no studies showing differences depending on the anastomosis type between the vein and the prosthesis. The prosthesis should be between 20 and 40 cm in length to ensure a large needling segment. Prosthesis diameter must range between 6 and 8 mm. According to some authors,142 bigger diameters are associated with better long-term outcomes in this type of VA.
2.4Fall-back techniquesPatients who have exhausted all their venous capital for VA in the upper limbs, including nAVF and pAVF, are a small, but growing, percentage of patients on HD.
In view of the greater morbidity and mortality discussed when performing HD through CVC, surgical techniques known as “fall-back” techniques have been described. These allow permanent access when there are no viable veins in the upper limbs. These techniques make it possible to avoid the use of CVC despite the higher level of complications, the greater operative morbidity and lower patency than conventional VA.93,97,122,132,133,143
2.4.1 Vascular access at lower limbs
There is widespread evidence regarding VA creation in the lower limbs, using a vascular prosthesis (proximal in the thigh or mid-thigh section) or else using nAVF (AVF in femoral vein with transposition). pAVF creation in the lower limb is the most widely used of all the fall-back techniques described, since it offers acceptable patency rates and is the least complex surgical technique.111,122,132,133
2.4.2 Prosthesis-tunnelled catheter device
The hybrid prosthesis-tunnelled catheter device Haemodialysis Reliable Outflow (HeRO-device) is indicated in cases where there is a central venous obstruction which prevents the creation of any other VA in the upper limb. It consists of a VA that is created in a mixed way. On the one hand, it is a tunnelled catheter which is inserted through the internal jugular vein to the atrium; on the other, it is connected to an ePTFE prosthesis that is anastomosed at the level of the brachial artery. This means that the needling area is the prosthesis which is subcutaneously tunnelled and distal drainage is carried out directly in the atrium. The objective is to go through the stenosis and central vein occlusions which would prevent AVF creation.
The advantage of using this in clinical practice is that it is a VA that can be implanted in patients without adequate central venous drainage, in which all nAVF and pAVF options have been depleted, without compromising future accesses; main disadvantages include the technical complexity of the operation and its high cost.
2.4.3 “Exotic” vascular access
“Exotic” VA are those considered when there are extensive occlusions of venous trunks, both in upper and lower limbs. They have two great advantages: firstly they allow a new VA to be created in a theoretically exhausted territory; secondly, they can sometimes be used to salvage VA which have failed due to occlusion in the drainage veins and where endovascular therapy has not been effective.144,145
Given the exhaustion of the venous bed in these patients, these accesses are created using ePTFE prosthetic grafts, in the form of artery bypass between the donor artery and recipient vein.
Unfortunately, despite the initial advantage of providing access when the venous bed is depleted, there is a higher incidence of complications than in nAVF, because they are made of heterologous material. This is particularly serious in infection, because it involves central vessels which are difficult for the surgeon to access.
Furthermore, they are complex surgical procedures which are not exempt from morbidity and mortality and are performed on patients with significant associated comorbidities, who frequently have a history of multiple failed VA.
Therefore, as a general rule, the benefit/risk of the surgical intervention should be assessed on a case-by-case basis, the intervention required and the existing access options.
Prosthetic accesses in the anterior chest wall
These VA are created either as a loop, placing a prosthesis between the vein and axillary artery on the same side, or in a straight line, placing the prosthesis between the vein and contralateral axillary artery. These VA can be considered for patients with an exhausted venous bed but with central vein patency; they especially benefit those patients at high risk of ischaemia in the limb. Results report patency rates similar to more conventional pAVF in the arm.146,147
Bypass to central veins
In the case of distal axillary vein thrombosis, the technique consists of creating a bypass between the brachial artery and proximal axillary vein, while in cases of extensive axillosubclavian thrombosis with internal jugular vein and brachiocephalic trunk patency, the technique of choice is to perform a bypass between the brachial artery and internal jugular vein.144
Bypass to leg veins
If the two brachiocephalic venous trunks or the superior vena cava are occluded, the surgical alternative is to perform a bypass between the axillary artery and iliac vein144,148 or else to the popliteal vein.145,149
Other derivative techniques
Other alternative derivative techniques have been described, such as bypassing to the right atrium,150 femorofemoral crossover bypass,145 axillo-renal bypass,151 or axillo-inferior vena cava bypass.144 In all cases, these are techniques which are considered extraordinary, and evidence is restricted to a few documented cases.
Types of vascular access
- •
nAFV at wrist and in forearm.
- –
Radiocephalic AVF in the wrist (Brescia-Cimino AVF).
- –
AVF in the anatomical snuffbox.
- –
Radiocephalic AVF in forearm.
- –
Radiobasilic transposition.
- –
Other venous transpositions.
- –
- •
nAVF in antecubital fossa (elbow) and arm.
- –
Brachiocephalic AVF.
- –
Brachioperforating AVF (Gracz AVF).
- –
AVF using the proximal radial artery.
- –
Brachiobasilic AVF.
- –
Brachiobrachial AVF.
- –
Other venous transpositions.
- –
- •
pAVF in upper limbs.
- –
Radioantecubital Straight graft.
- –
Brachio/radioantecubital loop.
- –
Brachiobrachial/axillary straight graft.
- –
Brachiobrachial/axillary loop.
- –
- •
Fall-back techniques.
- –
VA in lower limbs.
- –
Proximal femorofemoral (groin) graft.
- –
Femorofemoral graft in the middle third of the thigh.
- –
Transposition of femoral vein.
- –
Prosthesis tunnelled catheter device (HeRO).
- –
“Exotic” vascular access.
- –
- •
Central venous catheter.
Recommendations
- (•)
NEW R 2.5.1) We recommend that a native arteriovenous fistula be created in the non-dominant upper limb, and as distal as possible, as the vascular access of first choice
- (•)
NEW R 2.5.2) After exhausting radiocephalic vascular access along the forearm, we recommend that a native arteriovenous fistula be created using the available veins in the elbow, a brachiocephalic or proximal radiocephalic fistula should be considered as the first alternative
- (•)
NEW R 2.5.3) If a radiocephalic or brachiocephalic native arteriovenous fistula cannot be performed, we recommend a brachiobasilic fistula with superficialisation or venous transposition in the arm or forearm as an option prior to the use of a prosthetic arteriovenous fistula
- (•)
NEW R 2.5.4) We recommend restricting the creation of a prosthetic arteriovenous fistula in the upper limb to the following criteria:
- A.
Patients without anatomically appropriate veins in the arm or forearm
- B.
Patients requiring immediate commencement of haemodialysis where a tunnelled central venous catheter placement is to be avoided
- A.
- (•)
NEW R 2.5.5) If a prior arteriovenous fistula has failed, we recommend that both physician and patient agree on the location of the following fistula to be created, in order to decide whether to give priority to distal location or to the non-dominant limb criterion
- (•)
NEW R 2.5.6) Where all vascular accesses have been exhausted in both upper limbs, we suggest using fall-back techniques, and that priority be given to prosthetic arteriovenous fistula in the thigh and the prosthesis tunnelled catheter device as first choice options
→ Clinical question V Can an order of preference be recommended when performing the arteriovenous fistula?
(See fact sheet for Clinical question V in electronic appendices)
| Summary of evidence | |
| nAVF versus pAVF | Moderate quality |
| Several published randomised clinical trials (RCTs) show better outcomes for nAVF than for pAVF in terms of greater functional duration and lower rates of complications | |
| Order of creation of the different VA | Low quality |
| There are no comparative studies, randomised or not, that compare the efficacy and safety of different orders in performing successive VA for HD | |
Evidence synthesis development
Preferred arteriovenous fistula location
Experts and guidelines both indicate that the procedure should be started by placing an AVF as distally as possible to preserve the option of future, more proximal accesses if necessary.6,10,109,111 However, no study has been found comparing the results of different AVF locations for HD in patients where any of these options would initially seem viable.
According to published clinical guidelines,6,10,111 arteriovenous radiocephalic and brachiocephalic nAVF are the first and second choice for VA, respectively. If these options are not possible, they recommend the creation of an autologous brachiobasilic nAVF in the upper arm or a radioantecubital pAVF in the forearm.
Using the non-dominant limb
Although the first VA is generally recommended in the non-dominant upper limb, no studies have been found which explicitly compare the option of prioritising the dominant or the contralateral hand.
In this respect, Koksoy et al.152 document, in an RCT on efficacy and safety in brachiocephalic and brachiobasilic nAVF, that the use of the dominant arm may increase the risk of fistula failure. However, this trend could not be confirmed in any other study conducted to date.
Distal versus proximal location
Moreover, no studies have been found specifically comparing whether it is more effective or safer to prioritise the most distal locations possible, alternating between non-dominant limb and dominant, or, on the other hand, to continue using the same limb until all other surgical options have been exhausted. Given the lack of solid evidence clearly favouring either of the options, it seems reasonable to leave the decision on a future AVF proximally in the same limb or distally in the contralateral limb to the patient, with professional advice.
Reinhold et al.8 point out that the first VA should be placed as distally as possible. The main disadvantages of a distal radiocephalic nAVF in the anatomical snuffbox or wrist are the relatively high rates of occlusion and non-maturation, which are affected by patient risk factors such as age, diabetes mellitus and cardiovascular disease.
A previous review with meta-analysis66 based on 38 observational studies estimated a primary failure rate of 15.3%, and primary and secondary patency rates of 62.5% and 66.0%, respectively, for radiocephalic fistula in the wrist.
Options prior to placing a prosthetic arteriovenous fistula: role of the brachiobasilic native arteriovenous fistula
As an option prior to pAVF use, a brachiobasilic nAVF with superficialisation or venous transposition in the arm is indicated.
Brachiobasilic arteriovenous fistula. Results
The systematic review of Dukkipati et al.128 analyses the results of brachiobasilic nAVF, based on several observational studies and an RCT.134 This review finds acceptable rates for primary failure (15% to 20%), and primary patency after one year (72%) and 2 years (62%).
Brachiobasilic arteriovenous fistula versus prosthetic arteriovenous fistula
2 RCTs and 2 retrospective studies compare results between the two procedures.134,135,153,154 All of them report similar results, with significantly better primary patency rates and primary assisted patency rates in the group of brachiobasilic AVF patients. However, when secondary patency results are analysed, these differences disappear, although the number of surgical interventions required to maintain this secondary patency is markedly greater in the case of pAVF.134
Complications are more frequent in pAVF,134,135,153 especially those with infection; however, maturation time is higher in brachiobasilic AVF.154
Brachiobasilic versus brachiocephalic arteriovenous fistula
The RCT conducted by Koksoy et al.152 comparing the efficacy and safety of brachiocephalic AVF versus brachiobasilic AVF found no differences in relation to mortality, wound complications, immediate thrombosis, post-operative bleeding, AVF maturation and time to AVF maturation, and there were no significant differences regarding patency rates. Other authors155,156 reported similar results. In the aforementioned studies, brachiobasilic AVF also show a tendency to present better VA maturation rates, albeit with no statistically significant differences. This may be due to the better preservation of the basilic vein than the cephalic vein in most patients.
All these results make the brachiobasilic fistula a safe technique with good results when considering permanent VA.
Brachiobasilic arteriovenous fistula versus brachiocephalic arteriovenous fistula versus prosthetic arteriovenous fistula
There are also several published studies which analyse the results by comparing the three main types of AVF in the arm.156-159 All of them concur in describing better statistically significant patency in autologous VA, even though they present a greater primary failure rate.
Likewise, they also find a higher rate of complications and number of interventions needed to maintain patency in pAVF, but no significant differences between both types of nAVF.
There is no consensus between the different groups on the suitability of transposing the basilic vein during the same surgical procedure or after dilatation and arterialisation. Nor do they agree on which the technique of choice should be (transposition with subcutaneous tunnel, transposition with flap or simple superficialisation).128
One-stage procedure versus two-stage surgeries
In El Mallah’s RCT,160 which offers the best evidence to date, significantly better primary patency is described after two-stage surgery (50% versus 80%), although the number of patients is not high (n = 39). Similar results are subsequently described by Ozcan et al.,161 who found a higher rate of maturation and lower number of complications when surgery is performed in two stages. Finally, the case series published by Pflederer et al.158 highlighted that most complications in two-stage surgery occurred in the interval between both stages, so the authors recommend this technique to minimise surgical aggression.
Superficialisation versus transposition
There is agreement among authors that transposition through a subcutaneous tunnel is associated with a lower rate of complications,117,162 but not a better maturation rate. Finally, Hossny117 describes a greater level of satisfaction among nursing staff responsible for needling in the cases where transposition is performed by creating a subcutaneous tunnel.
Prosthetic arteriovenous fistula in an upper limb
Results of prosthetic arteriovenous fistula
The primary patency of prostheses is between 20% and 50% at 24 months and, through successive surgical interventions, can reach a level of assisted patency of between 45% and 70% at 2 years.163-167
The best available evidence comes from Huber’s systematic review with meta-analysis,93 which found thirt y-four studies, mostly case studies and some non-randomised controlled studies, comparing outcomes in nAVF and pAVF in the upper limb. Primary patency rates for nAVF were 72% at 6 months and 51% at 18 months, and for pAVF 58% and 33%, respectively. Secondary patency rates for nAVF were 86% and 77%, and for pAVF, 76% and 55%, respectively. It must be noted that there is significantly much greater patency in nAVF across all categories analysed (arm/forearm and primary/secondary patency).
To improve this patency, technical improvements, such as the inclusion of bioactive surface with heparin, have been introduced in the prostheses. So far, it has not been possible to demonstrate improvements in patency or in the need for fewer re-interventions.168,169
Prosthetic arteriovenous fistula indication
There is overall consensus among authors that nAVF are superior to pAVF,93 and this is reflected in the various clinical practice guidelines published.6,10,111 Thus, there is currently no controversy regarding pAVF indication in cases where venous capital in the patient has been exhausted and no other nAVF can be created.93,132,136
However, there is debate among authors related to the possible use of pAVF as the first choice in patients where the venous bed has not been exhausted.132,136,170
In recent years there has been a progressive increase in the average age of patients starting RRT with HD, and underlying pathology, which has meant there is a growing percentage of nAVF with impaired maturation and of nAVF which do not become functional.78 In some studies these have reached 60%.171 Inevitably, this results in a growing dependence on CVC in these patients, thus increasing the risk of sepsis and related complications.132
This has made several authors re-assess the suitability of prioritising nAVF in all cases over pAVF.78,132,136,170 They have also proposed assessing clinical situations in which a pAVF may be indicated as the technique of first choice, where the potential benefits of pAVF (shorter maturation time, lower rate of primary failure) would outweigh the advantages of nAVF (higher patency, lower rate of complications).
According to Sgroi et al.,136 clinical situations in which a pAVF would be the VA of first choice would be the absence of anatomically appropriate veins in the forearm or arm, a patient with end-stage kidney disease with limited life expectancy, the urgent need to start HD and patients with clinical risk factors for nAVF failure.
Urbanes132 recommends deciding on a case-by-case basis approach, and considering pAVF in cases of limited life expectancy, absence of suitable vessels in forearm and previous failed nAVF. He also considers the possibility of constructing “bridge” pAVF in patients with an urgent need for HD to avoid CVC placement.
Other authors170,172 propose an algorithm that decides between nAVF and pAVF based on the calculation of the likelihood of primary failure on the basis of three basic parameters: if HD has commenced, life expectancy of above or below 2 years, and a history of previous failed VA.
Fall-back techniques
As mentioned previously, once “conventional” VA have been exhausted, other fall-back VA may be performed. There is limited evidence available on the results of these techniques, so their role remains uncertain in VA choice in clinical practice.
Vascular access in the lower limb
The main recorded evidence comes from the systematic review carried out by Antoniou et al.173 in 2009. Patency and complications of the following types of AVF were assessed: pAVF in the upper thigh (inguinal region), pAVF in mid-thigh and nAVF with femoral vein transposition. These studies obtained acceptable results in terms of patency of these techniques, with a primary patency at 12 months of 48%, 43% and 83%; and a secondary patency at 12 months of 69%, 67% and 93%, respectively. The patency study found greater patency in the nAVF with femoral vein compared to the pAVF, with statistically significant differences, while there were none between both types of pAVF. Infection-related complications are described with more frequency in pAVF while femoral vein nAVF present the highest rate of ischaemia in the limb.
Other observational studies published compare the patency and complications of pAVF in the lower limbs with those created in the upper limbs. Miller et al.174 also show similar patency rates between both territories but with a higher incidence of primary failure and of infectious complications in lower limb VA. In turn, Harish and Allon175 report more serious infections arising from pAVF in these lower limbs.
Prosthesis-tunnelled catheter device
The first study published176 described a reduced incidence of infection compared to tunnelled CVC, obtained via a review of the literature conducted by the same authors (0.7 VA-associated bacteraemias per 1000 days versus 2.3 VA-associated bacteraemias per 1000 days).
Steerman et al.143 conducted a comparative study between this device and pAVF in the thigh, but found no differences in terms of secondary patency, infection and mortality rate. The main advantage of this device is therefore considered to lie in the use of the arm, which allows the thigh to be preserved for future accesses, and in possible use in patients with peripheral arterial disease.
Currently, the best available evidence refers to a meta-analysis published by Al Shakarchi et al.177 in which various published case series are referenced. Likewise, in two studies the results of this device are compared versus pAVF in lower limbs.143,178 Overall VA patency results described show a 1-year primary patency of 21.9% and a secondary patency of 59.4%, while in the comparison of pAVF there were no significant differences in patency. With regard to infection rate using the device, the authors report an incidence of 0.13 - 0.7 VA-associated bacteraemias per 1000 days, which is significantly better than the rates associated with CVC.177
“Exotic” vascular access
As mentioned previously, once “conventional” VA have been exhausted, fall-back VA may be constructed. These include pAVF in the anterior chest wall, central vein bypasses, lower limb vein bypasses, and other derivative techniques.
In all cases, the available evidence refers to the publication of case series.144-151 They all provide acceptable results considering that these are fall-back techniques, but there are no studies with a sufficient level of evidence showing what the first choice is in each case.
Assessment of the preferred vascular access in the elderly patient
As mentioned previously, the VA of choice is nAVF, due mainly to much higher primary, primary assisted and secondary patency rates than pAVF and CVC.93 Likewise, they have a lower complication incidence than other accesses, especially in terms of infections and thrombosis. In contrast, the major drawback of nAVF lies in their low maturation rate and in the lengthy period required for them to mature, especially in cases where secondary procedures are needed to induce them.
This high incidence of primary failure offered by nAVF, which in some studies reaches 60%,171 is considered its real Achilles’ heel. Moreover, it is even more pronounced in elderly patients, where there is an increased risk of primary failure (OR = 1.79) compared to non-elderly patients.64
Added to this is the low survival of this type of patient, due to their age and the frequent presence of significant comorbidities, with a mortality rate > 50% at 2 years for patients older than 75 when starting HD.179 A mortality rate of 30% is described in octogenarians even before they started RRT.180
In the light of these facts, it is the common opinion among several authors that the suitability of nAVF in the geriatric patient and/or with limited life expectancy should be reconsidered, as in these cases, attempting to start RRT through nAVF can lead to a greater dependency on CVC, with their associated complications.64,128,132,136,170,180
As a result, one of the major issues being debated today, due to the increased average age in the population in HD, is whether nAVF should be created in elderly patients, despite potential maturation issues, or pAVF, despite problems related to infection and medium-term patency. Direct placement of a tunnelled CVC is even considered in patients with limited life expectancy.
When performing a systematic search, no randomised controlled studies have been found regarding this issue. The best available evidence currently consists of a meta-analysis,64 a retrospective study with a cohort of patients from the United States Renal Data System,181 as well as several literature reviews and expert opinions.132,136,170
In a retrospective study of a cohort of 82,202 patients aged 70 and older when starting HD and whose data were collected in the United States Renal Data System, DeSilva et al.181 analysed the global mortality and survival of these patients. They found a lower mortality rate and better survival in patients who started HD with nAVF. He also highlights that pAVF results are better than CVC. Only in the group of patients over 90, although the trend described is maintained, the differences between nAVF and pAVF did not reach statistical significance. This leads to the consideration that, in general terms, nAVF is also valid as the VA of first choice for most elderly patients, even for those with comorbidities.
With regard to nAVF of choice in the elderly, the review with meta-analysis conducted by Lazarides et al.,64 based on retrospective cohort studies, finds a higher risk of failure for radiocephalic nAVF in elderly patients compared to younger patients. When comparing the results according to nAVF location, they notice a lower failure rate in brachiocephalic nAVF than in radiocephalic nAVF. They consider that the advantage of conserving proximal access sites for possible future accesses found in distal nAVF has minimal importance in patients with a short life expectancy. Therefore, the authors consider that brachiocephalic nAVF should be the first choice in elderly patients with short life expectancy or with a late start in HD. The main limitation of this study is the heterogeneous definition of old age, ranging between 50 and 70 years, depending on the study in question.
Finally, articles based on the literature review and expert opinion concur in considering a patient’s life expectancy as a main parameter rather than specific age as a criterion for the VA of choice. In this respect, they recommend pAVF in cases of patients with a life expectancy of less than 2 years, since this is the average accumulated patency for pAVF for HD.132,136,170
From evidence to recommendation
Preferred arteriovenous fistula location
Using the non-dominant limb
Although there are no studies in this respect, it is widespread practice to create nAVF as the first VA in the non-dominant limb, based on the reasonable assumption that the patient will prefer to have the dominant hand free during the HD session, and also because an AVF in the non-dominant limb will interfere less in daily activities.
Distal versus proximal location
As mentioned above, there are currently no studies that allow an unequivocal indication of which VA should be the first to be taken into consideration. Nevertheless, experts and guidelines unanimously agree on recommending the most distal AVF possible to preserve the option of future, more proximal VA if necessary.6,10,109,111 This broadly accepted criterion, based on good clinical practice, has prevailed in the recommendation put forward. However, clinical situations may occur in which other considerations could take priority (elderly patient, patients with short life expectancy in HD).
As a logical exception, in cases where the matured venous bed previously developed for a former, more distal AVF could be exploited to create a proximal AVF, the use of the aforementioned bed must be prioritised.
Vascular access of choice in the arm
After all nAVF options have been used up in the forearm, the next access to consider is the AVF in the arm/antecubital fossa. There are three conventional options: brachiocephalic nAVF, brachiobasilic nAVF or pAVF.
There is currently no discussion among authors on the suitability of nAVF (brachiocephalic AVF and brachiobasilic AVF) over pAVF, given their greater patency and their lower rate and severity of complications. However, there is currently a debate on specific cases in which pAVF may be a reasonable first indication. In accordance with the literature review and the majority opinion of the authors, GEMAV has decided to consider the recommendation to propose pAVF in cases of:
- 1.
Patients without anatomically appropriate veins in the arm or forearm.
- 2.
Patients requiring urgent HD (placement of immediate needling pAVF).
The first assumption is the main indication for pAVF, given the greater patency and the lower rate of complications versus CVC. For patients who require urgent HD without a mature nAVF, the indication of pAVF is restricted to those cases where the patient’s overall status does not allow for the consequences of potential CVC complications to be accepted. In this case, an immediate cannulation pAVF may be indicated, although the patient’s status should be carefully assessed, since pAVF placement without having used up the venous bed may lead to the early exhaustion of the limb’s veins.
Where life expectancy is short (as described above) some experts are of the opinion that an elective pAVF may be suitable. The choice should also be made carefully, as the available evidence does not allow for a minimum value of life expectancy from which to indicate pAVF to be established. In other words, the appropriateness of such a choice must be considered on a case-by-case basis. In any case, pAVF should not be indicated to the detriment of nAVF when life expectancy is over 2 years, as this is the average secondary patency of pAVF.
With regard to the convenience of prioritising brachiocephalic nAVF over brachiobasilic nAVF, the available evidence detects no significant differences in patency, so the decision to propose brachiocephalic AVF as first choice has been based on its lower surgical aggressiveness, greater comfort for the patient and the shorter maturation period required, especially when compared to those brachiobasilic AVF created with two-stage surgery.
Finally, concerning second-choice access in the arm (after brachiocephalic AVF), published studies are clear that there are better rates of primary and primary assisted patency for brachiobasilic AVF, as well as a lower incidence and severity of infections. Thus, although some groups have not documented differences in secondary patency and it takes longer to mature, the evidence recommends prioritising the use of brachiobasilic AVF over pAVF.
Fall-back techniques
After having exhausted access options in the forearm and arm, a fall-back VA can be considered as an alternative to tunnelled CVC. Except in the case of AVF in the thigh, the other techniques lack the casuistry to provide sufficient evidence to support their usefulness and safety in practice. Consequently, their use is recommended selectively, on a case-by-case basis.
Vascular access at the lower limbs
As discussed, AVF use in the thigh is a valid alternative to CVC, supported by the available evidence, with patency results comparable to pAVF in the upper limb.
From the three techniques described (transposition of femoral vein, prosthetic loop in groin and prosthetic loop mid-thigh), the transposition of the superficial femoral vein offers better patency at the expense of an increased risk of ischaemia and greater technical complexity, while the mid-thigh loop shows a non-significantly lower rate of infections in pAVF. In any case, as each technique has different advantages and disadvantages, no recommendation as to the technique of choice has generally been made; it is the patient’s clinical condition and individual preferences which advise their use.
Prosthesis-tunnelled catheter device
As this is a relatively new technique, there are no RCTs supporting its usefulness and safety. Existing evidence reports lower rates of complications than CVC. For this reason, its indication should be assessed after all AVF options have been exhausted prior to the catheter placement.
The only published meta-analysis to date describes rates of complications without significant differences compared to pAVF in the lower limbs, so it can be considered an alternative indication.
However, as with other fall-back techniques, there is currently insufficient evidence to be able to indicate its general use.
The order of sequence for creating VA in function of the location and type of VA is summarised in Figure 1.
Preferred vascular access in elderly patients
As mentioned in the evidence synthesis development, there is a debate on the VA of first choice in elderly patients. As a result, the high primary failure rate of autologous fistulae in wrist and the older patient’s limited life expectancy, the advisability of prioritising the use of pAVF over nAVF, and of nAVF in arm over nAVF in wrist, are being discussed.
A priori, pAVF is considered as a good option in these patients, since it has a low primary failure rate and drastically shortens the complex process of maturation. The disadvantage to be found in their worse patency rates and higher incidence of complications would be minimised because these are patients with low or very low life expectancy; for this reason, it has been included in the proposals put forward by several authors. Despite this, the studies which validate them have a small number of patients, and studies with a large number of elderly patients continue to confirm the benefits of nAVF across all age groups compared with pAVF and CVC, even in cases with significant comorbidities, with the possible exception of nonagenarian patients. For this reason, GEMAV believes it is important to put forward carefully thought-out indications for this group of patients, while highlighting that the main aim is still the need to achieve HD through nAVF, even in the advanced age group.
Available evidence on the possibility of considering VA in the arm from the outset confirms the worse prognosis for nAVF in the forearm compared to the general population. However, it is difficult and subjective to assess whether this justifies a general recommendation in this regard. In contrast, GEMAV suggests a careful assessment of the elderly patient, including ultrasound mapping, before deciding on the type of nAVF to be created. We consider there is insufficient evidence to be able to recommend constructing nAVF in the arm as a first option in all cases for this group, although in the same way we advise not creating AVF of dubious feasibility where possible, given the greater importance that morbidity/mortality associated with primary VA failure has in this group of patients. As already mentioned, ultrasound mapping is considered to be the most useful tool in this regard.
Finally, GEMAV considers that no time limit can be established to be able to classify patients as elderly. This is due, on the one hand, to the great heterogeneity of inclusion criteria in the main studies, which range from 50 to 90 years of age, and on the other, to the subordination of age criterion to life expectancy. The latter is the factor that will be most important when indicating VA.
Clinical question V. Recommendations
R 2.5.1) We recommend that a native arteriovenous fistula be created in the non-dominant upper limb, and as distal as possible, as the vascular access of first choice
R 2.5.2) After exhausting radiocephalic vascular access along the forearm, we recommend that a native arteriovenous fistula be created using the available veins in the elbow, a brachiocephalic or proximal radiocephalic fistula should be considered as the first alternative
R 2.5.3) If a radiocephalic or brachiocephalic native arteriovenous fistula cannot be performed, we recommend a brachiobasilic fistula with superficialisation or venous transposition in the arm or forearm as an option prior to the use of a prosthetic arteriovenous fistula
R 2.5.4) We recommend restricting the creation of a prosthetic arteriovenous fistula in the upper limb to the following criteria:
- A.
Patients without anatomically appropriate veins in the arm or forearm
- B.
Patients requiring immediate commencement of haemodialysis where a tunnelled central venous catheter placement is to be avoided
R 2.5.5) If a prior arteriovenous fistula has failed, we recommend that both physician and patient agree on the location of the following fistula to be created, in order to decide whether to give priority to distal location or to the non-dominant limb criterion
R 2.5.6) Where all vascular accesses have been exhausted in both upper limbs, we suggest using fall-back techniques, and that priority be given to prosthetic arteriovenous fistula in the thigh and the prosthesis tunnelled catheter device as first choice options
Recommendations
Rationale
Infection is one of the most significant complications associated with VA and in many cases leads to VA loss. Added to this, as these are superficial structures, infection of the surgical wound can lead to infection of the whole AVF relatively easily.
However, nAVF have a very low rate of peri-operative infection, so there is no evidence to justify systematic preoperative prophylaxis in these patients.
In contrast, a higher incidence and greater severity of infections is reported in pAVF, which in many cases necessitate their withdrawal in a patient who has very limited options for creating further VA. The micro-organisms that most often colonise or infect the pAVF are usually part of the cutaneous microbiota (staphylococci, streptococcus and corinebacterias), the most common being Staphylococcus aureus. For this reason, numerous studies advocate the pre-operative administration of prophylactic antibiotics, the most commonly accepted being a single dose of vancomycin.133,182
3Arteriovenous fistula careCONTENTS
- 3.1.
Care in the immediate post-operative period
- 3.2.
Care in the maturation period
- 3.3.
Use of the arteriovenous fistula
- 3.4.
Arteriovenous fistula care by the patient in the interdialytic period
- 3.5.
Antiplatelet treatment in arteriovenous fistula
Preamble
Arteriovenous fistula (AVF) care, both for native (nAVF) and prosthetic (pAVF), includes all the actions undertaken by the multidisciplinary team and the patients themselves, whose main aim is to achieve optimal development and appropr iately maintain a funct ioning arteriovenous access (VA). Care must begin in the immediate post-operative period, and continue during the maturation period and the whole time the AVF is used.
3.1Care in the immediate post-operative periodRecommendations
Care in the immediate post-operative period. Prevention and early diagnosis of complications
Strict monitoring of the patient with a newly-created AVF must allow for any possible complication that may arise to be prevented and diagnosed in the early stages and be treated appropriately. The main complications associated with VA creation include haemorrhage, seroma, infection, distal ischaemia, neuropathy and thrombosis.
In the operating theatre, once the AVF has been performed, before concluding the surgical procedure, the surgeon must check the presence of peripheral pulse and AVF function by palpating the thrill.183
A functioning AVF has a palpable thrill and an audible bruit on auscultation at the level of the anastomosis. If there is any doubt about functioning, a Doppler ultrasound (DU) can be performed183 to demonstrate its permeability. To this end, some authors have proposed intra-operative flowmetry.184 The absence of bruit at the end of the procedure in conjunction with end-diastolic velocity values under 24.5 cm/s, obtained by intraoperative DU, represent an effective predictive test for AVF thrombosis, which is better than the absence of thrill.185
It is important that the surgeon includes a clear diagram of the newly-created AVF in the patient’s medical record. The more information the nursing staff have about the AVF, the greater the likelihood of successful cannulation and improved VA patency.184
Most AVF can be created in major outpatient surgery without the need for hospital admission. During the time that the patient remains in the health centre, the AVF must be observed carefully in case any of the three major complications, i.e. bleeding, ischaemia and thrombosis, appear.
Care in the immediate post-operative period
- 1.
Monitor vital signs. Blood pressure (BP), heart rate, and body temperature should be checked. BP must never be taken in the arm with the AVF.183,186 The patient’s haemodynamic stability must always be maintained, minimising the risk of AFV thrombosis.183,186.
- 2.
Physical examination of the AVF (see section “Monitoring and surveillance of arteriovenous fistula”). The existence of bruit and thrill in the AVF should be checked in order to detect early failure and thrombosis. There are various pre-operative factors related to lower patency immediately after nAVF creation, associated with age over 65, female gender, diabetes, coronary disease and the patient’s peripheral vascular disease history, which are discussed in section 1 of this guide.58,185,187,188
In the case of pAVF, Monroy-Cuadros et al. observed lower patency in patients with the aforementioned clinical history and an access flow (QA) < 650 mL/min when starting needling.189 QA values < 500 mL/min in the nAVF represent an independent risk factor associated with lower primary patency.71
- 3.
Monitor the dressing for signs of bleeding. No compression dressings should be placed on the arm with the AVF.
- 4.
The limb with the arteriovenous fistula should be raised, resting on a pillow to promote venous return and prevent oedema.183,186
- 5.
Examine the limb where the AVF has been created and check the patient’s blood flow. Distal pulses of the AVF limb must be palpated and the capillary refill checked. Distal areas of the limb should be observed to rule out signs of ischaemia, such as the occurrence of pain, coldness, pallor and motor and sensory changes in the affected hand.
The distal hypoperfusion syndrome (steal syndrome) associated with AVF during the post-operative period is an uncommon but important complication. It is caused by a sudden drop in distal perfusion pressure, due to the occurrence of a preferred flow or diversion of arterial blood flow through the VA, causing symptomatic ischaemia in the affected limb. It occurs more frequently in arm nAVF with an incidence of between 1% and 20%, which is higher than in forearm or radiocephalic AVF85,87,87a,87b (see section “Complications of arteriovenous fistula”). Although less common, it may also be caused by an obstruction of the artery proximal to the anastomosis due to a technical failure.
If a distal pulse to the AVF is observed, a differential diagnosis should be made with ischaemic monomelic neuropathy (IMN). This is a neurological pathology that affects the three nerves in the forearm: the radial, ulnar and median nerve, without other signs that suggest arterial ischaemia. The main risk factors for steal syndrome and IMN are common (diabetes, female gender and brachial artery flow). In any case, the ischaemic hand of an AVF, whether due to arterial steal syndrome or the existence of IMN, may necessitate a revascularisation procedure or the complete ligation of the AVF.190
- 6.
Post-operative bleeding and/or haematoma should be checked (see section 5) and whether an immediate surgical review is required assessed. Although bleeding complications are uncommon, we should not forget that this is a surgical procedure which involves a vascular anastomosis and, therefore, it is important to check there is no haematoma in the surgical area which might necessitate an urgent review of the VA before discharge.191
Initial care during the outpatient follow-up
The first outpatient check-up should be carried about 7 days after the procedure. Depending on the status of the wound, the suture may be substituted by adhesive strips for some more days, or half of the stitches removed alternately. Antihypertensive medication should be reviewed and adjusted in order to avoid hypotensive episodes and minimise the risk of thrombosis of the AVF.191
This check-up should assess AVF patency and rule out the presence of complications. Skin and subcutaneous tissue should be examined to rule out any signs of infection, which can occur in between 1% and 5% of cases.192 If swelling, erythema, cellulitis or skin induration is observed, DU can help us to diagnose the specific existing pathology. Treatment of complications is discussed in section 5.
In the case of oedema in the AVF arm, venous hypertension should be ruled out. This complication occurs in 3% of patients and is usually associated with a central venous stenosis secondary to a previous CVC placement.190
Moreover, in pre-dialysis patients with ACKD, episodes of decompensated heart failure are not uncommon following nAVF creation. Up to 17% of cases of heart failure in patients with stage 4-5 CKD have been reported after AVF surgery related to an increase in cardiac output.10,193 It should be suspected when the AVF flow is > 2 L/min or ≥ 30% of the cardiac output.190,194 This is described in detail in section 5.
Medical and nursing staff are responsible for informing the patient about the characteristics of the AVF, its importance for their future haemodialysis (HD) treatment and the self-care that they should give their newly-created AVF (see self-care plan in point 3.4 of this section).191
3.2Care in the maturation periodRationale
Inadequate nAVF maturation may increase the incidence of complications associated with needling (haematoma, thrombosis) and reduce patency. In addition, when needling begins, a non-matured nAVF may require CVC placement in the incident patient in order to start the HD programme or CVC withdrawal should be delayed in the prevalent patient. Therefore, it is important to establish strategies that encourage the maturation process so that the nAVF can be cannulated at the right time.
Recommendations
- (•)
NEW R 3.2.1) We suggest that the patient do exercises before and after the creation of native arteriovenous fistulae to promote maturation
- (•)
NEW R 3.2.2) We recommend that cannulation of the native arteriovenous fistula not be initiated in the first two weeks following creation and that the optimal time for the first cannulation be decided on a case-by-case basis
- (•)
NEW R 3.2.3) We recommend that cannulation of the prosthetic arteriovenous fistula be initiated between 2 and 4 weeks following construction, except in those of immediate cannulation
→ Clinical question VI Are exercises useful for developing arteriovenous fistulae?
(See fact sheet for Clinical question VI in electronic appendices)
| Summary of evidence | |
| There are very few studies which offer data on the effectiveness of exercise for improving nAVF maturation or patency. In the existing clinical practice guidelines, only the KDOQI guidelines recommend dilation exercises in nAVF10 | |
| With regard to exercise prior to surgery, there are three observational studies with the participation of a small number of patients. The KDOQI Guide is based on two of these studies. They all show increased venous size | Very low quality |
| In terms of exercise following nAVF creation, a national randomised clinical trial has recently seen significantly higher maturation using clinical criteria but no difference in maturation criteria using DU | |
Evidence synthesis development
The study by Leaf et al.195 showed that the performance of a simple programme of exercises can cause a significant increase in cephalic vein diameter prior to the creation of the VA (n = 5). The diameter of the cephalic vein in the exercised arm increased significantly compared with the control arm when measured, both without (0.048 ± 0.016 versus 0.024 ± 0.023 cm2) and with a tourniquet (0.056 ± 0.022 versus 0.028 ± 0.027 cm2).
Order et al.196 analysed the impact of physical exercise in 20 patients prior to surgery. The mean change seen in the diameter of the nAVF was 0.051 cm or 9.3% (p < 0.0001).
The study by Uy et al.197 included 15 patients with small cephalic vein diameter (< 2.5 mm). After four weeks of exercise, the average diameter of the vein increased significantly, both proximally (1.66 to 2.13 mm) and distally (2.22 to 2.81 mm).
The prospective randomised study by Salimi et al.198 analysed the influence of a regulated scheme of exercises on nAVF maturation in 50 patients in a HD programme (25 patients in control group). Checks were performed by DU at 24 h and 2 weeks after AVF creation. Significant increases in the diameter of the efferent vein, wall thickness, venous area and QA were observed in the study group after exercise. Although there were no significant differences with regard to criteria of ultrasound maturation, significantly higher maturation was observed by clinical criteria. Its beneficial effects include the increase in venous diameter, as well as the increase in muscle mass and the decrease in the amount of fat tissue.
Fontseré et al.199 carried out a prospective randomised controlled study on the effect of a post-operative programme on nAVF maturation 1 month after creation in 69 patients with CKD in the pre-dialysis stage (65.2%) and in chronic HD programme. After 1 month, an assessment was made using criteria of adequate clinical maturation (specialist nursing staff) and ultrasound (QA > 500 mL/min, diameter > 5mm and depth < 6mm) in all patients. The rates of clinical and ultrasound maturation 1 month after nAVF construction were 88.4% and 78.3% respectively (Kappa coefficient = 0.539). The exercise group showed a non-significant trend towards better clinical and ultrasound maturation compared with the control group (94.7% versus 80.6%, p = 0.069; 81.6% versus 74.2%, p = 0.459). Logistic regression analysis identified nAVF location as a confounding factor so that, in distal nAVF, the exercise group showed significantly higher clinical maturation, but not in ultrasound (odds ratio [OR]: 5.861, 95% confidence interval [CI], 1.006-34.146, and OR: 2.403, 95% CI, 0.66-8.754, respectively).199
From evidence to recommendation
Although there are few studies on this subject, performing isometric exercises on the limb, before and/or after AVF construction, may promote the maturation process of the nAVF.
GEMAV suggests advising patients with ACKD to do exercises before and after nAVF creation in order to promote muscle and vascular development, and, consequently, to accelerate the maturation process, increase nAVF patency and development, and reduce morbidity associated with lack of maturation.
However, further clinical research is needed to analyse the advantages of doing exercises as a factor promoting the correct nAVF maturation process.
→ Clinical question VII What is the minimum maturation time required for a native or prosthetic arteriovenous fistula to be mature enough for needling?
(See fact sheet for Clinical question VII in electronic appendices)
Rationale
The maturation period for the VA is the time needed from the creation of the AVF until the moment when the first HD session can be carried out with the minimum risk of complications arising from needling. Although timing to begin needling is a controversial issue, both in nAVF and pAVF, it is accepted that excessively early use of any AVF may lead to a significant reduction in patency in relation to associated complications. Therefore, it is very important to determine the ideal time to initiate AVF cannulation.
The criteria for mature AVF are discussed in section 2. The lack of nAVF maturation has been associated with: a) insufficient arterial dilatation, present in patients with severe vascular disease and diabetes mellitus in the context of accelerated arteriosclerosis; b) deficiency in venous vasodilation secondary to the existence of collateral venous circulation; c) presence of a central stenosis, and d) development of accelerated neointimal hyperplasia secondary to juxta-anastomotic stenosis after the surgical procedure in areas of low tangential force.58,187,188
| Summary of evidence | |
| There are only two observational studies, which suggest that, while nAVF cannulation would not be advisable within 2 weeks of its creation, a first cannulation between 2 and 4 weeks may be considered following close clinical assessment without this necessarily increasing the risk of nAVF failure. In the case of pAVF, bearing in mind they are usually made of expanded polytetrafluoroethylene, cannulation is not recommended before 2 weeks due to the high risk of haematoma. From this date, pAVF needling should be started between 2 and 4 weeks after construction, except for immediate cannulation pAVF, once the subcutaneous tissue swelling has disappeared and the whole of its trajectory can be palpated without difficulty | Low quality |
Evidence synthesis development
There are only two observational studies that deal with this question.200,201 The first, based on data provided by the DOPPS study (Dialysis Outcomes and Practice Patterns Study), showed that the first nAVF puncture was performed within 2 months of construction in 36% of North American patients, 79% of European patients and 98% of Japanese patients.200 In the study by Rayner et al.,201 nAVF cannulation within 2 weeks after creation was associated with a significant decrease in patency, with a relative risk of 2.27 (p = 0.02). DOPPS studies200,201 suggest that, while nAVF cannulation is not recommended within 2 weeks of creation, first cannulation between 2 and 4 weeks afterwards may be considered following close clinical evaluation without it necessarily increasing the risk of nAVF failure.
In the case of pAVF, according to the data provided by the DOPPS study,200 needling starts between 2 and 4 weeks in 62% of North American patients, 61% of European patients and 42% of Japanese patients. No significant reductions in pAVF patency were observed in this study when cannulation started before 2 weeks or after 4 weeks following surgical placement, taking the 2-3-week subgroup as reference. However, needling a polytetrafluoroethylene pAVF within 2 weeks of its construction is not recommended due to the high risk of haematoma. Except those immediate cannulation pAVF, the remaining pAVF may usually be cannulated 2-4 weeks after construction once the subcutaneous oedema has disappeared and the graft can be easily palpated along its entire length.
From evidence to recommendation
In the case of nAVF, we recommend that needling not be started within the first 2 weeks after creation. From this date onwards, risks must be studied on a case-by-case basis in order to decide the ideal moment to perform the first cannulation.
For those patients with pAVF, we recommend that needling starts between two to four weeks after construction, except immediate cannulation pAVF. In this subgroup of patients, it is important to be familiar with the type of prosthetic material used.
Clinical question VII. Recommendations
R 3.2.2) We recommend that cannulation of the native arteriovenous fistula not be initiated in the first two weeks following creation and that the optimal time for the first cannulation be decided on a case-by-case basis
R 3.2.3) We recommend that cannulation of the prosthetic arteriovenous fistula be initiated between 2 and 4 weeks following construction, except in those of immediate cannulation
Recommendations
NEW R 3.3.1) We recommend that a complete physical examination of the arteriovenous fistula be performed in all advanced chronic kidney disease outpatient check-ups to assess degree of maturation and to detect any inter-current pathology before the first cannulation
NEWR 3.3.2) We recommend that Doppler ultrasound be performed if insufficient development of a native arteriovenous fistula is observed during physical examination in regular advanced chronic kidney disease outpatient check-ups
NEW R 3.3.3) We recommend that all universal asepsis measures be strictly adhered to during arteriovenous fistula cannulation to prevent the development of infections
- (•)
NEW R 3.3.4) We recommend that the rope ladder needling technique be used as the method for cannulating a prosthetic arteriovenous fistula
- (•)
NEW R 3.3.5) We recommend that the rope ladder technique be used as the preferred method for cannulating native arteriovenous fistula
- (•)
NEW R 3.3.6) We recommend that the buttonhole technique be reserved for cannulating tortuous or deep native arteriovenous fistulae, and/or those with an extremely short venous length
Rationale
A direct relationship has been described between a premature start to needling in nAVF and shorter patency.202,203 An AVF should only be cannulated when an optimal level of maturation has been reached. Therefore, nAVF must be monitored in all ACKD check-ups and, if insufficient development is observed, the nAVF must be examined using DU for diagnosis and the corrective treatment applied using percutaneous transluminal angioplasty (PTA) and/or surgery.
Basic examination to be performed prior to the first arteriovenous fistula cannulation
Physical examination is the most commonly used method for nAVF monitoring in the ACKD outpatient check-up to detect a deficiency in maturation and to attempt to identify its cause at the earliest possible moment. Different studies have shown that where this is done exhaustively, there is an increased diagnostic capability and an extraordinary cost-benefit relationship in the detection of significant stenosis and collateral venous circulation.204,205 The procedure for physical examinations is described in section 4.
DU is an essential tool in ACKD outpatient clinics and should be used both to perform pre-operative vascular mapping and to identify the cause of any post-surgical maturation deficit observed in physical examination. In the presence of any nAVF with insufficient clinical maturation, which is highly unlikely to be ready to use in the first HD session, GEMAV considers it necessary to carry out a DU to diagnose the precise cause of the lack of maturation. The objective is to repair any non-matured nAVF using an endovascular and/or surgical procedure in the pre-dialysis stage so that it can be cannulated in the first HD session.
Asepsis during arteriovenous fistula cannulation. Use of local anaesthetics
It must not be forgotten that AVF cannulation is an invasive procedure and, therefore, extreme care must be taken with asepsis measures. Before placing the sterile field and disinfecting the needling area, the arm or the needling area in the leg must be washed with soap and water, taking particular care where patients have used anaesthetic cream and if there are highly prominent aneurysms. Alcoholic chlorhexidine, alcohol 70% or povidone-iodine can be used to disinfect the area. The first takes effect after 30 seconds and lasts for up to 48 h. Alcohol has a shorter bacteriostatic effect and should be applied 1 min before needling. Povidone requires 2-3 min to fully develop its bacteriostatic capability. In an international survey conducted at 171 HD centres on 10,807 cannulations with two needles in patients mainly dialysed with nAVF (91%), an alcohol-based disinfectant was used for most cannulations (69.7%) and certain specific preferences were observed depending on the country: chlorhexidine in the United Kingdom, Ireland, Italy and South Africa, and povidone-iodine in Spain.206
Some patients with hypersensitivity to pain on needling the AVF may benefit from topical local anaesthetics. The most commonly used are the combination of lidocaine with prilocaine (cream) and ethyl chloride (spray) which need to be applied at least 1 hour before and 20 seconds before needling, respectively. In the same study by Gauly et al., the use of local anaesthetics was uncommon (overall, in 8.5% of cases), except in the United Kingdom, Ireland and Spain, where 29.4%, 31.7% and 27.2%, respectively, of cannulations were performed with their prior application.206
Characteristics of the dialysis needles
Types of needles
HD needles may have a sharp or a blunt tip. They have a silicone coating to facilitate insertion and reduce their resistance to QA.207 The bloodstream can be accessed through AVF via 2 needles with a different structure to carry out the HD session207: a) conventional stainless steel needle; this type of needle is the most commonly used, and b) catheter-fistula; made up of a polyurethane cannula and an internal metal needle designed to cannulate nAVF. Upon withdrawal of the needle, the cannula remains inside the arterialised vein for the entire HD session.208-210 This type of cannula can reduce pain both cannulating and removing the needle,209 besides decreasing the risk of extravasations and haematoma,208 especially in the case of nAVF in the elbow flexure in elderly patients.
Gauge and length of needles
HD prescription should be adapted to the needle type used.211 As a general rule, the smallest gauge and shortest needle which allows an adequate blood flow (QA) must always be chosen to suit the specific needs of each individual patient.212
With regard to needle gauge, these are available from 17 G up to 14 G, with the numbering being inverse to the gauge, i.e. a 17 G needle is the smallest and, on the other hand, a 14 G needle is the largest.213 After the first nAVF cannulations without complications, the choice of a higher-sized needle (lower number) depends on the diameter of the arterialised vein and the existing QA.213 In the study by Gauly et al., the needle gauge most commonly used was 15 G (61.3%), followed by 16 G in one-third of cases. 14 G and 17 G needles were used in less than 3% of cases.206
Moreover, the needle length chosen should be the shortest possible to reach the centre of the AVF lumen and thus reduce the risk of perforating the posterior wall.213
Only if we consider the relationship between a particular needle gauge, the maximum blood pump velocity and the duration of the HD session will we be able to adequately use nAVF without causing haematoma.211 Again, in the survey by Gauly et al., when larger needles were used (14 G) most patients were dialysed using a high pump flow (QB) (> 400 mL/min) and, on the other hand, when small needles were used (17 G) more than 80% of patients were dialysed with a QB ≤ 300 mL/min.206
Arterial needle backeye
In the study by Gauly et al., the arterial needle with backeye was used in most cases (65%).206 An arterial needle with backeye should always be used to maximise the flow aspirated through it and to prevent the adhesion of the bevel to the vessel wall due to negative pressure, which could cause damage.10,212
First cannulations of a new native arteriovenous fistula
Use the needle with the smallest gauge available (usually, 17 G)10. The selection of this “arterial” needle gauge ensures a sufficient blood flow to meet a demand for 200 mL/min from the blood flow pump of the HD machine and, simultaneously, minimises resulting haematoma if extravasation occurs during the HD session.213 Pre-pump arterial pressure monitoring (–250 mmHg or less) is recommended to ensure that the blood pump velocity does not exceed what the “arterial” needle can provide.10
Arteriovenous fistula cannulation. Methodology
Arteriovenous fistula cannulation
All professionals involved in a kidney patient’s care are aware of the existing difficulty in ensuring that the incident patient is dialysed through a mature nAVF from the very first HD session.214 Perhaps the main barrier lies in the maturation period but, undoubtedly, the final crucial hurdle that must be overcome for the nAVF to be usable for HD is its cannulation.214 Inadequate cannulation of the nAVF may require the placement of a CVC to carry out the first HD session and, therefore, all the previous work done in the pre-dialysis phase to achieve the best VA to start the HD programme will have been lost.
There is a relationship between cannulation practices (technique used, needle gauge, direction of the arterial needle), patient factors (age, comorbidity) and centre factors (QB, duration of the session), all of which may influence one of the key issues in any HD programme in a prevalent patient: the AVF patency.215,216 In this respect, Parisotto et al.,217 applying a Cox multivariate regression model on the results of an international survey on cannulation practices (n = 7058, majority nAVF), showed that AVF patency was significantly lower in the case of HD through pAVF, small-gauge needle (16 G), which may have been due to the endothelial damage caused by the increased velocity of the blood return, retrograde direction of the arterial needle and cannulation with the bevel down, QB < 300 mL/min, venous pressure < 100 mmHg (perhaps due to inflow stenosis) or progressively increasing pressure (perhaps due to outflow stenosis) and, finally, without compression of the arm at the time of cannulation or with compression using a tourniquet (versus compression of the arm by the patient). In addition, AVF patency was significantly greater if needling was performed using the rope ladder or the buttonhole technique compared to the area method.217
Repeated venous cannulation itself may damage the AVF due to direct trauma of the needle and/or to increased endothelial damage by shear forces created during blood return.207,211,212,218 These factors may stimulate the development of intimal hyperplasia, which could decrease patency of the AVF and, probably, also the survival of the patient.207,211,212,218 In this respect, the Frequent Hemodialysis Network Trial Group has conducted two controlled randomised trials: a) diurnal trial, comparing patients in in-centre HD during the day (6 days a week) and patients in conventional HD (3 days per week) for 1 year, and b) nocturnal trial, comparing patients in nocturnal home HD (6 nights per week) and patients in conventional HD (3 days per week) for 1 year.218 In both diurnal and nocturnal trials, the HD regimen of 6 times per week significantly increased the risk of AVF complications compared to the HD regimen performed 3 times per week. The authors concluded that frequent HD increases the risk of VA complications, largely because of the need for more repair procedures in patients with AVF. In other words, the more frequent nAVF use itself causes VA dysfunction.218
Cannulation practices are key factors in the process of AVF care and attention. An inadequate AVF cannulation technique could lead to short and long-term complications, such as infiltration-haematoma, infection, formation of aneurysms and pain at the cannulation site, resulting in situations of anxiety and fear in the patient, which often lead to a refusal to remove the CVC.207,211,212 These complications have a number of direct consequences, such as the need for additional needling, suboptimal or missed HD sessions, patient discomfort due to interruption of their regular treatment regimen and the need for longer sessions, the need to use CVC to bridge the gap between the creation and maturation of a new AVF, increase in hospital admissions and interventions, as well as higher HD treatment costs.207,211,212 These complications and their consequences can reduce VA patency and patient survival.207,211,212
Lee et al. analysed the risk factors and consequences of extravasations caused by needling the nAVF comparing 47 patients with a sufficiently significant nAVF infiltration to prolong CVC dependence for HD with 643 patients in the control group without nAVF infiltration.208 These authors showed that nAVF infiltration caused by needles is more common in elderly patients (aged 65 years or above) and those with recent nAVF (less than 6 months).208 In addition, as a result of these infiltrations, numerous diagnostic studies and interventions on the nAVF were carried out. There was a notable percentage of thrombosis (26%) and CVC dependence for HD was prolonged for more than 3 months.208 Finally, the financial impact of CVC-related bacteraemia, linked to the increase in the days of CVC dependence secondary to major nAVF infiltration, was estimated at US $ 8 million per year.208
Van Loon et al. published two prospective observational studies in 2009 (from the first cannulation until 6-months follow-up) on incident HD patients with nAVF and pAVF using the rope ladder technique.219,220 In most patients miscannulations (defined as the need to use more than one needle for the arterial and venous connection) were recorded between 1 and 10 times, being always greater the percentage of miscannulations for nAVF than for pAVF219. Although miscannulations were recorded on over 10 occasions for 37% of patients with nAVF and 19% of patients with pAVF, ultrasound-guided needling of the AVF was only used in 4% of patients.219 The percentage of patients with haematoma secondary to inadequate cannulation was always higher for nAVF than for pAVF, and it was higher for AVF in the arm than in the forearm.219 In the multiple regression model applied, complications associated with cannulation (need to use a CVC or carry out the HD session using single needle) were predictive of AVF thrombosis.219 In addition, these authors showed that these complications depend on the type of existing AVF so the percentage of AVF without complications was always significantly lower for pAVF than for nAVF.220
The use of the portable DU has been recommended in all HD Units in Spain for several years.216 There is no doubt that ultrasound guided cannulation is a tool of invaluable assistance for successful cannulation in difficult nAVF and, therefore, it can reduce errors in needling.221,222 In a national study covering 119 examinations using portable DU performed by the same nephrologist on 67 AVF, 31 previously unsuspected stenoses were identified in 44 cases where needling was difficult.223
Methodology of arteriovenous fistula cannulation
- •
The AVF must be used exclusively to carry out HD treatment.
- •
Cannulation of any AVF must be performed exclusively by specialised nursing staff in HD units who have demonstrated a high level of knowledge and specific skills.222
- •
The initial cannulation of all new AVF should be performed exclusively by experienced nursing staff members of the HD Unit.212,213,222,224,225
- •
All needling incidents should be recorded for investigation and for appropriate corrective measures to be adopted in order to ensure that the patient will receive the best nephrological care possible.213,225
- •
Multiple unsuccessful needling attempts made by the same cannulator represent unacceptable practice.213
- •
Prior to AVF cannulation, it is essential to know type, anatomy and the direction of QA in order to plan the location of the needling areas. For this purpose, it is extremely useful to have an AVF map in the patient’s medical record. All nursing staff needling an AVF for the first time should have prior knowledge of the map to correctly needle the area.
- •
Before starting each HD session, an exhaustive physical examination of the AVF is needed, as detailed in section 4.
- •
Needling should not be performed without first checking whether the AVF functions properly.225
- •
Needling must be avoided at all times in areas of redness or areas with signs of infection, in areas with haematoma, crusting or altered skin and in apical areas of aneurysms.
- •
In difficult or first cannulations, it is advisable to check for correct AVF cannulation using a syringe with physiological saline solution to avoid blood extravasations and the subsequent formation of a haematoma.
- •
Topography of the needles. The “venous” needle should always be inserted proximal to the “arterial” needle to avoid recirculation.
- •
Direction of the needles. The tip from the “venous” needle should always point in the same direction as QA (anterograde direction) to ensure optimal venous return.222 Whether the “arterial” needle tip should be oriented in the same direction (anterograde direction) or in the opposite direction to QA (retrograde direction) has been the subject of debate.213 In the Gauly et al. study, the “arterial” needle was placed in an anterograde direction in most cases (63%),206 but this situation does not necessarily increase the risk of recirculation as long as QA in the AVF is significantly higher than QB.222 According to recent data, anterograde direction of the “arterial” needle is associated with higher AVF patency217 as it leads to a lower turbulent QA and, probably, less intimal vascular damage.212
- •
Orientation of the needle bevel. In the Gauly et al. case series, the bevel pointed upwards in most cases (72.3%).206 Although the upward or downward orientation of the bevel has been associated with the degree of pain at the time of needling,226 it has recently been shown that the bevel-up orientation is associated with higher AVF patency.217
- •
Rotation of the needle (180º) at the time of cannulation. In the survey by Gauly et al., this manoeuvre was performed in around 50% of cases206 but nowadays it is discouraged since it enlarges the needle entry hole. It may also tear the body of the pAVF or damage the endothelium of the arterialised vein, and give rise to blood infiltrations in the lateral wall of the arterialised vein during the HD session.212,213 In addition, performing this rotation is unnecessary if backeye needles are used.10,213
Process of native arteriovenous fistula cannulation
- •
After preparing the skin, proximal compression (manual, tourniquet) should be performed to cause venous stasis, and to stretch the skin in the opposite direction from the cannulation in order to fix but not obliterate the arterialised vein. The vein should always be compressed even if it is very well developed and/or the buttonhole method is used.213,217 In the study by Parisotto et al., compression by the patient at the time of nAVF cannulation had a favourable effect on its patency compared with no compression or the use of a tourniquet.217
- •
Using the rope ladder method, the insertion angle of the needles in the nAVF must be approximately 25°, although this may vary according to the depth of the arterialised vein. The needles should be located at a distance of at least 2.5 cm from the anastomosis and should maintain a distance of at least 2.5 cm between their tips.207,212 In an international survey covering more than 10,000 cannulations with two needles in patients undergoing dialysis, mostly by nAVF (91%), the average distance between the two needles was 7.0 ± 3.7 cm and very similar to the distance recorded in a national study (7.3 ± 3.1 cm).206,227
Prosthetic arteriovenous fistula cannulation process
- •
The angle of insertion of the needles in pAVF should be approximately 45°, although this may vary depending on their depth. The needles should be located at a distance of at least 5 cm from the anastomosis and should maintain a distance of at least 2.5 cm between tips.207,212 Rotation of needling sites for each HD session is particularly important in pAVF and consequently new needling sites should be selected between 0.5 cm and 1.25 cm from the previous sites to preserve the fullest integrity of the pAVF wall.212
- •
Once the pAVF is needled, the angle must decrease to avoid needling the posterior wall, and it is then cannulated, making sure the tip of the needle is located in the centre of the pAVF lumen.
- •
The area should not be proximally compressed when needling.
Fixing the needles and haemodialysis blood lines
- •
Needles must be securely fixed on the skin of the limb to prevent accidental dislodgement and should remain visible for the entire treatment. The needle tip must be checked so that it does not damage the vessel wall.
- •
The lines can be fixed onto the VA limb. It is not recommended that they be attached to anything mobile (armchair, bed or pillow). The main aim is to prevent extravasations as the patient moves.
- •
Accidental needle dislodgement during HD session.228-230 This is a serious complication that may have catastrophic results.228 The reasons why needles may accidentally be dislodged are the following: poor fixation of the needles to the skin, defective adhesive tape, traction on any of the circuit lines or sudden movement of the AVF-bearing arm.230 To prevent needle dislodgement, the needles and blood lines must be properly secured with enough space so as to avoid dangerous traction.230 The limb must always be kept in view and, if necessary, kept still. If one of the needles is dislodged, the bleeding exit site must be immediately compressed, the blood pump stopped if this has not happened automatically, and the corresponding line clamped.230 The volume of blood lost must always be estimated and the patient’s haemodynamic stability must be checked.
→ Clinical question VIII What is the needling technique of choice for the different types of arteriovenous fistula: the 3 classical ones and self-cannulation?
(See fact sheet for Clinical question VIII in electronic appendices)
Rationale
Three different types of AVF cannulation techniques have been described.207,211-213,231
Rope ladder or rotating needling technique (sharp needle tip)
This is the needling method of choice for most patients. The needling sites are distributed regularly throughout the length of the arterialised vein for nAVF or pAVF body. In each HD session, two new sites are chosen for needle placement, thus allowing the skin to heal between HD sessions. With this technique, there is a moderate increase in diameter over the entire length of the arterialised vein with no or very little development of aneurysms (this avoids progressive weakening of the vein wall secondary to the blood return flow when this always occurs at the same point). The main problem is that it requires an arterialised vein which has a long enough trajectory to allow needling to rotate.
Area technique or needling circumscribed to the same area (sharp needle tip)
The main reasons for the use of this method are: short length of the arterialised vein, difficult trajectory for cannulation, nursing assessment that needling in another area will fail and patient refusal to be needled in another area. This technique involves repeated needling in a very restricted area of the arterialised vein, which causes damage to the venous wall and forms aneurysms in nAVF, as well as causing risk of pseudoaneurysms and thrombosis in pAVF. Therefore, this method is to be avoided whenever possible. However, the current situation in the “real world” is disappointing: according to an international survey mentioned above, the most commonly used technique (61%) was the area needling method.206
Buttonhole technique or constant needling at the same site (blunt needle tip)
This method must be used exclusively in nAVF and never in pAVF. The same hole is used to needle vessels in all HD sessions (the same entry into the skin, same angle of entry and same depth of entry into the vein). Following the creation of a subcutaneous tunnel of fibrous scar tissue, access to blood circulation is obtained with a blunt needle tip which eliminates the risk of internal tearing and bleeding.
The technique is based on repeatedly inserting a sharp needle tip into the same site and at the same angle of entry, preferably by a single cannulator, over six to ten HD sessions. This strategy allows a tunnel of fibrous scar tissue to be built up to the vein wall, which can then be cannulated with blunt-tipped needles. An arterial and a venous buttonhole are created. Once the tunnel is well formed, any trained member of the nursing staff or the patients themselves can needle the nAVF. In addition to the conventional sharp needle tip, the construction of the subcutaneous tunnel by other methods has been reported.232,233
It is very important to follow strict aseptic protocol. Before inserting the blunt-tipped needle into the subcutaneous tunnel, the two buttonhole sites must be carefully disinfected both before and after every HD session (double aseptic method), making sure that the scar crusts are completely removed from the previous session. The scab should never be removed with the same blunt needle which will subsequently be used for cannulation. Most blunt needles have a specially designed cap for removing the scab safely, without the need to use an additional needle and without damaging the walls of the hole.
All highly motivated patients with sufficient capability, treated in an HD unit or else in home HD, are offered the option to self-needle using the rope ladder method and, in some selected cases, using the buttonhole method.213,224
| Summary of evidence | |
| The evidence review was based on various observational studies and several randomised controlled trials (RCTs). The quality of evidence was low in the observational studies and moderate in the RCTs | |
| There are no differences in nAVF patency when comparing the rope ladder and buttonhole techniques | Low quality |
| Buttonhole is associated with lower rates of haematoma and formation of aneurysms compared with rope ladder | |
| Pain caused by needling is not significantly reduced by the buttonhole method | |
| Buttonhole is associated with a higher risk of local and systemic infectious events compared with rope ladder | Moderate quality |
| Buttonhole should be reserved only for needling in selected nAVF which present tortuosity and/or there is a short vein segment available for needling | |
Evidence synthesis development
The initial enthusiasm generated by the buttonhole method, which was even reflected in some clinical guides,13 has been curbed by the evidence which subsequently appeared.234,235 For example, regarding the degree of pain perceived by the patient using the buttonhole method, studies have been published which report less pain,236-238 greater pain239,240 and the same amount pain versus rope ladder technique.241,242 In other words, there is equivocal evidence regarding the degree of pain using the buttonhole method, so taking all combined observational studies into account, buttonhole method is associated with a significant reduction in pain but this benefit disappears when analysing the randomised controlled trials.235 Therefore, with the currently available evidence, we cannot state that the pain produced by needling is significantly reduced using the buttonhole method, either in in-centre HD or in home HD with self-cannulation.235
Van Loon et al. published a prospective observational study with a 9-month follow-up in 2010 comparing 145 prevalent patients on HD using rope ladder technique (n = 70) and buttonhole method (n = 75).240 Despite seeing a significantly greater number of miscannulations in the buttonhole group compared with the rope ladder group, the number of haematomas was significantly lower in the buttonhole group, probably because an unsuccessful cannulation with a blunt-tipped needle causes less tissue damage than a sharp-tipped needle.240 In addition, the buttonhole group required significantly fewer interventions on the nAVF at the expense of fewer PTA; no differences were noted in the number of thrombectomies and surgical procedures between both groups of patients.240 The formation of aneurysms was significantly lower in the buttonhole group but, on the other hand, this group of patients received antibiotic treatment for nAVF-related infection significantly more frequently.240 Finally, patients in the buttonhole group experienced significantly greater pain and fear compared to the rope ladder group, although the application of local anaesthetic cream was significantly more frequent in this latter group.240
MacRae et al. carried out a randomised controlled trial in 2012 comparing 140 prevalent patients on HD using the standard (rope ladder, n = 70) and buttonhole needling (n = 70) methods. There was no difference in the perception of pain on needling between both groups of patients241. In the same study, although haematoma was significantly higher in the standard group, the signs of local infection and episodes of bacteraemia were significantly higher for the buttonhole group; no differences were found in post-dialysis bleeding between both groups241. Finally, the degree of difficulty in needling by the nursing staff was significantly higher in the buttonhole group (for both the arterial and the venous needle) compared with standard needling from 4 weeks, which coincided with the use of the blunt needle by multiple nurses.241
Subsequently, in 2014 MacRae et al. published the follow-up results of these patients (17.2 months with standard and 19.2 months with buttonhole needling): no differences were found in nAVF patency between HD patients using standard (rope ladder, n = 69) and buttonhole technique (n = 70).243 However, the total number of infections, both local and Staphylococcus aureus bacteraemia, was significantly higher in the buttonhole group.243 They found no differences in thrombosis rates, fistulography, PTA and surgical procedure when comparing the two groups of patients.243 The conclusions of this RCT were that the lack of patency benefit in nAVF and the increased risk of infection should be taken into careful consideration when promoting buttonhole technique.243
In 2013 Vaux et al. carried out a prospective randomised clinical trial comparing 140 prevalent patients on HD using the standard method (n = 70) and buttonhole technique (n = 70) with 1 year follow-up. They showed a significantly higher nAVF patency rate, significantly fewer procedures to maintain nAVF function (due to a lower number of PTA in stenosis) and no episodes of nAVF-related bacteraemia in the buttonhole group.233 The beneficial effects of the buttonhole method seen in this study may be explained by the different methodology used in constructing the subcutaneous tunnel, as a polycarbonate peg was used as a tutor inserted into the tunnel between HD sessions during the tunnel creation stage using a sharp-tipped needle.233,241,244
Muir et al. conducted a retrospective review of 90 consecutive patients in home HD comparing rope ladder (n = 30) and buttonhole needling (n = 60). No difference was found between the two groups with regard to definitive nAVF loss or the need for surgical intervention (any surgical revision or event requiring the definitive loss of the nAVF and/or the creation of a new nAVF).245 However, the total number of infections was significantly lower for rope ladder compared to buttonhole: 0.10 versus 0.39 events per 1000 days of nAVF use, respectively245. In addition, these authors also conducted a systematic review of 15 studies (4 randomised controlled trials and 11 observational studies) and found that, compared to the rope ladder method, the risk of infection was approximately three times greater using the buttonhole method.245
The rate of total recorded infections in the group of patients on dialysis using buttonhole in the study by Muir et al.245 was very similar to the rate of CVC-related bacteraemia (0.40 episodes of bacteraemia/per 1000 days CVC) recorded in HD units with optimal CVC management.246 Therefore, one of the main benefits of nAVF compared to CVC, i.e. its low infection rate, is thrown into serious doubt when using the buttonhole method.244
This increased risk of local and systemic infection when using buttonhole has been confirmed in other studies and systematic reviews234,235,239,247,248 and calls into question the use of this method in routine clinical practice.245 Favourable results have been reported in the prevention of bacteraemia caused by Staphylococcus aureus via the application of topical mupirocin in each buttonhole after performing haemostasis.249 However, the fundamental cornerstone for reducing infectious episodes using this technique is the continuing education of nursing staff and/or the patient through periodic step-by-step review of the asepsis protocol used.248
In 2013, Grudzinski et al. carried out a systematic review of 23 full text articles and 4 abstracts on the buttonhole method: 3 were open-label trials and the rest were observational studies of different methodological design and quality.234 The main conclusions of these authors were as follows: a) there were no qualitative differences in the results obtained between home HD patients and those who were dialysed using this method in HD centres; b) studies which considered nAVF patency, hospital admission, quality of life, pain and the formation of aneurysms had serious methodological limitations with an impact on the analysis of the results considered; c) bacteraemia rates were generally higher when using buttonhole cannulation, and d) the buttonhole method may be associated with an increased risk of infection.234
More recently, Wong et al. published another systematic review of 23 articles, 5 randomised trials and 18 observational studies on the buttonhole method, in which they highlighted the following main aspects235: a) this method does not significantly reduce pain during cannulation and appears to be associated with an increased risk of local and systemic infections; b) considering nAVF patency, interventions in the nAVF, hospital admissions or nAVF-related mortality, haemostasis, and hospital admission or mortality for any other cause, there are no data that enable us to impose one needling technique over the other, and c) the buttonhole method is only beneficial in reducing the formation of haematomas and aneurysms. The final conclusion of these authors235 was that: a) the evidence does not support the preferred use of the buttonhole method over rope ladder, either in a conventional in-centre HD or in home HD, and b) the evidence does not exclude buttonhole cannulation as appropriate for some patients with nAVF which are difficult to cannulate.
Although experience is limited, there is a surgical placement device250 which allows deep nAVF, at a depth of up to 15 mm, to be needled using the buttonhole method without the need for surgical superficialisation. This is a funnel-shaped titanium guide sutured over the vein. Its use is also indicated in nAVF which have a very limited space for needling and are difficult to cannulate. This device can only be used with the buttonhole technique.
From evidence to recommendation
According to the scientific evidence reviewed, there are no conclusive data to recommend one cannulation technique for all HD patients. However, rope ladder technique has been proven to offer fewer complications in both nAVF and pAVF. Buttonhole technique results in terms of reduction in the number of aneurysms, duration of AVF, local and systemic infections, pain on needling and post-dialysis bleeding time vary from one study to another. These data reinforce the idea that it is a “centre and cannulator-dependent” needling technique. However, the incidence of infections reported in controlled studies contraindicates its systematic use in the AVF, and hence GEMAV considers that the buttonhole method should be reserved exclusively for selected nAVF with great tortuosity and/or a short vein segment available for needling.
Clinical question VIII. Recommendations
R 3.3.4) We recommend that the rope ladder needling technique be used as the method for cannulating a prosthetic arteriovenous fistula
R 3.3.5) We recommend that the rope ladder technique be used as the preferred method for cannulating native arteriovenous fistula
R 3.3.6) We recommend that the buttonhole technique be reserved for cannulating tortuous or deep native arteriovenous fistulae, and/or those with an extremely short venous length
Single needle cannulation
The single needle cannulation technique is occasionally used in routine clinical practice when nAVF cannulation with two needles is impossible. It is a transitory fall-back puncture technique in order to avoid CVC placement, when the arterialised vein only presents a very short segment for double needling, as there has been some kind of complication during cannulation and/or withdrawal of the needles (haematoma) in the preceding HD session, or to attempt to continue the development of an incomplete arterialised vein, especially in a brachial location. A Y-shaped dual-exit bevel 14G or 15G needle and a double pump system in the HD monitor are required. HD adequacy should be monitored strictly, increasing the surface of the dialyser and/or duration of the HD session if necessary.
Needle withdrawal
The technique used to withdraw needles is just as important as cannulation as it must protect the AVF, avoid any additional injury (tears) and facilitate appropriate haemostasis.207 Each needle should be removed at approximately the same angle as it was inserted.10 No pressure should be placed on the exit site until the needle has been completely withdrawn in order not to damage the AVF.207
At the time of cannulation, 2 holes are created for each needle: one that goes through the skin (external) and another through the arterialised vein wall of the nAVF or of the pAVF body (internal).207 Therefore, even though they are not on the same plane, both external and internal holes should be compressed after removing the needle to ensure that there is no bleeding.207 If the internal orifice is not adequately compressed, bleeding will occur in the subcutaneous tissue with subsequent haematoma development.207,251 As previously mentioned in AVF cannulation methodology,208 this haematoma may jeopardise the AVF as it may hinder subsequent cannulation, limit the options for future cannulations and cause thrombosis due to increased extrinsic pressure or the development of stenosis.207 Therefore, 2 fingers should always be used for haemostasis after removing the needle, one intended to compress the outer hole and the other the inner hole.207 During haemostasis, the pressure exerted must be constant, without interruption and intense enough to stop bleeding at the exit sites but without interrupting the QA in the AVF.207,251 To minimise the risk of re-bleeding through the “arterial” needle hole once haemostasis has been achieved at this point (due to a sudden backward increase in pressure inside the AVF secondary to compression of the “venous” needle hole), it is preferable to first remove the “venous” needle, carry out the corresponding haemostasis at this level and then remove the “arterial” needle.251
Manual compression must be maintained for at least 10 min before checking if there is still bleeding at the needling site.252 In general, the time of haemostasis is higher for pAVF than for nAVF.207 When there is no excessive anticoagulation, a prolonged haemostasis time (more than 20 min) may indicate increased pressure inside the AVF secondary to a stenosis as detailed in section 4.251-253 If there are problems of bleeding and/or patients with a prolonged bleeding time, haemostatic dressings may be effective.251 A transparent micro-perforated dressing, which significantly reduces haemostasis time in both “arterial” and “venous” holes compared with conventional manual compression, has recently been introduced.254
Haemostasis in the first needling sessions must always be carried out by an expert member of the nursing staff. Subsequently, if the patient characteristics and AVF allow it, the patient himself should be taught how to perform haemostasis with a non-sterile glove.251 If this is not possible, a staff member of the HD Unit must be responsible for haemostasis.251 Clamps should not be used to perform haemostasis on pAVF and their use is discouraged in nAVF.207 If their use is necessary, they should only be applied to a well-developed nAVF with an adequate QA and the continuing nAVF function should be continually checked while the clamp is placed.207
Adhesive dressings or bandages should be applied to needling sites but never before haemostasis has been fully achieved.252 The bandage should never cover the whole circumference of the limb.207 AVF patency should always be checked after applying the dressing.207 The patient will be instructed to remove the dressing 24 h after application.207
3.4Arteriovenous fistula care by the patient in the interdialytic periodRationale
The self-care plan involves fully training the patient to take all the actions needed to help maintain a correctly functioning AVF, prolong its patency and acquire the necessary habits to allow them to detect, avoid and prevent AVF complications.
Training the patient to look after the arteriovenous fistula
This section describes the AVF self-care plan from its creation and the steps to be taken in the interdialytic period.10,255
Monitoring arteriovenous fistula function
Where possible, depending on their characteristics, patients must be taught to perform a daily physical AVF examination as detailed in Figure 2 of section 4.
Detection of possible complications
- •
Signs and symptoms of infection such as redness patches/irritations, warmth, pain and suppuration.
- •
Signs and symptoms of ischaemia on the AVF-bearing arm such as coldness, pallor and pain.
- •
Signs and symptoms of thrombosis such as the appearance of hardening or pain, and absence of bruit and thrill.
- •
Signs and symptoms of decreased venous return such as the presence of oedema.
Local care
- •
From the first 24-48 h after AVF creation, gentle movements should be made with the fingers and arm of the AVF to promote blood circulation, but no brusque movements should be made when doing the exercises as they are likely to lead to bleeding from the wound or hinder venous return. In elbow nAVF and in pAVF created in the flexure, the arm must not be flexed.
- •
The dressing should be kept clean and dry at all times and changed if dirty or wet.
- •
In these early stages, situations that may contaminate the surgical wound are to be avoided and, if necessary, adequate protective measures should be taken (work in the countryside, work with animals.).
- •
After the surgical stitches have been removed, the whole arm of the AVF should be thoroughly cleansed with warm water and soap on a daily basis. Skin should be kept hydrated to prevent the appearance of wounds.
- •
When the patient has started HD therapy, the dressing covering the needling sites must be removed the day after the HD session. If the dressing is stuck to the skin, it is advisable to wet it with saline solution to prevent any injury which might lead to bleeding or infection of the AVF. The scab covering the wound must never be lifted.
- •
If bleeding occurs through the needling hole in the skin, a gauze should be applied and compressed gently with the fingers as in the HD session. If bleeding does not stop in a reasonable amount of time, the patient should attend a healthcare facility for assessment. A circular compression bandage should never be used.
Acquiring certain habits in order to preserve arteriovenous fistula function
- •
Blood pressure must not be taken or venipunctures be performed on the same arm as the AVF.
- •
The AVF must not be knocked or compressed. Tight clothing, watches, bracelets and occlusive bandages should not be worn and the patient should not sleep on the arm of the AVF.
- •
Weights must not be lifted or brusque movements made during exercise with this arm.
- •
Sudden changes of temperature must be avoided.
If complications are detected, the nearest medical centre of reference must be contacted.
3.5Antiplatelet treatment in arteriovenous fistulaRecommendations
- (•)
NEW R 3.5.1) We suggest that antiplatelet therapy for thrombosis prophylaxis of native arteriovenous fistula be indicated on a case-by-case basis, because although it shows a decrease in the risk of thrombosis, we consider that adverse effects have not been studied with sufficient accuracy
- (•)
NEW R 3.5.2) We suggest that antithrombotic prophylaxis not be used in patients with prosthetic arteriovenous fistula, because there is no benefit in preventing thrombosis and adverse effects have not been studied with sufficient accuracy
Rationale
AVF failure may be early or late. Early AVF failure is common, with an incidence of 9 to 53%.94,256 Late failure is associated with acquired stenosis in the arterial and mainly venous territory. The physiopathology of the failure is not at all well-defined, but it has been associated with different triggers that initially cause a stenosis that can lead to thrombosis and VA loss.257 Thrombosis is therefore the common factor in both early and late failure.
There are vascular diseases where the territory affected by a thrombosis has severe clinical repercussions, such as coronary or cerebral arteries.258 As antithrombotic medication may be beneficial in these diseases, for this reason it has been suggested that it could also reduce AVF thrombosis and, therefore, VA loss.
The first time this type of drug was proposed for VA thrombosis prevention was with the Scribner cannula in 1967.259 This cannula connected vessels in the wrist (radial artery and cephalic vein or ulnar artery and basilic vein) or in the lower third of the leg (posterior tibial and internal saphenous), through a permanently installed external bridge made of synthetic material (external AVF), so that the artificial kidney might be connected as often as necessary. Since then the result of the use of antiplatelet agents to reduce AVF failure has not been conclusive. Salicylates have been linked to a decrease in early failure, but observational studies such as the DOPPS found no increase in the proportion of usable AVF for HD.260 A clinical trial comparing clopidogrel with placebo demonstrated a reduction in early thrombosis in incident AVF but the proportion of AVF useful for HD did not change.171 Moreover, another DOPPS review found a lower risk of AVF failure in patients taking acetylsalicylic acid for at least one year261 and a meta-analysis including studies with the short-term use of different antiplatelet drugs also demonstrated a reduction in thrombosis in nAVF and pAVF.262
However, the follow-up period of these studies is limited, usually less than one year, and they fail to clearly show the benefit on patency without showing an increased risk of bleeding.
The HD patient presents a higher risk of bleeding as a result of multiple factors, including platelet dysfunction, anaemia or heparin use during HD. Added to this is the uncertainty of the extra risk due to the use of antiplatelet agents or oral anticoagulants, knowing that bleeding risk scores developed for the general population have not been validated for patients on HD.
This greater tendency towards haemorrhage has been observed in one of the DOPPS study reviews in patients with specific antiplatelet indications, such as rhythm disorders, in which the use of both antiplatelet agents and anticoagulants was associated with an elevated risk of mortality, both from cardiovascular and all-cause mortality.263 In a retrospective study that included a 5-year follow-up of 41,000 patients, there was also a higher association with a higher mortality with antiplatelet or anticoagulants in HD patients, although the confounding factor of the treatment indication could not be totally ruled out.264 When studies assessing the risk of bleeding are analysed using the results of antiplatelet therapy in AVF patency, these are limited and without conclusive results. Although appearing to show a decrease in the risk of thrombosis in nAVF and not in pAVF, a systematic review assessing the risk of bleeding in HD patients cannot find agreement on an indication in antiplatelet therapy in the presence of increased risk of bleeding in kidney patients.265
It is therefore considered necessary to assess whether antithrombotic therapy can be indicated in the prevention of AVF dysfunction.
→ Clinical question IXa In which situations is it necessary to indicate antithrombotic prophylaxis after creating/repairing the arteriovenous fistula?
(See fact sheet for Clinical question IXa in electronic appendices)
→ Clinical question IXb Does the use of antiplatelet agents prior to arteriovenous fistula creation have an impact on patency and reduce the risk of thrombosis?
(See fact sheet for Clinical question IXb in electronic appendices)
| Summary of evidence | |
| In HD patients with nAVF, treatment with antiplatelet agents after surgery and for 6 months subsequently reduces the risk of failure (due to thrombosis and loss of patency) and is not accompanied by negative effects in other outcome measures | Moderate quality |
| In patients pending AVF creation as the VA, antithrombotic prophylaxis prior to surgery and extended from four to six weeks post-surgery reduces the risk of fistula failure (due to thrombosis or loss of patency) and is not accompanied by negative effects in other outcome measures | Low quality |
| In HD patients with pAVF, treatment with antiplatelet drugs shows no effect on the prevention of thrombosis, maintenance of VA patency or on any of the outcomes of interest | Moderate quality |
| In patients pending pAVF creation, antithrombotic prophylaxis before and for several weeks after does not show a positive effect on any of the outcome measurements | Low quality |
Evidence synthesis development
In which situations is it necessary to indicate antithrombotic prophylaxis after creating/repairing the arteriovenous fistula?
The systematic review of Palmer et al.266 (arising from the Cochrane review of Palmer et al.267) analyses the effect of antiplatelet therapy on the rate of thrombosis and patency of the VA in HD patients, including both nAVF and pAVF. In 12 trials (with 3118 participants), antiplatelet therapy started at the time of surgery; in 6 trials, 1-2 days before; in 2 trials, 7-10 days before; in 2 trials, 1-2 days after; in 1 trial 1 month after, and was not specified in another. The median of intervention was 3 months (interquartile range, 1.25-6). Ticlopidine, acetylsalicylic acid and clopidogrel were the most commonly used antiplatelet drugs.
Limitation: risk of high or unclear bias in most trials and limited data for analysis of some effects, especially in pAVF and VA adequacy for HD.
Results
- •
AVF failure due to thrombosis or loss of patency. Antiplatelet therapy reduced the thrombosis or loss of patency in nAVF to half (6 trials, 188 events, 1242 participants; relative risk [RR]: 0.49; 95% CI, 0.30-0.81; I2 = 29%). In absolute terms, the treatment of 100 individuals with antiplatelet agents for 1-6 months (acetylsalicylic acid, ticlopidine or clopidogrel) would prevent failure of the fistula in between 6 and 21 individuals, assuming a baseline risk of 30% of one or more events.
However, antiplatelet therapy had little or no effect on pAVF thrombosis or patency (3 trials, 374 events, 956 participants; RR: 0.94; 95% CI, 0.80-1.10).
- •
AVF failure due to thrombosis or early loss of patency. VA failure was assessed in 5 trials (1105 participants) in the 8 weeks after surgery. In this subgroup, treatment with antiplatelet drugs significantly reduced early thrombosis or failure in AVF patency in 57% compared with placebo treatment or no treatment (177 events, RR: 0.43; 95% CI, 0.26-0.73; I2 = 25%). There were no data in the review on patients with pAVF.
- •
Failure to achieve a suitable VA for HD. The effect of antiplatelet therapy on the adequacy of VA for HD was investigated in 5 trials (1503 participants). The differences were not statistically significant, either in nAVF (2 trials, 470 events, 794 participants; RR: 0.57; 95% CI, 0.13-2.51) or in pAVF (1 trial, 12 events, 649 participants; RR: 0.51; 95% CI, 0.16-1.68).
- •
Need for intervention to maintain AVF patency or maturation. There were no statistically significant differences in the need for intervention to maintain patency or maturation of the AVF, in nAVF (1 study, 17 events, 866 participants; RR: 0.69; 95% CI, 0.26-1.83) or in pAVF (1 study, 196 events, 649 participants; RR: 0.89; 95% CI, 0.64-1.25).
- •
Risk of bleeding. Information is provided on bleeding events in 10 trials (3930 participants). There were no statistically significant differences in severe bleeding—retroperitoneal, intraocular, intra-articular, cerebral or gastrointestinal—(10 studies, 3930 participants; RR: 0.93; 95% CI, 0.58-1.49) or minor bleeding (4 studies, 237 participants; RR: 1.22; 95% CI, 0.51-2.91).
- •
Abandonment of treatment. There were no statistically significant differences in abandoning treatment compared with the control group (8 studies, 1973 participants; RR: 1.01; 95% CI, 0.84-1.20).
- •
Antithrombotic prophylaxis after VA repair. No studies have been found that analyse the effects of antithrombotic prophylaxis after VA repair.
Does the use of antiplatelet agents prior to arteriovenous fistula creation have an impact on patency and reduce the risk of thrombosis?
No studies were found on the use of antiplatelet agents prior to VA creation and the impact it has on patency and the risk of thrombosis in the publications by Palmer et al.,266,267 so there is no comparison of antiplatelet use prior to or post VA creation, or prior and post versus only post VA creation. Since the studies found analyse peri-operative, i.e. both prior to and post, treatment in all cases, the evidence available is considered to be indirect.
Results
- •
AVF failure (due to thrombosis or loss of patency). Reviews provide information on 5 RCTs in which antithrombotic therapy begins before AVF creation and continues for up to four or six weeks after creation; however, there is a great deal of variability in the number of days the drug is received prior to the operation in each study. In one study, the drugs were given one day before and for 28 days after creation; in another, 2 days before and for one month afterwards; in another two, 7 days before and then for 28 days; in yet another, 7 to 10 days before and then for 6 weeks. This meta-analysis also includes another study in which drugs are administered on day 1 of the operation and continue for 6 weeks.
Antiplatelet therapy reduced the risk of thrombosis or patency failure by almost 50% (6 trials, 218 events, 1365 participants; RR: 0.54; 95% CI, 0.39 to 0.74; I2 =10%).
- •
Early thrombosis of the VA (within 8 weeks) in AVF. Antiplatelet therapy reduced the risk of early VA thrombosis by close to half (6 trials, 218 events, 1365 participants; RR: 0.54; CI: 95%, 0.39 to 0.74; I2 = 10%).
There were no significant differences between treatments relating to: all-cause mortality; cardiovascular-related mortality; fatal or non-fatal infarcts; fatal or non-fatal strokes; minor, major or fatal bleeding; loss of primary patency; need to perform any intervention to maintain patency and hospital admission.
- •
pAVF. No differences were found between treatments for any outcome measure in patients who undergo VA creation using pAVF.
- •
pAVF failure (due to thrombosis or loss of patency). No significant differences were found between treatments (2 trials, 266 events, 756 participants; RR, 0.94; 95% CI, 0.79 to 1.11; I2 = 0%).
In a systematic Cochrane review on the use of medical treatment to improve nAVF and pAVF patency,268 the antiplatelet ticlopidine showed a significant reduction in the risk of failure of nAVF due to thrombosis compared with placebo, which in relative terms was 48% (3 clinical trials, 339 participants; OR: 0.45; 95% CI, 0.25 to 0.82). No significant differences were seen when comparing other treatments such as acetylsalicylic acid, clopidogrel or warfarin with placebo. According to the authors of the review, the quality of evidence was low due to the limited follow-up of the studies and the low availability of studies to test the efficacy of the treatment.
From evidence to recommendation
VA thrombosis is the consequence of both early and late failure leading to the loss of the vascular access. Based on other vascular territories, where antiplatelet therapy is effective in reducing risk of thrombosis, it has been proposed that this benefit might even be applied to improve AVF patency. However, patients on HD present a greater risk of multifactorial component bleeding, meaning the introduction of antiplatelet therapy could potentially increase this risk.
The evidence review shows that in HD patients with nAVF antiplatelet therapy reduces the risk of thrombosis, and there are no differences in the effects on maturation and use of the nAVF for HD. It must be noted that bleeding risk analysis gives uncertain results. The authors point out that not every adverse effect was reported accurately, because the number of events identified in both groups was limited. In addition, episodes of serious haemorrhaging events were defined a priori and systematically described in only 2 out of 21 trials. Therefore, GEMAV interpreted that the use of antiplatelet therapy should be studied on a case-by-case basis, due to the potential side effects in this population.
On the other hand, in HD patients with pAVF, antiplatelet treatment is not effective in preventing thrombosis or maintaining VA patency.
Clinical question IX. Recommendations
R 3.5.1) We suggest that antiplatelet therapy for thrombosis prophylaxis of native arteriovenous fistula be indicated on a case-by-case basis, because although it shows a decrease in the risk of thrombosis, we consider that adverse effects have not been studied with sufficient accuracy
R 3.5.2) We suggest that antithrombotic prophylaxis not be used in patients with prosthetic arteriovenous fistula, because there is no benefit in preventing thrombosis and adverse effects have not been studied with sufficient accuracy
CONTENTS
- 4.1.
Rationale
- 4.2.
Clinical monitoring
- 4.3.
Monitoring and surveillance of arteriovenous fistula pressure
- 4.4.
Recirculation of arteriovenous fistula
- 4.5.
Unexplained decrease in haemodialysis adequacy
- 4.6.
Dilution screening methods for indirect determination of arteriovenous fistula flow
- 4.7.
Imaging tests. Arteriovenous fistula surveillance using Doppler ultrasound
- 4.8.
Predictive power of first- and second-generation methods for detecting stenosis and thrombosis of arteriovenous fistula
- 4.9.
Predictive factors of thrombosis in arteriovenous fistula with stenosis
Preamble
The aim of monitoring and surveillance of the arteriovenous fistula (AVF) is early diagnosis of its pathology both in native (nAVF) and prosthetic (pAVF). The AVF follow-up should permit the prevention of thrombosis through early detection of significant stenosis and increase its patency.
4.1RationaleRecommendations
R 4.1.1) We recommend that haemodialysis units have protocolised programmes for arteriovenous fistula follow-up, involving multidisciplinary participation. These programmes should include methods for early diagnosis of arteriovenous fistula dysfunction and locate its origin, as well as performing the elective treatment
R 4.1.2) We recommend that the application of programmes for arteriovenous fistula follow-up must involve periodic assessment of the parameters obtained by each monitoring and/or surveillance method applied
R 4.1.3) We recommend that the repeated alteration of any monitoring and/or surveillance parameter be used as a criterion to perform an imaging examination of the arteriovenous fistula in front of suspected pathology
Several obstacles have to be overcome to obtain a valid AVF which can be used to start a chronic haemodialysis (HD) programme.214 The biggest of them is to achieve an adequate maturation, particularly in the case of nAVF, since the current percentage of maturation failures is about 40%.214 Once this difficult objective has been achieved, we must remain in a state of alertness and use all available means at our disposal to prevent thrombosis and to maintain AVF patency in the prevalent patient.
Irreversible thrombosis of the AVF results in a series of negative consequences for the prevalent patient included on an HD programme269: reduction in venous capital, need for central venous catheter (CVC) placement, lower HD efficacy, possible central vein stenosis or thrombosis, chronic inflammation in the case of pAVF, and construction of a new AVF. All of this increases the frequency of hospital admission, morbidity and mortality and healthcare spending for the chronic HD patient.270 Therefore, preventing thrombosis of the AVF is paramount for these patients.
Regarding AVF thrombosis, it must be taken into account that:
- •
It is not always technically possible to restore AVF patency in all cases of thrombosis of the AVF, even in the hands of experienced specialists.271
- •
Secondary AVF patency is significantly lower after restoring AVF patency post-thrombosis when compared with elective repair of AVF stenosis before thrombosis (see section 5 “Complications of arteriovenous fistula”, recommendation 5.2.6).272,273
Therefore, it is very important to note that thrombosis should preferably be prevented through early diagnosis and treatment of significant stenosis rather than be salvaged via interventional radiology or vascular surgery.
The most common cause of thrombosis is severe stenosis of the AVF.10,253 Currently, in order to qualify a stenosis as significant, it is necessary to demonstrate a reduction in the vascular lumen greater than 50% using ultrasound and/or angiography together with the repeated alteration of one or several parameters obtained by monitoring and/or surveillance methods.10 The diagnosis of significant stenosis is an indication that corrective treatment by percutaneous transluminal angioplasty (PTA) and/or surgery should be performed electively or preventatively to avoid thrombosis.10
AVF follow-up programmes comprise two key aspects: a) the early diagnosis of significant stenosis using different screening methods or techniques, and b) its elective or preventive correction to prevent thrombosis and improve AVF patency.10
The philosophy of these programmes is based on the fact that, in the vast majority of cases, AVF stenosis develops over varying time intervals and, if diagnosed and corrected in time, sub-dialysis can be avoided and the rate of thrombosis reduced by between 40% and 75%.10,274 These follow-up programmes should be developed in every HD unit systematically, protocolised and with multidisciplinary participation involving nursing staff, nephrology, radiology and vascular surgery.253Table 8 shows the AVF follow-up programme objectives for both nAVF and pAVF.275-277
Theoretical aims for arteriovenous fistula (AVF) follow-up programmes, for both native and prosthetic fistula
|
According to DOPPS (Dialysis Outcomes and Practice Patterns Study I and II, 1996-2004) study data, the likelihood that a prevalent patient be dialysed through a CVC is directly related to the number of permanent AVF previously placed.32 It is likely that if AVF follow-up programmes had previously been introduced in these DOPPS centres, many cases of thrombosis could have been avoided; therefore, the prevalence of patients on HD with CVC would have been reduced.32 In this respect, an inverse relationship between the rate of preventive intervention and the rate of AVF thrombosis has been demonstrated in Spain for both nAVF and pAVF.278
The screening methods or techniques for the early diagnosis of significant stenosis are classified in 2 major groups279 (Table 9):
- 1.
First-generation methods.
- •
Clinical monitoring:
- –
Physical examination.
- –
Problems during the HD session.
- –
Blood pump (QB) stress test for nAVF.
- –
- •
Monitoring and surveillance of AVF pressure: dynamic venous pressure (DVP), intra-access pressure (IAP) in its normalised static version.
- •
Determining the percentage of recirculation.
- •
Unexplained decrease in HD adequacy: Kt/V index, urea reduction ratio (URR), Kt index.
- •
- 2.
Second-generation methods. These allow the calculation of AVF blood flow (QA).
- •
Dilution screening methods.
- •
Doppler ultrasound (DU).
- •
Monitoring and surveillance techniques of the arteriovenous fistula (AVF)
| I. First-generation methods |
|
| II. Second-generation methods |
They allow the non-invasive estimation of the QA in two ways:
|
HD, haemodialysis; Kt/V and Kt, dialysis index—K, dialyser clearance; t, duration time; V, urea distribution volume—; nAVF, native arteriovenous fistula; QA, blood flow; QB, pump flow; URR, urea reduction ratio. *Surveillance methods.
In addition, these techniques can also be classified as “monitoring methods” and “surveillance methods” depending on whether special instrumentation is not or is required, respectively. All first-generation methods, except static venous pressure, fall within monitoring methods.274 Static venous pressure (see section 4.3.2.) and second-generation methods are considered to be surveillance methods. With regard to periodicity of determination, although these methods should be applied monthly,10 it is acceptable to measure QA in the nAVF every 2-3 months.14,15
Regarding the different monitoring and surveillance techniques used, it is important to consider that:
- •
The prospective analysis of any monitoring or surveillance parameter used has greater predictive power to detect AVF dysfunction than any isolated values.10 In this respect, it is essential to have a record of each AVF in the HD unit to allow it to be assessed over time.
- •
They are not exclusive but complementary. The application of several monitoring or surveillance methods simultaneously increases the performance of the follow-up programme.280,281 In addition, it has been demonstrated that the precision of each AVF monitoring and surveillance technique is related to stenosis location.282
Many of the screening methods described, both first- and second-generation, can be used to non-invasively assess the functional outcome of elective procedure performed on the AVF stenosis.282-284 In this regard, QA measurement has also been used in situ immediately after performing PTA on the AVF stenosis to check the functional outcome of the elective treatment.285
4.2Clinical monitoringAlthough clinical monitoring lost a certain amount of importance when dilution methods were introduced for non-invasive QA determination and DU use became more widespread, its central role in AVF monitoring is currently undisputed.282,286,287 The AVF’s clinical monitoring takes into account two key aspects253,270,282,286-293: physical examination and problems during the HD session. The stress test for nAVF according to QB (QB stress test) has recently been described, and seems to be effective in diagnosing so-called inflow stenosis.294
4.2.1 Physical examination
This should be carried out regularly using inspection, palpation and auscultation10,282,286,293 (Table 10). This is an easy method to learn and perform; it takes very little time, does not require any special instrumentation or additional staff and, therefore, is a low cost test. In addition to nursing staff and the nephrologist, it is advisable that this examination should be partially carried out by the patients themselves daily (Figure 2).295 The detection of changes in the characteristics of the pulse, bruit and thrill of the AVF, compared with prior checks, makes it possible to diagnose a stenosis and specify its location.282,286,293 Unlike other AVF follow-up methods, physical examination also allows the identification of pathologies other than stenosis, such as aneurysms or infection.286
Systematics of physical examination of arteriovenous fistula (AVF)
| Inspection |
|
| Palpation |
|
| Auscultation |
|
CVC, central venous catheter. Modified from references 293-300.
Table 11 summarises the findings obtained by physical examination for the differential diagnosis between inflow stenosis (located in the feeding artery, in the anastomosis itself or in the initial segment of the arterialised vein up to 5 cm post-anastomosis), outflow stenosis (located in the arterialised vein segment from the needling area to the right atrium) and nAVF thrombosis. Central venous stenosis is an outflow stenosis which is located in the venous segment from the cephalic vein arch at the level of the first rib to the right atrium. The nAVF without stenosis has a smooth or soft, easily compressible pulse, a predominant thrill over the anastomosis and a continuous bruit (systolic and diastolic) of low intensity (Table 11).
Differential diagnosis between inflow stenosis, outflow stenosis, central venous stenosis and thrombosis according to the data obtained from physical examination
| Normal | Inflow stenosis | Outflow stenosis | Central venous stenosis | Thrombosis | |
|---|---|---|---|---|---|
| Inspection | Normal arterialised vein | Poorly defined arterialised vein | Distended | Oedema | Hyperaemia can be visualised on the thrombosed area |
| Excessive collapse with arm elevation | Absence of collapse with arm elevation | Proximal collateral veins | |||
| Absence of collapse with arm elevation | |||||
| Palpation: pulse | Soft and easily compressible | Reduced | Increased | Variablec | Absent or increased |
| Pulse augmentation test: weak | |||||
| Palpation: thrill | Continuousa | DiscontinuousbReduced | DiscontinuousbIncreased at site of lesion | VariablecIt may be present below the clavicle | Absent |
| Auscultation: bruit | Continuousa | DiscontinuousbReduced | DiscontinuousbHigh pitched or acute toneIncreased at site of lesion | VariablecIt may be present below the clavicle | Absent |
aSystolic and diastolic.
bSystolic only.
cNormal or increased.
- •
Inspection. Table 10 summarises the basic information to be taken into account during AVF examination. It is very important to observe the entire limb where the vascular access (VA) is located. In the case of AVF in the upper limbs, oedema and collateral circulation are signs that suggest total or partial central venous stenosis. The extension of an oedema can help us to locate the level of the central stenosis: if the oedema only involves the arm, this suggests that the stenosis is in the subclavian vein; if the oedema includes the chest, breast and/or ipsilateral face, stenosis is more likely in the brachiocephalic vein; bilateral oedema (chest, breasts, shoulders and face) suggests a superior vena cava stenosis.286 The distal areas of the limb must be assessed for signs of ischaemia (coldness, pallor and ischaemic digital ulcers) and signs of venous hypertension (hyperpigmentation and stasis digital ulcers).286,296-298 The entire AVF segment must be inspected to detect the presence of haematomas, aneurysmal dilatations and signs of swelling.299,300 Any arterialised vein which does not collapse, at least partially, after lifting the arm, probably has a proximal stenosis (Tables 10 to 12).10,282,286,293
Table 12.Main tests used for physical examination of arteriovenous fistula (AVF)
Arm elevation test - •
It consists of raising the arm where the nAVF is located, above the heart level, and then observing whether the arterialised vein collapses
- •
The test is considered normal when the nAVF collapses after arm elevation, thus ruling out an outflow stenosis
- •
In the case of a venous stenosis, only the segment of arterialised vein proximal to the lesion will collapse during the test, while the segment distal to the stenosis will remain distended without collapsing
Pulse augmentation test - •
This test allows assessment of the inflow segment of the arteriovenous access
- •
It consists of transient arterialised vein occlusion several centimetres above the arterial anastomosis with one hand and, simultaneously, the evaluation of the pulse intensity at the anastomosis level with the other
- •
This test is considered normal when the arterialised vein segment distal to the occlusive finger (between the finger and the anastomosis) presents an increase in pulse rate
- •
This test is based on the fact that if the vascular access is completely occluded at a distance from the arterial anastomosis, the soft pulse intensity will increase. The degree of this increase is directly proportional to the QA in the inflow segment of the nAVF
- •
The presence of arterial system pathology retrograde to the anastomosis (feeding artery stenosis) conditions the degree of pulse increase obtained when using this test
Sequential occlusion test - •
It is similar to the Pulse Augmentation Test but focuses on the thrill disappearance with the arterialised vein occlusion of the nAVF
- •
Its purpose is to detect the collateral venous branches that arise from the arterialised vein. Often, a collateral vein may be visible and, therefore, previously detected by examination
- •
This test is based on the relationship between the thrill and the QA of the arterialised vein
- •
It involves occluding the arterialised vein close to and proximally to the anastomosis with one hand, while the normal thrill is palpated over the anastomosis with the other. The thrill, usually palpable in the arterial anastomosis, indicative of QA, disappears when the arterialised vein is manually obstructed proximally by causing a transient QA interruption. Next, the entire arterialised vein segment should be examined by progressively changing the occlusion point proximally. If the thrill does not disappear at any point along the venous pathway, it means that a collateral vein is present below the occlusion point
nAVF, native arteriovenous fistula; QA, blood flow.
- •
- •
Palpation.282,286,293,301 Pulse is observed more correctly using the finger tips. Under normal conditions, the nAVF pulse is of low intensity, soft and easily compressible. Usually, an increased nAVF pulse is indicative of proximal stenosis (hyperpulsatile nAVF) and the amount that this increases is directly proportional to the existing degree of stenosis. In contrast, a pulse which is excessively weak (hypopulsatile nAVF, flat access), with little increase through transitory manual occlusion, suggests the presence of inflow stenosis (pulse augmentation test, Table 12).
Thrill is a palpable nAVF vibration, which is more easily explored using the palm of the hand, and reflects the QA circulating along the arterialised vein.282,286,293 The absence of thrill indicates a deficit of QA. This sign, together with the absence of pulse, is characteristic of AVF thrombosis. Two different types of thrill can be palpated:
- –
A diffuse basal thrill in a normal AVF. This is gentle, continuous (systolic and diastolic), palpable throughout the whole AVF segment but more intense at the level of the venous anastomosis.
- –
A locally increased thrill. This reflects the presence of turbulent flow located at a stenosis area in the arterialised vein. As the degree of stenosis progressively increases, with a concomitant increase in resistance to the QA, thrill shortens and loses its diastolic component. The whole trajectory of the arterialised vein should be examined to detect the presence of abnormal thrill. In the event of a stenosis in the subclavian vein or cephalic vein arch, thrill can be detected below the clavicle.286
- –
- •
Auscultation. The normal AVF bruit and the temporary changes that may occur in this bruit, as well as the occurrence of abnormal bruits,282,286,293 must be assessed. This is the auditory manifestation of thrill. Two different types of bruit can be heard:
- –
A diffuse basal bruit in a normal AVF. This has a low tone, like a soft and continuous murmur (systolic and diastolic).
- –
An abnormal bruit associated with stenosis. The increased resistance caused by a progressive stenotic lesion will lead to the gradual loss of the diastolic component of the bruit and a simultaneous increase in its tone. The whole trajectory of the arterialised vein, including the area below the clavicle, should be examined to assess the presence of an abnormal bruit.286
- –
Juxta-anastomotic or peri-anastomotic nAVF stenosis, i.e. the stenosis located in an area of 2-3 cm immediately adjacent to the anastomosis, which can affect both the afferent artery and the efferent vein, behaves like an inflow stenosis and may be diagnosed easily by exploring the anastomosis and the most distal segment of the arterialised vein286. At anastomosis level, thrill is only palpated during the systole and the pulse is greatly increased (defined as “water-hammer” according to English-speaking authors) but it suddenly disappears when the examiner’s finger moves proximally along the trajectory of the vein and finds the precise location of the stenosis; proximally to the stenosis, the pulse is very weak and may be difficult to detect. On occasions, the stenosis can be seen as a gap related with a sudden decrease in vein size.
Several prospective observational studies have shown that physical examination diagnoses stenosis with a high degree of sensitivity and specificity, as well as precision, and therefore, it should have a prominent position among AVF screening methods.204,286,287,301-308 The efficacy of physical examination carried out by qualified staff is equivalent to other more sophisticated screening methods287,301,302; the key lies in the examiner’s judgement.302 In this respect, in the study by Coentrão et al., conducted on 177 consecutive prevalent patients with nAVF dysfunction, diagnostic agreement of physical examination with fistulography for diagnosis of stenosis at all locations was always higher when a resident nephrology doctor with six months’ training performed the study compared with several general nephrologists without any specific training in nAVF examination (overall agreement 86% versus 49%, respectively).302
4.2.2 Problems during the haemodialysis session
These could be indirect signs of AVF stenosis if they appear persistently (three consecutive HD sessions), compared with the previous HD sessions253:
- •
Difficulty in AVF needling and/or cannulation.
- •
Aspiration of clots during needling.
- •
Increase in negative pre-pump arterial pressure.
- •
Failure to reach prescribed QB.
- •
Increase in the return or venous pressure.
- •
Prolonged haemostasis time, without excessive anticoagulation.
4.2.3 Native arteriovenous fistula stress test according to the pump flow
This test has proved effective in diagnosing inflow nAVF stenosis (positive predictive value of 76.3%) and is based on the decrease that occurs in QA when raising the upper limb from 0° to 90° for 30 s and QB of 400 mL/min.294 To carry it out, with the arm in this raised position, QB is reduced progressively to 300, 200 and 100 mL/min and the test is considered positive when the alarm on the HD machine is triggered because negative arterial pressure falls below -250 mmHg. The existence of a positive test with low QB values (100-200 mL/min) involves the presence of decreased QA and, therefore, high probability of relevant stenosis.
4.3Monitoring and surveillance of arteriovenous fistula pressureThe presence of significant AVF stenosis may cause a retrograde increase in pressure inside it and can be detected by monitoring and surveillance of AVF pressure.10,274,283,303,309-315Table 13 provides details of how to determine AVF pressure.
Determination of venous pressure
| Monitoring dynamic venous pressure (DVP) |
| General characteristics |
|
| Dynamic venous pressure (DVP) is the pressure generated by the venous return of the dialysed blood through the arteriovenous fistula (AVF) via the venous needle. It is measured by the venous pressure (VP) transducer of the HD monitor. It reflects the pressure inside the AVF and the resistance offered by the venous needle. However, the DVP may be affected by other factors that lead to an error in measurement such as: a) QB, which may vary, according to various studies, between 50 and 425 mL/min; b) the length and size of the needle used; c) the viscosity of the blood (haematocrit), which affects DVP; d) the patient’s blood pressure (BP), and e) the development of collateral veins, in some AVF, which may lead to failure in the detection of stenosis using this method. For these reasons there is a conviction that DVP does not reflect intra-access pressure (IAP) and, therefore, the resistance caused by stenosis, which leads to lower sensitivity and specificity than other methods, such as measurement of AVF flow (QA) and static VP |
| Surveillance of intra-access or static pressure |
| Currently, a simplified method is used to determine intra-access venous pressure (IAVP). This does not require a special device or imply additional costs and is reproducible and easy to perform. It is based on the determination of pressure reflected in the HD machine transducer and the hydrostatic pressure created by the blood column between the AVF and the venous chamber. IAVP does not depend on changes in QB, blood viscosity or size and distribution of the needles; it is only related to systemic blood pressure (BP), which is why normalised IAVP value (nIAVP) is used as the ratio between IAVP and mean systemic arterial pressure (MAP) determined simultaneously:IAVPn=IAVP/MAP |
| The nIAVP determination does not allow stenosis located distally to the venous needle to be detected, either in the body or at the arterial anastomosis level of the graft. For this purpose, it is useful to measure intra-access arterial pressure (IAAP), which considers the pressure obtained in the pressure transducer connected to the arterial line (simultaneously with the measurement of pressure in the venous line) and height in centimetres between the arterial needle and the arterial chamber If a stenosis develops in the body of the prosthetic graft, between the two needles, IAVP remains normal or decreased while IAAP increases. A difference between the normalised IAAP and nIAVP ≥ 0.5 may be indicative of intra-access stenosis |
| Requirements for intra-access pressure determination |
|
| Calculation of the static or intra-access venous pressure |
|
| Pressure threshold values suggesting stenosis | |||
| a) Native arteriovenous fistula | |||
| Stenosis degree and location | Arterial segment (nIAAP) | Venous segment (nIAVP) | |
| Stenosis absent or < 50% | 0.13-0.43 | or and | 0.08-0.34 |
| 1. Outflow stenosis > 50% | > 0.43 | > 0.35 | |
| 2. Stenosis > 50% between both needles of arterialised vein | > 0.43 | ≤ 0.35 | |
| 3. Inflow stenosis > 50% | < 0.3 + clinical findings | Clinical findings | |
| b) Prosthetic arteriovenous fistula | |||
| Stenosis degree and location | Arterial segment (nIAAP) | Venous segment (nIAVP) | |
| Stenosis absent or < 50% | 0.35-0.74 | or and | 0.15-0.49 |
| 1. Stenosis > 50% at venous anastomosis | > 0.75 | > 0.5 | |
| 2. Stenosis > 50% at pAVF body (between both needles) | ≥ 0.65 | < 0.5 | |
| 3. Stenosis > 50% at arterial anastomosis | < 0.3 | Clinical findings | |
These methods are preferred for the follow-up of proximal nAVF and, especially, pAVF.10,283 Collateral veins of a radiocephalic nAVF can cause decompression and a decrease in the sensitivity of these techniques when used for detecting distal nAVF stenosis.10,283
The pioneering work of Besarab et al.309 showed that the sensitivity to diagnose significant pAVF stenosis by determining normalised static pressure (see section 4.3.3.) was 91%. In the presence of the most common stenosis diagnosed in pAVF, i.e. the stenosis located in the anastomosis between the venous end of the graft and the efferent vein, there is a retrograde increase in pressure throughout the whole pAVF and the pressure level reached is directly related to the existing degree of stenosis.316
4.3.1 Dynamic venous pressure
DVP is the pressure needed to return the dialysed blood into the AVF through the venous needle recorded by the venous pressure transducer of the HD monitor. In fact, it is the sum of the pressure required to overcome resistance exerted by the venous needle and the pressure existing inside the AVF (Table 13).10
There are contradictory results in the literature with regard to DVP efficacy in detecting AVF with significant stenosis and high risk of thrombosis.311,312,317-321 In the classic Schwab et al.312 study, the incidence of thrombosis obtained when comparing AVF with electively corrected significant stenosis (previous DVP > 150 mmHg) and AVF with normal DVP with no suspected stenosis was similar (0.15 versus 0.13 episodes/patient/year). Smits et al.311 showed a significant reduction in the incidence of pAVF thrombosis by the application of a follow-up programme which included DVP measurements, static venous pressure and QA. However, this same Dutch group failed to previously demonstrate the efficacy of DVP in predicting pAVF thrombosis.322
To sum up, the current available data suggesting the usefulness of DVP to diagnose stenosis and predict thrombosis are limited and inconclusive. It is not acceptable to use DVP as a screening method for AVF stenosis in a non-standardised way.
4.3.2 Intra-access pressure or static pressure
This is determined by the presence of QB = 0 mL/min (pump stopped). Unlike DVP, IAP is not influenced by the type of needle used, QB or blood viscosity.
The simplified determination by Besarab et al. is used to calculate it. This takes into account the pressure obtained by the pressure transducer connected to the venous or arterial line of the HD monitor (mmHg) and the height between the venous or arterial needle (or the arm of the patient’s armchair) and the level of blood in the venous or arterial chamber (cm).10,314
In a national study, referring to 24 brachial pAVF, the VA with stenosis had a significantly higher IAP than the other AVF (48.7 ± 22.2 versus 27.6 ± 0.1 mmHg).283 It is considered that a DVP ≥ 150 mmHg with a QB = 200 mL/min (PV200) corresponds to an IAP > 60 mmHg.313 In the aforementioned study by Besarab et al., pAVF surveillance using static pressure achieved a 70% decrease in the incidence of thrombosis.309
4.3.3 Equivalent or normalised static intra-access pressure
As IAP relates to mean arterial pressure (MAP), the results are expressed in an equivalent or normalised form using the IAP/MAP ratio.10 In the absence of significant stenosis and because of existing collateral circulation, the IAP/MAP ratio will always be lower in nAVF than in pAVF. In another study by Besarab et al.,315 the IAP/MAP ratio in cases without stenosis was higher in pAVF (0.43 ± 0.02, n = 414) compared with nAVF (0.26 ± 0.01, n = 286), but without significant differences in relation to QA.
Normalised intra-access pressure profiles have been described according to the situation of the stenosis in the pAVF at the level of the arterial anastomosis, body (between the 2 needles) or venous anastomosis. It is considered that, when faced with a stenosis located in the venous anastomosis of the pAVF, the IAP/MAP ratio at the level of the venous and arterial needles is > 0.5 and 0.75, respectively.10,315 In the aforementioned study by Caro et al.,283 there was a significant difference between the IAP/MAP ratio determined in pAVF with and without stenosis: 0.5 ± 0.2 and 0.3 ± 0.1, respectively.
When there is a significant stenosis located in the venous anastomosis of a pAVF, there is an inverse relationship between normalised IAP and the QA of AVF.315,323 In this functional situation of raised normalised IAP and low QA, the AVF comes fully within the area of high risk for thrombosis.274
4.4Recirculation of arteriovenous fistulaWhen significant stenosis is present, the QA of AVF decreases and the percentage of already dialysed blood re-entering the dialyser through the arterial needle increases. In the absence of technical errors, recirculation occurs as a consequence of a severe AVF stenosis when QA is close to or decreases below the planned QB (300-500 mL/min).10,313,315
Therefore, the measurement of recirculation is not the best method for early detection of stenosis.279,324 Above all, it is not recommended that it be applied to monitoring pAVF.10,274 In this type of AVF, recirculation occurs late when there is severe stenosis and a very high risk of thrombosis.274 In addition, we must remember that the presence of a localised stenosis between the two AVF needles does not cause recirculation.324
The recirculation percentage can be determined using the following two methods10:
- •
Determination of urea recirculation.325 This is described in Table 14. The presence of a percentage of urea recirculation > 10% is a criterion for investigating a possible AVF stenosis by means of an imaging test.10
Table 14.Determination of urea recirculation
- •
This should be performed at the start of the haemodialysis session—HD—(during the first 30-60 min), provided that the haemodynamic stability of the patient is checked.
- •
In order to calculate it, the ultrafiltration (UF) rate should be decreased to zero. If the online haemodiafiltration technique is being used, it must be disabled.
- •
To obtain the samples, withdraw blood simultaneously from the dialyser entry in the arterial line and at the exit of the dialyser in the venous line at the programmed pump flow (QB). Immediately afterwards, reduce the QB to 50 mL/min, wait 20 seconds and take another sample from the arterial line to determine peripheral or systemic urea (low-flow method). Then, continue with the scheduled HD.
- •
The recirculation percentage calculation (R) is performed according to the following formula:R=( UREAp−UREAa / UREAp−UREAv)×100
UREAa, urea of the arterial line; UREAp, peripheral urea; UREAv, urea of the venous line.
- •
- •
Determination of recirculation using dilution screening techniques (Table 15).326-330 These methods present higher sensitivity and specificity than the urea recirculation method.328,329 There are published studies using the ultrasound dilution method, the thermodilution method with BTM (blood temperature monitor) sensor and the glucose perfusion method.327,328,330 In this respect, Wang et al.330 demonstrated that recirculation values higher than 15% obtained using the BTM sensor provided a high sensitivity (81.8%) and specificity (98.6%) in the detection of nAVF requiring elective intervention. The presence of AVF stenosis should be investigated in the case of a recirculation percentage greater than 5% and 15% using the ultrasound dilution and thermodilution methods, respectively.10,330
The decrease, for no apparent reason, in HD adequacy assessed by the Kt/V index or PUR may be an indirect sign of AVF dysfunction.279,331 In one study, patients with significant nAVF stenosis (n = 50) presented a Kt/V index lower (1.15 ± 0.20) than the remaining patients (1.33 ± 0.16) (p < 0.0001).303 It is considered that HD efficacy is affected at a late stage during the natural development of AVF stenosis when a high percentage of recirculation becomes evident.279
However, it has been published that the persisting decrease in the Kt index, determined online using the ionic dialysance method in each HD session, makes it possible to detect early recirculation caused by significant nAVF stenosis.332
4.6Dilution screening methods for indirect determination of arteriovenous fistula flowRationale for dilution methods
The objective follow-up of AVF function should be carried out regularly by determining its QA.14 In the presence of a significant stenosis, QA always decreases irrespective of the AVF type (nAVF or pAVF), its location (upper or lower limb) or the topography of the stenosis (feeding artery, anastomosis, arterialised vein, central vein) .10,279,333,334 This is very important and is a notable advantage compared with first-generation methods. For example, in the presence of a significant nAVF stenosis in the arterialised vein, QA will decrease but, depending on the venous needle position in the arterialised vein, it is possible that the venous pressure (determined by DVP) does not increase.279
The introduction of the ultrasound dilution method by Nicolai Krivitski in 1995 meant a qualitative change in the field of AVF study as, for the first time, it was possible to perform non-invasive QA estimation.335 Since then, several dilution techniques that allow the indirect determination of QA during HD and, therefore, the functional follow-up of the AVF have been described (Tables 15 and 16).336-343
– Blood flow (QA) determination of the arteriovenous fistula (methods requiring the reversal of blood lines)
| 1. Ultrasound dilution method |
| This was the first dilution method described. An external monitor, a Doppler sensor placed on each haemodialysis (HD) line and an isotonic saline bolus (indicator) administered for 6-8 seconds in the arterial line, with the HD lines in the normal and inverted positions, are all required to calculate QA. This is calculated using the software incorporated in the external monitor (Transonic) using the following formula:QA=QB×(Sv/Sa−1)=QB×(1/R−1)Where QB is the effective blood flow, Sv/Sa is the ratio between the areas registered by the 2 Doppler sensors in the venous and arterial lines respectively, after the injection of the indicator bolus, and R is the existing recirculation with the blood lines reversed |
| 2. Haematocrit dilution method or Delta-H |
| This is an optodilution method which employs the Crit-Line III monitor (ABF-mode). It is a photometric technique based on the inverse relationship between blood volume and haematocrit. QA is determined during the first hour of the HD session based on the changes in haematocrit in relation to abrupt changes of ultrafiltration (UF), from 0.1 to 1.8 L/h, with the HD lines in normal and reversed configuration. Haematocrit changes are recorded continuously by an optical sensor in the form of a clamp that is attached onto a blood chamber inserted between the dialyser and the arterial line. QA is calculated using the following formula:QA=(UFmax−UFmin)×Hctmaxrev/D Hct rev −D Hct norWhere UFmax is the maximum UF, UFmin is the minimum UF, Hctmax rev is the maximum haematocrit obtained with HD lines in the reversed position, D Hct rev is the change in the haematocrit with the lines reversed, and D Hct nor is the change in the haematocrit with HD lines in the normal position |
| 3. Thermodilution method |
| QA is determined using the blood temperature sensor BTM (Blood Temperature Monitor) incorporated in some HD machines. This dilution method calculates QA from the recirculation values obtained with the HD lines in the normal and reversed positions. The determination process starts from the production of a temperature bolus secondary to the self-limited decrease (2 °C for 2 minutes) from the temperature of the dialysate. Initially, this thermal decrease is captured by the venous line temperature sensor and, after travelling through the cardiopulmonary circulation system of the patient, it returns, already reduced, to the dialyser and is captured by the arterial line temperature sensor. The quantification of the last bolus of arterial temperature, compared to the bolus of venous temperature generated initially, makes it possible to calculate the existing recirculation percentage with HD lines in the normal position; the same procedure is carried out with HD lines in the reversed position. QA is calculated from both recirculation values using the following formula:QA=(QS−UFR)×(1−RX−RN+RX×RN)/RX−RX×RN−(QS−URF/QS)×(RN−RX×RN)Where QS is the effective blood flow (mL/min), UFR is the ultrafiltration rate (mL/min), RN is the recirculation obtained with HD lines in the normal position and RX is the recirculation obtained with HD lines in the reversed position. To correctly obtain recirculation values, both QS and UFR must be maintained constant throughout the whole period used to make the determination |
| 4. Temperature gradient method |
| QA is determined using the BTM sensor incorporated in some HD machines. The temperature gradient technique makes it possible to calculate QA from temperature values obtained with HD lines in the normal and reversed positions, without the need to generate a temperature bolus. The value of QA is obtained by applying the following formula:QA=(QB,x−UFR)×Tart,x−Tven,x/Tart,n−Tart,xWhere QB, x is the effective blood flow with HD lines in the reversed position (mL/min), UFR is the ultrafiltration rate (mL/min), Tart, n is the temperature of the arterial line with HD lines in the normal position, Tart, x is the temperature of the arterial line with HD lines in the reversed position and Tven, x is the temperature of the venous line with HD lines in the reversed position. To correctly calculate QA, the QB, UFR and the temperature of the dialysate fluid (35.5 °C) must be kept constant throughout the whole determination period |
| 5. Ionic dialysance method |
| Ionic dialysance is equivalent to “effective” urea clearance. Its application, requires HD machines to have incorporated sensors which allow ionic dialysance to be automatically read by analysis of the conductivity of the HD fluid at the entry and exit of the dialyser. After obtaining measurements of ionic dialysance or urea clearance (K) in lines in both the normal and reversed positions, QA value can be obtained by applying the following formula:QA= Drev ×(Dnor − UFR)/( Dnor − Drev)Where Drev is the ionic dialysance value with HD lines in the reversed position, Dnor is the reading of the ionic dialysance with HD lines in the normal position and UFR is the ultrafiltration rate in mL/min |
QA is calculated by quantifying the difference in recirculation before and after the dilution of a particular indicator (haematocrit, temperature), with or without inversion of the HD blood lines. If both arterial and venous needles have been inserted into the same arterialised vein, artificial recirculation is created when reversing the blood lines, with the dilution of the indicator that enables us to calculate QA according to the formulae shown in Table 16.
The dilution methods requiring the HD blood lines to be reversed are those most commonly used today. However, in some cases, they cannot be applied: when we insert the venous needle through which blood returns into a vein other than the AVF-bearing arterialised vein, AVF recirculation is zero and, therefore, QA calculation is impossible.338,344
Dilution techniques that calculate QA during HD should be performed within the first hour of the session to avoid haemodynamic changes secondary to ultrafiltration.270
According to the European guidelines, there is no clear preference for any of these methods14 and most studies have shown similar results for QA after applying different dilution techniques.345-348 Indeed, all of them have advantages and disadvantages when used. For example, the time required to determine QA using the Delta-H method is long (more than 20 min) but, in contrast, it is a completely examiner-independent method and QA value automatically displays on the Crit-Line monitor screen immediately after the completion of the examination.270,348 Other methods, like thermodilution and temperature gradient, have an advantage over those previously mentioned, as the sensor (BTM) is already incorporated into the HD machine, but QA value is not obtained automatically and needs to be calculated subsequently345,348; both methods are only validated for high-flux HD with QB of 300 mL/min.349 The use of certain devices allows the instant inversion of the HD blood lines and, therefore, the time required to obtain QA value is significantly reduced.348
Interpreting the results
When any functional AVF alteration is detected by any of these screening methods, an imaging test should be carried out in the event of suspected AVF stenosis. The functional criteria for this are as follows10,15,350:
- •
Absolute QA value. The threshold value or cut-off point of QA which indicates the need for an imaging test varies according to the ROC curves of sensitivity-specificity obtained in several studies: < 500, 650, 700 or 750 mL/min.269,288,327,350,351 The KDOQI guide considers a QA < 600 mL/min for pAVF and < 400-500 mL/min for nAVF,10 whereas the European Guide indicates elective intervention in the case of a QA < 600 mL/min in pAVF or < 300 mL/min in nAVF of the forearm.14
- •
Temporary decrease in QA > 20-25%, regardless of whether nAVF or pAVF, in relation to the baseline QA.10,15,227,352,353
As mentioned previously, prospective analysis of QA evolution over time is of higher value for diagnosing AVF stenosis than isolated determinations.10 In a longitudinal study by Neyra et al.,353 involving 95 AVF, QA decrease over time was a powerful predictive variable of thrombosis, so that the relative risk (RR) of thrombosis increased when there was a drop in QA higher than 15% and was maximum (34.7%) when the decrease in QA was > 50%. Paulson et al.354 consider that a 20% to 25% decrease in QA percentages may be secondary only to haemodynamic changes and that only a decrease in QA greater than 33% should be considered significant.
It has been demonstrated that QA is related to AVF type (for example, radiocephalic versus brachiocephalic nAVF) as well as various demographic and clinical factors of the patient345,349,352,355,356. An inverse relationship between QA of the AVF and patient age has been demonstrated269,349 so that the application of a multiple linear regression model showed a reduction of 11.6 mL/min in baseline QA of the AVF for every year of the patient’s life, with the rest of the variables considered remaining constant.269 The functional AVF profile also depends on its location, as demonstrated in a case series by Treacy et al.,356 referring to 53 nAVF studied using the thermodilution method: the functional result obtained differed depending on the nAVF topography in the snuff box, distal forearm, proximal forearm, brachiocephalic and brachiobasilic, from the lowest to the highest QA.
In some studies, better AVF function, that is to say, a higher QA, has been shown in patients with a history of some previous ipsilateral AVF.345,357 The existence of previous venous arterialisation may explain this functional difference. In other words, a previous functional distal AVF in the same limb may determine the function of a secondary nAVF of proximal location. In this respect, in a study by Begin et al.,357 referring to 45 patients with nAVF, QA of patients with brachiocephalic nAVF, measured by the ultrasound dilution method, was higher in cases of a previously functioning radiocephalic nAVF in the same arm compared with the remaining patients (1800 ± 919 versus 1167 ± 528 mL/min).
Functional AVF surveillance through QA determination has allowed a higher incidence of pathology to be shown in the feeding artery than that reported in historical studies and currently estimated at around 30% of all dysfunctional AVF cases.333,358,359 In addition, through this AVF surveillance, the radial artery pathology in radiocephalic nAVF could be classified in 3 differentiated groups.333
In addition to diagnosing AVF stenosis, AVF surveillance through periodic QA measurements allows the identification of hyperdynamic AVF with excessive QA which may cause heart failure.360,361 There is increased risk of heart failure secondary to AVF when its QA is ≥ 2000 mL/min or 20% of cardiac output.194 In such cases, it is reasonable to perform strict cardiological follow-up by periodic echocardiograms. On the other hand, cardiac decompensation can also occur with a QA< 2000 mL/min in patients with a reduced myocardial reserve.360
In some cases, the estimation of both QA of the AVF and the systolic pulmonary artery pressure jointly using non-invasive methods (Delta-H method and Doppler echocardiogram, respectively) has allowed a diagnosis to be made, surgical indication to be established (banding) and post-operative follow-up of the AVF with haemodynamic repercussions to be carried out.361
4.7Imaging tests. Arteriovenous fistula surveillance using Doppler ultrasoundRecommendations
- (•)
NEW R 4.7.1) We recommend that both Doppler ultrasound and dilution screening methods be used interchangeably to assess arteriovenous fistula function, as they have an equivalent performance for blood flow determination
- (•)
NEW R 4.7.2) We recommend that Doppler ultrasound be used as the first-choice imaging test in the hands of an experienced examiner, without the need for confirmatory fistulography, to indicate elective treatment in the event of suspected significant stenosis
- (•)
NEW R 4.7.3) We recommend that fistulography be reserved as a diagnostic imaging exploration only for cases with inconclusive Doppler ultrasound findings and persistent suspicion of significant stenosis
DU is an imaging technique that allows examination of both nAVF and pAVF using a linear sender and receiver ultrasound transducer applied on the different AVF planes (Table 17). Despite some drawbacks (operator-dependent technique, impossible to use in case of bandages and/or wounds and difficulties in assessment in the case of vascular calcification), the use of ultrasound image together with Doppler is growing as an AVF surveillance method since this is a second-generation non-invasive method which does not use ionising radiation or iodinated contrast media and which, in addition, is inexpensive and readily available.362 In Table 18 other imaging methods for studying AVF are described.
Surveillance of the arteriovenous fistula (AVF) by Doppler ultrasound (DU)
|
Other imaging examinations of the vascular access
| Angiography or fistulography with iodinated contrast is a precise technique in the diagnosis of AVF stenosis, as it explores the entire venous pathway to the central vessels. This examination also allows immediate percutaneous treatment if the characteristics of the lesion meet the criteria for it. On the other hand, it is an invasive imaging examination compared to DU, which gives exposure to ionising radiation, as well as possible side effects that can be caused by the iodinated contrast agent and which, in pre-dialysis cases, may cause a renal function impairment due to nephrotoxicity. In addition, it does not provide information on AVF function (QA) or on possible underlying soft tissue lesions (for example haematomas, abscesses and seromas). Therefore, fistulography for purely diagnostic purposes should be avoided if it does not include the possibility of a therapeutic approach at the same time. In patients who are allergic to the iodinated contrast or at risk of nephrotoxicity, using CO2 as a contrast agent is a valid alternative to the iodinated contrast, although it carries lower rates of accuracy, sensitivity and specificity in estimating the degree of stenosis |
Among the advantages of fistulography compared to other imaging techniques, especially DU, it is possible to highlight the assessment of central vessels and the possibility of performing the diagnostic study and the treatment in the same act. The indications for it to be carried out would be:
|
| In relation to conventional fistulography, computed tomography and magnetic resonance offer an unfavourable cost-benefit profile. In addition, the development of nephrogenic systemic fibrosis has been described after the administration of gadolinium as magnetic resonance contrast agent in patients with renal impairment |
AVF, arteriovenous fistula; DU, Doppler ultrasound; PTA, percutaneous transluminal angioplasty; QA, blood flow.
DU has the following benefits in AVF surveillance52,363,364:
- •
Method for quick diagnosis that can be used in situ in the HD room (portable ultrasound machine) when any change in the AVF is detected by a first-generation method or by a decrease in QA recorded by a dilution method.223 In a Spanish study referring to 119 portable DU examinations carried out by the nephrologist on 67 AVF, 31 stenoses were diagnosed in 44 cases of needling difficulty with no other warning signs for stenosis,223 demonstrating the usefulness of DU in the hands of a well-trained professional.
- •
Regulated surveillance method for periodic AVF assessment. DU allows direct visualisation of the AVF and, therefore, makes it possible to perform morphological surveillance.365-368
- •
Haemodynamic information related to the AVF. DU allows direct QA determination and, therefore, functional AVF surveillance.369 QA (mL/min), preferably in the brachial artery, is calculated using the following formula367:QA=Time-averaged mean velocity (m/s)× cross-sectional area (mm2)×60
Various authors have found that QA determined by DU is significantly lower in AVF with stenosis compared with other AVF without stenosis.303,369 A positive correlation has been shown between QA of the arterialised vein in nAVF determined by DU and diameter and parameters of the feeding artery (diameter and arterial blood flow).369 A significant correlation has also been found between QA obtained by DU and by various dilution methods.369,370
- •
Imaging test of choice to confirm, locate and quantify AVF stenosis detected by screening methods prior to elective treatment.303,371-373 In this respect, a linear correlation has been described between DU and fistulography to diagnose significant AVF stenosis.373,374 In addition, it allows for the surveillance of stenoses which are considered non-significant.375 The ultrasound criteria described for the diagnosis of significant AVF stenosis are shown in Table 19.52,84,376
Table 19.Described ultrasound criteria for significant arteriovenous fistula stenosis
Morphological criteria - •
Vascular lumen reduction ≥ 50%
Functional criteria - •
Pronounced “aliasing” phenomenon as a sign of turbulent flow. It is a suspicion, not a diagnostic criterion
- •
PSV > 400 cm/s. Not valuable in the AVF anastomosis zone
- •
PSV ratio. This is the ratio between PSV in the stenotic and in the pre-stenotic area and is considered diagnostic when it is > 2
- •
Indirect characteristics in brachial artery: high resistive Doppler wave; resistive index: > 0.6
- •
QA values preferably obtained at brachial artery level: absolute < 500 (nAVF) or < 600 (pAVF) mL/min, or a decrease in QA > 25% over time
nAVF, native arteriovenous fistula; pAVF, prosthetic arteriovenous fistula; PSV, peak systolic velocity; QA, blood flow.
- •
- •
It allows the morphological and functional assessment of other AVF dysfunctions which are not related to stenosis or thrombosis, such as aneurysms and pseudoaneurysms, haematoma, abscesses, etc.
→ Clinical question X How reliable is Doppler ultrasound in determining blood flow in the arteriovenous fistula in comparison to dilution screening methods?
(See fact sheet for Clinical question X in electronic appendices)
| Summary of evidence | |
| No comparative studies have been found with different methods for determining QA and its relationship to AVF parameters (survival, patency, thrombosis), adverse effects and mortality | Low quality |
| The available studies mainly provide information about the outcome of different techniques (ROC curves) and the agreement of results between them | |
| Comparative studies of QA determination show a high degree of concordance of QA values between the ultrasound dilution method and DU, on the one hand, and between ultrasound dilution and thermodilution, on the other | Low quality |
Evidence synthesis development
Doppler ultrasound versus ultrasound dilution
The study by Weitzel et al.377 evaluated the comparability of QA measurements through DU with those taken by ultrasound dilution method in 24 patients with pAVF. In this study the reproducibility in 54 pairs of DU measurements was also assessed. Measurement variations by DU were 4% for pAVF with QA < 800 mL/min (n = 17), 6% for pAVF with QA flow between 801 and 1600 mL/min (n = 22), and 11% for pAVF with QA > 1600 mL/min (n = 15). The mean variation coefficient of measurement was 7% for DU compared with 5% for ultrasound dilution method. Correlation coefficients (r) between QA measurements by DU and by ultrasound dilution were 0.79 (n = 24, p < 0.0001), 0.84 for pAVF with QA < 2000 mL/min (n = 20, p < 0.0001), and 0.91 for pAVF with QA < 1600 mL/min (n = 18, p < 0.0001). They concluded that DU gives reproducible QA measurements which correlate with ultrasound dilution measurements.377
The study by Schwarz et al.378 compared both techniques using fistulography as a reference. They assessed 59 HD patients with forearm nAVF using ultrasound dilution, DU and fistulography, in that order, and diagnosed nAVF stenosis in 41 patients, who were treated with PTA. The accuracy of both techniques, assessed by ROC curves, was similar: average areas under the curve were 0.79 (95% CI, 0.66 to 0.91) for ultrasound dilution and 0.80 (95% CI: 0.65 to 0.94) for DU. The correlation between QA values obtained by ultrasound dilution and by DU measurements was 0.37 (Spearman = 0.004). The optimal cut-off value calculated for stenosis prediction was 465 mL/min for ultrasound dilution and 390 mL/min for DU. Both ultrasound techniques were valid for predicting nAVF stenosis (p < 0.01). In 13 patients restenosis occurred in the first 6 months after PTA. QA obtained by ultrasound dilution after PTA was significantly lower in these 13 patients, compared with the other 21 patients. The authors concluded that QA surveillance of nAVF for HD using ultrasound techniques provides a reasonable prediction of stenosis and restenosis.378
The study by Lopot et al.379 provided measurement comparative data for DU and dilution ultrasonography, which was used as the reference technique in 27 patients, and found a good correlation between both techniques (r = 0.8691).
The study by Lin et al.380 compared the reproducibility and correlation of QA measurements using a variable QBbased Doppler method combined with spectral analysis of Duplex Doppler images (VPFDUM), with the ultrasound dilution method, and conventional DU method, in 73 HD patients, 70 with nAVF and 3 with pAVF. The mean value of QA by VPFDUM (870.8 ± 412.0 mL/min) showed a high degree of similarity to that of measurements by ultrasound dilution (868.6 ± 417.9 mL/min) but was higher than measurements by conventional DU (685.1 ± 303.6 mL/min; p < 0.005). The mean coefficient of variation values was similar using VPFDUM (1.6%) and ultrasound dilution (1.4%) but lower than conventional DU (6.8 %, p < 0.01). The correlation coefficient and the intra-class correlation coefficient (ICC) of repeated QA measurements by VPFDUM (0.985 and 0.993, p < 0.001) were also similar to those by ultrasound dilution (0.992 and 0.995, p < 0.001), but slightly higher than those of conventional DU (0.917 and 0.948, p < 0.005).The reproducibility of the VPFDUM technique (r = 0.98, p < 0.0001) and the correlation between VPFDUM and ultrasound dilution (r = 0.99, p < 0.0001) for QA measurements were good. Unassisted AVF patency at 6 months was significantly lower in patients with a QA < 500 mL/min than in those with a QA > 500 mL/min (13.6 % versus 92.2 %, p < 0.0001). They concluded that the VPFDUM technique is a non-invasive, accurate and reliable procedure for measuring QA and has predictive power regarding AVF patency.380
Doppler ultrasound versus other dilution methods
Roca-Tey et al.369 carried out a functional study comparing DU and Delta-H methods for QA determination in AVF (84.8% of nAVF) in 33 prevalent patients on chronic HD. In diagnostic concordance analysis, the ICC between QA values of the AVF obtained using both methods was 0.74 (p < 0.0001). The authors concluded that DU and Delta-H methods are super-imposable for QA determination of the AVF.369
Fontseré et al.381 compared thermodilution and DU, which they used as the reference technique, to measure QA in a cross-sectional study conducted in 64 HD patients using nAVF (54) and pAVF (10). The mean QA obtained by DU was 1426 ± 753 mL/min for nAVF and 1186 ± 789 mL/min for pAVF. The values obtained by thermodilution were 1372 ± 770 for nAVF (bias: 54.6; ICC: 0.923) and 1176 ± 758 for pAVF (bias: 10.2; ICC: 0.992). In the subgroup of 28 patients with end-to-side radiocephalic nAVF, the QA obtained by DU was 1232 ± 767 mL/min.; in the radial artery, 942 (ICC: 0.805); radialulnar artery, 1103 (ICC: 0.973); cephalic vein, 788 (ICC: 0.772) and with thermodilution, 1026 (ICC: 0.971). They concluded that thermodilution is a useful indirect method for QA measurement. In the subgroup of patients with radiocephalic nAVF the sum of QA obtained in radial and ulnar arteries was more accurate. However, thermodilution also had an excellent correlation with the brachial artery.381
Sacquépée et al.382 studied the correlation of QA values obtained using thermodilution and DU in 15 patients dialysed through nAVF (14) and pAVF (1). The QA mean was 1088 ± 576 mL/min measured by DU and 1094 ± 570 measured by thermodilution. The comparison of QA values obtained with both techniques showed a strong linear relationship.
From evidence to recommendation
Due to their high concordance for determining QA of AVF, dilution screening methods such as ultrasound dilution, Delta-H and thermodilution are equivalent to DU.369,370,377-382
→ Clinical question XI Can regulated Doppler ultrasound performed by an experienced examiner replace angiography as the gold standard to confirm significant arteriovenous fistula stenosis?
(See fact sheet for Clinical question XI in electronic appendices)
| Summary of evidence | |
| The values of sensitivity and specificity of DU performed by an experienced examiner to confirm the diagnosis of significant AVF stenosis are high (89.3% and 94.7%, respectively) in contrast to fistulography, but not high enough to be able to replace it | Low quality |
| There are no controlled studies that have assessed the clinical consequences of studying patients with AVF dysfunctions for HD, which can lead us to suspect a possible significant stenosis, only using DU or through angiography | |
Evidence synthesis development
In order to formulate the recommendations in this guide, a meta-analysis was carried out (using the program MetaAnalyst, 11-11-2013) on four studies conducted in the last ten years. These studies provide complete data, thereby making it possible to calculate the sensitivity and specificity of regulated DU compared with fistulography to confirm diagnosis of significant AVF stenosis in patients with clinically suspected stenosis.364,374,383,384 Using data from 755 patients, of which 319 were diagnosed with significant stenosis by fistulography (prevalence: 42.3%), the meta-analysis provided high overall values of sensitivity of 89.3% (95% CI, 84.7-92.6) and a specificity of 94.7% (95% CI, 91.8-96.6) for DU (Figures 3 and 4). These levels are high, but they are insufficient to consider DU as a substitute for fistulography as the “gold standard” for confirming diagnosis of significant AVF stenosis. No diagnostic test which leaves 10% of cases undetected can be considered as a “gold standard”.
However, the answer to this clinical question leads us to ask other questions: Which test should patients initially be assessed with in cases of suspected AVF stenosis: DU or fistulography? Are DU findings enough to indicate elective intervention in patients with suspected stenosis, a suspicion arising from the use of other screening methods?
Clinical decisions are not solely dependent on the sensitivity and specificity of DU to correctly diagnose significant stenosis, but they also depend heavily on real prevalence of significant stenosis among patients with suspected stenosis that arise after applying methods of AVF monitoring and/or surveillance. For fixed sensitivity and specificity, incorrect and accurate diagnoses will be heavily influenced by the prevalence of the pathology being studied. As can be seen in Table 20, the positive predictive value of DU, i.e. the percentage of patients who really present a significant stenosis among those diagnosed by DU, progressively increases as prevalence of significant stenosis rises among patients who are suspected of having one. Thus, when the prevalence of significant stenosis is 50%, the positive predictive value of DU is 94.4%, and this percentage increases as higher prevalence is reached.
Positive (PPV) and negative (NPV) predictive values of Doppler ultrasound according to the prevalence of significant stenosis
| Prevalence of significant stenosis (%) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPV (%) | 0.0 | 65.2 | 80.8 | 87.8 | 91.8 | 94.4 | 96.2 | 97.5 | 98.5 | 99.3 | 100.0 |
| NVP (%) | 100.0 | 98.8 | 97.3 | 95.4 | 93.0 | 89.8 | 85.5 | 79.1 | 68.9 | 49.6 | 0.0 |
| Success (%) | 94.7 | 94.16 | 93.62 | 93.62 | 92.54 | 92 | 91.46 | 90.92 | 90.38 | 89.84 | 89.3 |
The option of using DU as an initial diagnostic test to assess possible significant stenosis would have a significant impact from the start: angiography would no longer be performed on all patients, as it is an invasive test, with potential side effects, and is more expensive for health services.
There is no doubt that there are two patient groups which clearly benefit from using DU without fistulography, because the same conclusion would be reached as in fistulography, patients would be exposed to lower risks and it is economically cheaper. On the one hand, there would be true positives: patients with significant stenosis, for whom preventive intervention of the stenosis would be directly indicated. On the other, there would be true negatives: patients without any stenosis who would be kept on the routine follow-up programme.
It is especially important to consider the false-negative cases, i.e. AVF with significant stenosis in which DU has been unable to establish the diagnosis. In these particular cases, the suspicion will persist despite the DU result and therefore, it seems reasonable to then carry out fistulography, which will end up providing the definitive diagnosis of the stenosis.
Therefore, the use of DU as a first choice diagnostic imaging test for patients with suspected significant stenosis seems to be a sensible decision, both clinically and economically. Given that false-positive rates are low, those who showed positive on the DU could be treated electively, without the need to undergo fistulography for confirmation. In patients with persisting suspected significant stenosis in whom a previous DU was not conclusive, it is advisable to perform fistulography and preventive treatment if the stenosis is then confirmed.
There are no controlled studies which have assessed the clinical consequences of testing patients with AVF dysfunctions that may make us suspect the possible presence of significant stenosis, by means of only DU or angiography.
No relevant studies have been identified regarding patient preferences. It seems logical to think that if there were equal clinical performances, patients would prefer the non-invasive techniques which do not imply exposure to radiation.
No relevant studies have been identified related to the use of resources and costs, either. DU is a less expensive technique than fistulography. The diagnostic approach of using DU at the beginning of the study and keeping fistulography for cases where stenosis is repeatedly suspected, but with a negative result in DU, is more cost-effective than performing fistulography on all patients with suspected stenosis.
From evidence to recommendation
Although it cannot replace fistulography as the “gold standard”, DU is a non-invasive imaging diagnostic method, does not harm the patient, and is highly sensitive and specific for the diagnosis of significant stenosis. Furthermore, it provides valuable additional functional information, its portable version can be used in situ in the HD room and, in addition, it offers a favourable cost-effectiveness profile. For all these reasons, GEMAV unanimously considers that the best diagnostic approach is to perform DU as the initial imaging examination if there is any suspicion of stenosis and keep fistulography for cases of negative outcome and persisting suspicion of stenosis.
Clinical question XI. Recommendations
R 4.7.2) We recommend that Doppler ultrasound be used as the first-choice imaging test in the hands of an experienced examiner, without the need for confirmatory fistulography, to indicate elective treatment in the event of suspected significant stenosis
R 4.7.3) We recommend that fistulography be reserved as a diagnostic imaging exploration only for cases with inconclusive Doppler ultrasound findings and persistent suspicion of significant stenosis
Recommendations
- (•)
NEW R 4.8.1) According to the current concept of significant stenosis, we do not recommend that surveillance of the prosthetic arteriovenous fistula be performed using second-generation screening methods, whether they be dilution methods to estimate the blood flow or Doppler ultrasound
- (•)
NEW R 4.8.2) According to the current concept of significant stenosis, we recommend that first-generation screening methods be used for monitoring the prosthetic arteriovenous fistula
- (•)
NEW R 4.8.3) According to the current concept of significant stenosis, we recommend that both first- and second-generation methods be used for monitoring and surveillance of the native arteriovenous fistula
→ Clinical question XII Which non-invasive monitoring or surveillance screening method for haemodialysis arteriovenous fistula presents predictive power of stenosis and thrombosis and increased patency of the prosthetic arteriovenous fistula in the prevalent patient and what is the frequency?
(See fact sheet for Clinical question XII in electronic appendices)
| Summary of evidence | |
| In 2008 two published meta-analyses questioned the role of AVF surveillance methods according to the current significant stenosis criterion (KDOQI).385,386 The benefit attributed by observational studies to pAVF surveillance methods, in relation to the reduction in the incidence of thrombosis and to the increase in patency rates, disappears in the randomised controlled clinical trials354 | Moderate quality |
| There was no evidence of significant differences in estimating the risk of thrombosis or the definitive loss of the pAVF if surveillance of the pAVF using regular QA determination is added to regular monitoring using first-generation methods. Active surveillance by DU implies a greater use of healthcare resources | |
| The meta-analysis by Tonelli et al.385 showed that active screening with ultrasound is associated with an increased use of PTA and a lower risk of CVC insertions. There were no significant differences in relation to the number of fistulograms, elective interventions or hospital admissions | |
| There are no studies on the cost-effectiveness or budget impact that the generalisation of continued and regular use of active screening techniques in HD patients with a pAVF would suppose in our environment | |
| No relevant studies related to patient preferences have been found | |
Evidence synthesis development
Two systematic reviews with meta-analysis have been found, both published in 2008, which address the clinical effects of pAVF monitoring and surveillance.385,386 The review by Tonelli et al.385 includes only randomised clinical trials (RCTs), whereas the Casey et al review386 also includes non-randomised studies. Both studies found the same clinical trials and come to similar conclusions. For this Guide, the Tonelli et al. meta-analyses were used as they provide more complete data in the stratified analysis for patients with pAVF.385
Clinical benefit of screening compared with usual practice
The systematic review with meta-analysis by Tonelli et al.385 included 6 clinical trials comparing active pAVF screening (using QA or DU measurements) versus usual follow-up by monitoring methods in 446 patients. In this study, there were no significant differences in the rate of pAVF thrombosis between active methods of surveillance and regular monitoring (RR 0.94, 95% CI, 0.77 to 1.16). Using data from 1 clinical trial and 126 patients, there were no statistically significant differences in the time to pAVF thrombosis between the two follow-up options (hazard ratio [HR]: 1.13; 95% CI, 0.71 to 1.80). Meta-analysis with data from 4 clinical trials and 381 patients did not show statistically significant differences in pAVF loss between the active methods of surveillance and those of usual monitoring (HR: 1.08; 95% CI, 0.83 to 1.40). Data from 2 clinical trials and 315 patients also showed no differences in the time to pAVF loss (HR: 0.51; 95% CI, 0.15 to 1.74; high statistical heterogeneity I2: 85%).
Some causes have been reported which may explain these disappointing results obtained by clinical trials in pAVF surveillance316,354,387-394:
- •
Diameter of the artery and the vein involved in anastomosis. This diameter controls the relationship between QA (or venous pressure) and the stenosis. In the event of a low artery/vein ratio, progression of the stenosis is so fast that regular surveillance is unable to detect a decrease in QA (or an increase in venous pressure) before thrombosis.
- •
MAP. The significant decrease in blood pressure can play a central role in some cases of pAVF thrombosis without prior suspicion of stenosis.
- •
Preventive intervention by PTA. In a stable stenotic lesion or slow growth PTA can stimulate intimal hyperplasia, lead to rapidly developing restenosis and have a negative impact on pAVF patency.
- •
Sample size. An insufficient sample size in the published studies could explain the results obtained.
From evidence to recommendation
There are no significant differences in the risk of thrombosis or in survival of the pAVF if surveillance methods are added to usual methods of monitoring. Therefore, according to the current concept of significant stenosis included in the KDOQI guide,10 we cannot recommend pAVF surveillance using second-generation screening methods, whether they be dilution techniques to estimate the QA or DU. The application of these methods in pAVF is not predictive of thrombosis and will not help increase their patency compared with first-generation methods, based on current criteria for significant stenosis. It is recommended that pAVF monitoring be performed using first-generation screening methods.287
Clinical question XII. Recommendations
R 4.8.1) According to the current concept of significant stenosis, we do not recommend that surveillance of the prosthetic arteriovenous fistula be performed using second-generation screening methods, whether they be dilution methods to estimate the blood flow or Doppler ultrasound
R 4.8.2) According to the current concept of significant stenosis, we recommend that first-generation screening methods be used for monitoring the prosthetic arteriovenous fistula
→ Clinical question XIII Which non-invasive monitoring or surveillance screening method for haemodialysis arteriovenous fistula presents predictive power of stenosis and thrombosis and increased patency of the native arteriovenous fistula in the prevalent patient and what is the frequency?
(See fact sheet for Clinical question XIII in electronic appendices)
| Summary of evidence | |
| Clinical monitoring by means of physical examination is a test with high sensitivity and acceptable specificity, providing high positive and negative predictive values for the diagnosis of significant nAVF stenosis. Active surveillance using dilution and DU methods reduces the risk of thrombosis and the need to use CVC for HD. Currently, there is no evidence against the routine application of second-generation screening methods (dilution techniques to determine QA and DU) for AVF surveillance. Both first- and second-generation methods are effective in reducing the incidence of nAVF thrombosis | Low quality |
| There is no reliable evidence to make any recommendations on the frequency of application of second-generation methods | |
| There are no studies on the cost-effectiveness or budget impact that the generalisation of continued and regular use of active screening techniques through QA measurements and DU would suppose in these patients in our setting | |
Evidence synthesis development
First-generation methods
A prospective study by Asif et al.204 of 142 patients with nAVF analysed the accuracy of physical examination in detecting stenotic lesions in comparison with fistulography, which is considered the gold standard test. The sensitivity and specificity of physical examination was 92% and 86%, respectively, for outflow stenosis and 85% and 71% for inflow stenosis.
A study by Campos et al.303 analysed the accuracy of physical examination and pressure measurement in detecting stenotic lesions in comparison with DU, which they used as the reference technique. Out of the 84 patients analysed, 50 of them, i.e. 59%, showed positive for stenosis by DU. Upon physical examination 56 patients showed positive, representing a sensitivity for the test of 96%, a specificity of 76%, a positive predictive value of 86% and a negative predictive value of 93%. Intra-access pressure measurement for 34 patients showed positive, i.e. 40%, representing a sensitivity for the test of 60%, a specificity of 88%, a positive predictive value of 88% and a negative predictive value of 60%.
Second-generation methods
With respect to second-generation methods, several published meta-analyses should be highlighted.385,386,395 On analysing data from four controlled clinical trials and 360 patients, Tonelli et al.385 describe that active screening by ultrasound causes a statistically significant decrease in the risk of nAVF thrombosis (RR: 0.47; 95% CI, 0.28-0.77). In addition, the time to reach nAVF thrombosis was significantly higher in the “surveillance” group compared to the “control” group (HR: 0.30; 95% CI, 0.16-0.56). Regarding AVF loss, when carrying out a meta-analysis with data from 2 RCTs and 141 patients, no statistically significant differences were found (RR: 0.65; 95% CI, 0.28 to 1.51), and, finally, with data from 1 RCT and 60 patients, slightly statistically significant differences were found with respect to time to access loss (HR: 0.38; 95% CI, 0.14 to 0.99).385
Moreover, after analysing the functional criteria selected for the diagnosis of stenosis in various controlled and uncontrolled clinical trials as well as observational studies, Tessitore et al.395 concluded that nAVF surveillance through QA determination significantly reduces the risk of thrombosis. In this respect, a case-control study from Spain demonstrates a significantly lower incidence of AVF thrombosis (mostly nAVF) in patients dialysed in a hospital HD unit and under QA surveillance compared with patients dialysed at a satellite HD centre without QA measurements352. Salman et al.396 analysed 4 RCTs (n = 395) to assess the benefit of nAVF surveillance using QA determination and the result was positive for the 3 trials in which the main aim was to reduce the rate of thrombosis.396 Muchayi et al.397 performed a meta-analysis on these same 4 studies and showed a non-significant reduction of 36% in thrombosis risk by nAVF surveillance.
Concerning nAVF patency, the meta-analysis by Tessitore et al.,395 carried out on two controlled clinical trials, demonstrated a 50% reduction in risk of nAVF loss using screening with QA determination, but the difference was not statistically significant given that they are two single-centre studies (Verona, Italy) with a limited sample size and follow-up.276,398 Recently, the preliminary results have been published from a controlled, multicentre clinical trial carried out in Spain (METTRO) on the effect of second-generation methods compared with conventional monitoring on the incidence of thrombosis and patency of nAVF. These results show a significantly lower rate of thrombosis and better primary assisted patency after 1 year of follow-up.399
The implementation of second-generation screening techniques for nAVF surveillance makes it possible to reduce the incidence of thrombosis and, therefore, decrease the rate of CVC placement and its associated morbidity/mortality.269,368,385
Use of resources and costs
No specific cost-effectiveness studies were found when these interventions were analysed for the setting in which this Guide is to be applied. Neither are there studies on the budget impact that the generalisation of continued and regular use of active screening techniques by DU would suppose on nAVF patients in our setting.
From evidence to recommendation
The regular application of second-generation screening methods (both dilution techniques to estimate AVF flow or QA and DU) is recommended for nAVF surveillance as existing evidence indicates a beneficial effect with relation to the reduction in thrombosis incidence and CVC placement rate. There are no arguments against such methods in relation to the rate of nAVF thrombosis and patency.
4.9Predictive factors of thrombosis in arteriovenous fistula with stenosisRecommendations
- (•)
NEW R 4.9.1) We recommend that a stenosis be considered significant when there is any reduction in the vascular lumen in native or prosthetic arteriovenous fistulae, shown by Doppler ultrasound, which meets all the criteria for high risk of thrombosis (the 2 main criteria and at least 1 additional criterion)
- (•)
NEW R 4.9.2) We recommend that an elective intervention be performed without delay by percutaneous transluminal angioplasty and/or surgery when the diagnosis of significant arteriovenous fistula stenosis is established because of the high risk of thrombosis
- (•)
NEW R 4.9.3) We recommend that a stenosis be considered non-significant when there is any reduction in the vascular lumen in native and prosthetic arteriovenous fistulae, shown by Doppler ultrasound, which does not meet all the criteria for high risk of thrombosis
- (•)
NEW R 4.9.4) We recommend that the elective intervention not be performed when a diagnosis of non-significant stenosis is established in an arteriovenous access because of the low risk of thrombosis
- (•)
NEW R 4.9.5) We recommend that all non-significant arteriovenous fistula stenosis be strictly controlled using second-generation screening methods due to the risk of progression to significant
→ Clinical question XIV What are the demographic, clinical and haemodynamic factors and variables with predictive power of thrombosis in an arteriovenous fistula that presents stenosis?
(See fact sheet for Clinical question XIV in electronic appendices)
| Summary of evidence | |
| No studies that analyse different possible thrombosis risk factors have been found, either separately or not, specifically in patients with AVF stenosis | Low quality |
| The research did not reveal any factor that separately is a good predictor of risk of AVF thrombosis | |
| The results of studies analysing the usefulness of different QA measurements in predicting thrombosis in the access, and using ROC curve analysis, indicate that the QA surveillance methods are poor at predicting pAVF thrombosis, and could make many patients undergo many unnecessary and costly procedures | |
| With regard to other factors not related to AVF follow-up, none of the published studies found increased risk of thrombosis in patients with higher levels of homocysteinemia | |
| No relevant studies related to patient values and preferences have been found | |
| No relevant studies related to the use of resources and costs have been identified | |
| However, based on currently existing data, there are some morphological and functional factors that are considered additional criteria with sufficient predictive power to discern whether an nAVF or a pAVF with stenosis has a high or low risk of thrombosis. Therefore, the concept of significant stenosis should be modified to include only those AVF with an increased risk of thrombosis because they have additional risk criteria for thrombosis and really require corrective intervention | |
Evidence synthesis development
The application of various methods of AVF surveillance in routine clinical practice has shown cases of AFV thrombosis preceded by a QA value > 600 mL/min without apparent stenosis, as well as cases of stenosis > 50% which are stable over time and never actually become thrombosed.400-403 Therefore, in the case of any vascular reduction > 50% in nAVF or pAVF, it is necessary to know its thrombosis risk. It is important to identify whether this stenosis involves a high risk of thrombosis, i.e. a high likelihood of progressing over time and leading to total vascular lumen occlusion of the AVF if an early elective intervention through PTA or surgery is not carried out. However, if we perform preventive treatment when there is a vascular lumen reduction > 50% of nAVF or pAVF with a low thrombosis risk, in addition to this being an unnecessary procedure with a noteworthy financial cost, we may cause an unwanted accelerated restenosis and AVF thrombosis which would not have occurred with therapeutic abstention.
It is necessary to identify factors or variables (demographic, clinical, haemodynamic) which are predictive of thrombosis in any AVF with stenosis. The presence or absence of these will define the existing risk of thrombosis and, therefore, make it possible to distinguish if any vascular lumen reduction > 50% in nAVF or pAVF has a high or low risk of thrombosis.
In this respect, Paulson et al.403 demonstrated in a prospective study in 2000, through ROC curve analysis, that the functional variable QA did not provide enough predictive value of pAVF thrombosis on its own for it to be used as an isolated criterion in decision-making. They thus concluded that the inclusion of other predictive variables in association with QA could provide the predictive value required.
In 2005, Malik et al.367 published a randomised clinical trial in 192 patients with pAVF comparing pAVF patency (mean follow-up 392 ± 430 days) between two subgroups of patients who were categorised according to the different monitoring and surveillance strategy applied: group 1 (n = 97) using traditional screening (clinical monitoring, venous pressure, and QA) associated with DU surveillance every 3 months and group 2 (n = 95) only by traditional screening. Cumulative pAVF patency was significantly higher in group 1 compared to group 2, which the authors attributed to the early diagnosis of stenosis and, therefore, more common elective procedures on the pAVF stenosis.367 Unlike other clinical trials on pAVF with negative results,404 why was this positive result obtained in this study? The answer lies in the methodology, as DU indications for carrying out fistulography and/or a therapeutic procedure using PTA or surgery were as follows:
- •
Finding a significant stenosis, defined by the presence of a peak systolic velocity (PSV) ratio > 2 with or without decrease in QA.
- •
Finding a non-significant stenosis in appearance but associated with a decrease in QA > 25% over time.
In the case of uncertainty over significant or non-significant stenosis, the existence of a residual diameter of less than 2.0 mm was an additional indication for fistulography. Patients that only met one of the above-mentioned criteria were examined after 4-6 weeks by DU.367 In other words, in this study a series of additional ultrasound factors were introduced which allowed preventive action to be taken on significant stenoses with the risk of thrombosis and pAVF patency to be prolonged.
In 2009 the same group published a retrospective study in which pAVF stenoses were classified by DU into 2 distinct groups375:
- •
Significant pAVF stenosis or high risk of thrombosis defined by a combination of the following criteria: reduction > 50% in the vascular lumen + PSV ratio > 2 + at least 1 additional criterion (residual diameter < 2 mm or QA < 600 mL/min or decreased QA > 25%).
- •
Borderline pAVF stenosis or low thrombosis risk (n = 102): defined with the same criteria but without any additional criteria.
The first group of stenoses was treated electively by PTA and the second underwent a strategy of “wait and see” with repeated DU after 6 – 8 weeks. After 14 ± 6 weeks, the follow-up of the 102 borderline stenoses was as follows: 55 without stenosis progression, 38 with an increase in the degree of stenosis, 8 treated using PTA because of clinical indication and 1 single case of thrombosis375; in other words, at the time of the next DU (14 ± 6 weeks), more than half of the borderline stenoses remained stable over time with a risk of thrombosis < 1%. The significant risk factors for progression of borderline stenosis to significant stenosis were a history of previous PTA and female gender. The authors concluded that delaying PTA in asymptomatic borderline stenosis is safe using this expectant management and stenoses remain stable, at least in the short term, but with a high risk of progression, especially if there is a history of previous PTA.37 In Spain, when a similar protocol for selecting AVF with a greater risk of thrombosis was implemented, a thrombosis rate < 0.05/patient/year was achieved.52
To sum up, according to current data, AVF surveillance methods could be optimised for both nAVF and pAVF by redefining the concept of significant stenosis, which would include only those AVF with an increased risk of thrombosis and, therefore, really require elective intervention (Figure 5).405 In this way, in addition to the criteria of stenosis according to the current KDOQI Guide, the haemodynamic repercussion of the stenosis should be assessed and some additional criteria added, both morphological and functional, that have enough predictive power to discern whether nAVF or pAVF with stenosis has a high or low risk of thrombosis.394 These criteria are:
- •
Two main criteria: vascular lumen reduction percentage > 50% + PSV ratio > 2.
- •
At least one of the following additional criteria: morphological criterion (residual diameter < 2 mm) or functional criterion (QA [mL/min] < 500 [nAVF]-600 [pAVF] or ∇QA > 25% if QA < 1000 mL/min).
From evidence to recommendation
Optimising AVF surveillance methods requires redefining significant stenosis. Some ultrasound variables, both morphological and functional, have been described that allow the risk of thrombosis of stenosis to be clarified and therefore, whether this stenosis really requires elective intervention. The new concept of significant stenosis would only include stenosis with high risk of thrombosis. As a result, some criteria were established to define it (Figure 5): some main criteria (reduction of vascular lumen > 50% + PVS ratio > 2) and some additional criterion should be added (residual diameter < 2 and/or QA < 500 mL/min in nAVF/< 600 mL/min in pAVF and/or reduction in QA > 25% if QA < 1000 mL/min). This redefinition of significant stenosis would result in a series of benefits over AVF, such as the decrease in unnecessary procedures that may endanger the AVF itself, the reduction in the thrombosis rate and the increase in patency (Table 21).
Theoretical implications in the diagnosis, treatment and prognosis of redefining significant stenosis in arteriovenous fistula (AVF) for haemodialysis
|
PTA, percutaneous transluminal angioplasty.
Clinical question XIV. Recommendations
R 4.9.1) We recommend that a stenosis be considered significant when there is any reduction in the vascular lumen in native or prosthetic arteriovenous fistulae, shown by Doppler ultrasound, which meets all the criteria for high risk of thrombosis (the 2 main criteria and at least 1 additional criterion)
R 4.9.2) We recommend that an elective intervention be performed without delay by percutaneous transluminal angioplasty and/or surgery when the diagnosis of significant arteriovenous fistula stenosis is established because of the high risk of thrombosis
R 4.9.3) We recommend that a stenosis be considered non-significant when there is any reduction in the vascular lumen in native and prosthetic arteriovenous fistulae, shown by Doppler ultrasound, which does not meet all the criteria for high risk of thrombosis
R 4.9.4) We recommend that the elective intervention not be performed when a diagnosis of non-significant stenosis is established in an arteriovenous access because of the low risk of thrombosis
R 4.9.5) We recommend that all non-significant arteriovenous fistula stenosis be strictly controlled using second-generation screening methods due to the risk of progression to significant
CONTENTS
- 5.1.
Treatment of stenosis
- 5.2.
Treatment of thrombosis
- 5.3.
Management of the non-matured fistula
- 5.4.
Treatment of infection
- 5.5.
Distal hypoperfusion syndrome (“steal syndrome”)
- 5.6.
Aneurysms and pseudoaneurysms
- 5.7.
High-flow syndrome
Preamble
The aim of treating arteriovenous fistula (AVF) complications is to address the different types of pathology that may affect AVF. These include, on the one hand, treatment of stenosis and thrombosis to achieve the greatest possible patency time and, on the other hand, complications not directly related with patency, such as infection, distal hypoperfusion, aneurysms or pseudoaneurysms and complications derived from high blood flow (QA).
5.1Treatment of stenosisRecommendations
- (•)
NEW R 5.1.1) We suggest surgical treatment of juxta-anastomotic stenosis of the native arteriovenous fistula be performed provided a central venous catheter does not need to be placed
- (•)
NEW R 5.1.2) We suggest venous juxta-anastomotic stenosis of the prosthetic arteriovenous fistula be treated indistinctly by angioplasty or surgical intervention
NEW R 5.1.3) We suggest non juxta-anastomotic stenosis of the native arteriovenous fistula initially be treated using angioplasty because it is less invasive than surgery
R 5.1.4) We recommend fistulography be performed if central venous stenosis is clinically suspected
- (•)
NEW R 5.1.5) We recommend only central vein stenosis that are symptomatic be treated
- (•)
NEW R 5.1.6) We recommend endovascular therapy be performed using percutaneous transluminal angiography with balloon as the first treatment option for central stenosis
- (•)
NEW R 5.1.7) We suggest the use of stents be limited to selected cases where there is technical failure of angioplasty and frequent relapse of stenosis, and we recommend they not be used in venous confluents
NEW R 5.1.8) We suggest that angioplasty be used as the initial treatment in stenosis in the cephalic vein arch. Treatment by stent placement or by surgical transposition of the cephalic vein may also be considered
Rationale
The aim of correcting stenosis requiring elective treatment is to ensure sufficient QA, proper haemodialysis (HD) adequacy, to prevent the occurrence of thrombosis and to increase AVF patency. Only significant stenosis should be treated electively as described in section 4.
Types of stenosis
Anatomically and functionally, vascular stenosis with haemodynamic repercussion in the AVF function can be located in the segment prior to the arteriovenous anastomosis (arterial stenosis), in the anastomosis itself or in the outflow vein of the AVF (venous stenosis).
- •
Arterial stenosis. Vascular lesions located in the arterial tree that feeds the vascular access (VA). The haemodynamic alteration they cause is a decrease in AVF flow. It is mainly due to the presence of stenosing or occlusive lesions arising from the progression of an existing underlying atherosclerosis.
- •
Stenosis in the arteriovenous anastomosis. They are usually due to a technical problem during anastomosis creation. Clinically, they present as immediate or early thrombosis of the access or as alterations in maturation (non-mature fistula).
- •
Venous stenosis. It is the most common cause of access dysfunction. Depending on location along the venous pathway, aetiology, frequency and response to treatment vary. Therefore, it is usually classified into four groups:
- –
Juxta-anastomotic or peri-anastomotic stenosis. This is the one located in an area covering the zone immediately adjacent to the anastomosis and up to 5 cm post-anastomosis. It is of complex aetiopathogenesis, involving haemodynamic factors and alterations in the inflammatory response of the endothelium.
- –
Stenosis of the cannulation segment. Stenosis located in needling areas. It usually occurs in response to mechanical trauma caused by cannulation of the vessel.
- –
Stenosis of the cephalic vein arch (CVA). Stenosis located in the cephalic vein segment immediately adjacent to its confluence in the axillary vein. Like stenosis located in the juxta-anastomotic region, it is usually due to haemodynamic factors, presenting a poor response to percutaneous treatment.
- –
Central venous stenosis. Stenosis located in the venous sector from the subclavian vein to its drainage in the right atrium, and covers the subclavian vein, brachiocephalic trunk and superior vena cava. It is usually associated with endothelial trauma caused by the presence of venous catheters inside the vessel.
- –
Another classification used in different publications prioritises a criterion of functionality in relation to the cannulation point, classifying them between inflow stenosis (arterial stenosis, arteriovenous anastomosis and the juxta-anastomotic venous segment) and outflow stenosis (venous stenosis of the cannulation segment, CVA, and central venous stenosis).374,406
As described, stenosis location is the main determining factor when considering therapeutic option. In this context, the success of the results should weigh up not only the efficacy of the treatment, but also any possible associated comorbidity and complications.
There are several examples that can demonstrate this factor, as will be seen below. There is consensus that central vessel (arterial or venous) stenosis be given endovascular treatment as it is difficult to access these vessels in surgery and there is high morbidity and mortality.10,407 Venous stenosis of the needling segment has traditionally been treated by percutaneous transluminal angioplasty (PTA) because HD can be continued using the same VA and without the need for CVC placement. On the other hand, there has arisen more controversy in the treatment of juxta-anastomotic stenosis, which entails most AVF stenosis, in both native arteriovenous fistula (nAVF) and prosthetic arteriovenous fistula (pAVF), since they can be approached from both a surgical and an interventionist point of view, although the first usually offers better overall results than percutaneous treatment.
Types of treatment
Stenosis can be treated either by an endovascular procedure consisting of PTA and/or endoprosthesis placement or by surgical review.
In general terms, percutaneous treatment is the least invasive alternative, has lower morbidity and does not require CVC placement to continue HD. However, a significant disadvantage is that it presents a high rate of restenosis which determines the need to periodically perform additional interventional procedures to maintain access patency.
On the other hand, surgical treatment usually has better primary patency in the medium and long term, although, in terms of drawbacks, it is more invasive and sometimes requires the use of venous capital and CVC placement for HD after the intervention. Thus, even though the technique provides better results overall, in daily clinical practice treatment must be decided on a case-by-case basis, precisely delimiting if the greater patency of the procedure justifies the possible consumption of venous segment and the possibility of CVC placement.
Percutaneous transluminal angioplasty
PTA is a percutaneous technique of intravascular dilation using a balloon that allows the treatment of vascular stenosis. In addition to the use of the conventional balloon, the technical improvements that have arisen in recent years for the treatment of stenosis have allowed the development of high-pressure balloons, cutting balloons and drug-coated balloons.
The advantages of PTA include the fact that it can be performed during diagnosis by fistulography, especially in the case of central venous stenosis, and preserves the vascular tree, unlike surgery. On the other hand, it has a higher recurrence rate versus surgery. The success of the procedure can be considered from the anatomical and functional perspective: anatomically, when residual stenosis is < 30% after balloon removal and, functionally, with the improvement of AVF haemodynamic parameters and the restoration of flow (QA).
The only absolute contraindication for this procedure is the active AVF infection. Relative contraindications would include allergy to the contrast, a shunt from pulmonary to systemic circulation and severe pulmonary disease.
High pressure balloons
High pressure balloons are those that bear an inflation pressure higher than 25-30 atm. Their use is indicated in the treatment of symptomatic stenosis that has not responded to dilation with conventional semi-compliance balloons. The use of high pressure balloons does not initially provide better patency results when compared to conventional balloons.408 High cost, the need to use thicker introducers, emptying difficulty and lower compliance and flexibility make it advisable not to use them as first choice in the treatment of stenosis
Cutting balloon
When small blades or atherotomes are incorporated into a conventional balloon, they are called cutting balloons. Its use is controversial and is not justified as initial treatment for stenosis. In a recent randomised study,409 no significant differences were found in the treatment of stenosis between cutting balloon and conventional balloon except for a greater primary assisted patency at 6 and 12 months, in favour of the cutting balloon, when treating juxta-anastomotic venous stenosis of pAVF (86% and 63% versus 56% and 37%). However, higher cost, management difficulties (they need finer guides) and the larger size of the introducer make them less indicated for the initial treatment of a dysfunctional access.
Eighty-five per cent of stenosis responds satisfactorily to conventional balloon angioplasty.410 In the rest, which do not have an appropriate response, both high pressure and cutting balloons would be useful. Existing studies comparing both procedures find no significant difference in immediate outcomes410-413 but there was an increase in the 6-month assisted patency of stenosis treated with cutting balloon versus high-pressure balloon (66.4% versus 39.9%).410-413
Angioplasty with pharmacoactive balloon
Drug coated balloons impregnated with paclitaxel have recently appeared as an alternative in the treatment of arterial stenosis. Application in nAVF stenosis is very low, though some randomised clinical trials (RCTs) with satisfactory results at 6 months414 and at 1 year follow-up415 have been reported.
Stents
Indications for stent placement are limited given the lack of evidence regarding improvement of secondary VA patency after their use. This controversial use is relegated to the treatment of stenosis with recoil, vascular ruptures after PTA or in dissections that condition stenosis > 30%.
It can be considered for use with refractory AVF following early recurrence (< 3 months) after several PTA and due to vascular recoil (elastic stenosis) following PTA.416 However, stent use is highly controversial in these two indications, given that on the one hand, a certain number of AVF maintain an adequate function even if there is residual post-PTA stenosis up to 50%, and on the other, there are early recurrences (< 3 months) in angioplasties with good immediate outcomes.417,418
Regarding stent use for vessel rupture treatment, it should be noted that this is the most frequent PTA complication. The initial treatment is tamponade with prolonged inflation at low pressure and external manual compression at the point of rupture. After three failed attempts, the placement of covered prostheses is considered indicated.417,418
Vascular endoprosthesis
Recently stent graft has been increasingly used in an attempt to improve outcomes. A controlled multicentre study found a patency of covered stents at 6 months that was significantly higher (51% versus 23%) than simple PTA in the treatment of venous anastomotic stenosis in pAVF.419 Use in other locations has been reported on several occasions,420,421 with better outcomes than those obtained with PTA alone or with placement of uncovered metal stents.422,423 In the most recent study of Schmelter et al.,424 conducted on 66 AVF (41 pAVF and 25 nAVF), found good initial results but with no increase in overall patency. They observed a high rate of restenosis and thrombosis, although not associated with the stent graft, which were responsible only for a minority of the new cases of dysfunction. The authors conclude that the placement of covered stents can be used to solve local problems but they do not improve the average patency of the VA because they are associated with lesions situated in other locations.
Regarding endoprosthesis-related disadvantages, it is important to emphasise that there is great difficulty in creating new accesses in the treated vein segment and can be associated with a not insignificant percentage of complications.420,425 Although stent placement may increase the interval between first dilatation and stenosis recurrence, once intra-stent neointimal hyperplasia stenosis is established, it is very difficult to treat. Recent studies must be added here, in which a high percentage of post-stent complications are observed (28.9%),426 as well as others describing migrations,427 fractures428 and infections.429,430
Ultimately, it can be concluded that further multicentre, randomised, prospective, multidisciplinary studies are required to adequately rate the advantages of the new versus traditional materials when performing PTA, the usefulness of stents and their benefit or disadvantages versus surgical treatment.
Surgical treatment
There are multiple surgical techniques described for AVF stenosis correction. The major advantage of this type of treatment is that it tends to have better patency rates than endovascular, but has higher morbidity, depletes the venous segment, may require CVC placement and is technically more complex, especially in central vessels.
Arterial stenosis
In the case of stenosis located in the arterial segment prior to the arteriovenous anastomosis, endovascular treatment through PTA presents low morbidity and acceptable results, which is why surgery is considered as a fall-back technique. Surgical revascularisation is performed through the interposition of a bypass of autologous material, presenting excellent patency in the medium and long term.
Anastomotic stenosis
In the case of stenosis located in the arteriovenous anastomosis (related to the surgical procedure to create the access), surgical review of the anastomosis, as well as the correction of the underlying technical defect, are indicated.
Juxta-anastomotic stenosis
In many cases, reanastomosis between the artery and the outflow vein in the area immediately proximal to the AVF is the surgical technique of choice. Likewise, the interposition of a bypass made of prosthetic material has been reported between the artery and the proximal sector of the outflow vein.
Stenosis of the cannulation segment
In the event of stenosis of the venous cannulation segment, the surgical treatment of choice consists of interposing a bypass made of prosthetic material, which can be placed in the shape of a loop, to allow the newly implanted segment to be cannulated.
Stenosis of the cephalic arch
As discussed below, the technique of choice consists of transposing the cephalic vein and its anastomosis with the proximal brachial or axillary vein. The surgical re-implantation of this cephalic arch has also been described.
Central venous stenosis
As surgery in central veins is complex and aggressive, it is considered a fall-back technique. Interventions using extra-anatomical derivative techniques to allow drainage to central venous trunks have been reported.
→ Clinical question XV Is there a treatment with better outcomes (percutaneous transluminal angioplasty versus surgery) in juxta-anastomotic stenosis, assessed in terms of patency and/or thrombosis and cost/benefit?
(See fact sheet for Clinical question XV in electronic appendices)
| Summary of evidence | |
| There is no conclusive evidence for mature nAVF with stenosis. The available data come from 2 comparisons of clinical studies,431,432 with non-homogeneous results. Both articles find better results in surgery but only in terms of primary patency. A meta-analysis of four clinical studies has shown similar results for the primary patency of AVF at 12 and 18 months follow-up433 | Low quality |
| There is little evidence in the literature on venous anastomotic stenosis in pAVF, a prevalent lesion in the thrombosis in these accesses. Only one RCT has been found comparing the results of surgery and PTA, in a study dating back to 1987,434 in which results for surgery were favourable. However, given the difficulties of performing surgery, these stenosis have traditionally been treated with PTA, with good primary results but with a high recurrence percentage. The use of stent graft could improve patency, although longer-term studies are needed to recommend its use419 | |
Evidence synthesis development
Native arteriovenous fistula
No clinical trials comparing PTA versus surgery for the treatment of stenosing AVF in patients with nAVF have been identified. Two publications comparing series of patients treated with surgery and patients treated with PTA have been identified.
In the study of Napoli et al.,432 conducted on 66 PTA and 68 surgical procedures with juxta-anastomotic stenosis of the AVF, the efficacy of the interventions was evaluated by measuring the brachial artery flow. The comparative analysis between the two options showed a significantly better primary patency for surgery, but with no difference in primary assisted patency, although PTA showed a greater tendency to restenosis.
Tessitore et al.431 conducted a retrospective analysis of clinical data of 64 patients with juxta-anastomotic stenosis of the fistula in the distal part of the forearm, of which 43 were treated with PTA and 21 with surgery. The restenosis rate was 0.168 and 0.519 events per year of fistula follow-up for surgery and PTA, respectively (p = 0.009), with an adjusted relative risk 2.77 times higher for PTA than for surgery. The cost profile was similar for both procedures. Both procedures show similar primary assisted patency and costs.
The other studies evaluate the two techniques individually. Thus, in an article from 2012435 evaluating the medium-and long-term results of surgery in juxta-anastomotic stenosis in 96 radiocephalic nAVF, the authors found very high results for immediate patency, without the need for CVC. Primary patency was higher than that recommended in international guidelines (89% versus 50%) with a low rate of maintenance procedures (0.035 procedures/patient/year). These patency data are superior to those shown in the study by Mortamais et al.,436 where the results of angioplasty are evaluated in 147 procedures performed on 75 radiocephalic nAVF. They obtained a primary patency at 1 and 3 years of 46.6% and 25.5%, respectively, with assisted patency in the same periods of 81.3% and 63.2%. They associate worse outcomes and early relapse of stenosis to the presence of post-PTA residual stenosis > 50%. They consider that in these cases evaluation and surgical repair would be indicated.
Although neither study conducted a comparative analysis with the other repair technique, their results support the use of surgery as an initial technique in the treatment of juxta-anastomotic stenosis, provided that there is a surgical team which has 24-hour availability and repair can be executed without the use of CVC.
Recently, a meta-analysis including the clinical series discussed in this section has shown results similar to those of the original studies. The combined results from the case study data showed significantly better primary patency of the AVF in patients treated with surgery at 12 (odds ratio [OR]: 0.42) and 18 months (OR: 0.33), an effect that seems to become moderate at 24 months of follow-up (OR: 0.53).433
Prosthetic arteriovenous fistula
Only one RCT has been identified in the literature comparing surgery and PTA in patients with pAVF and juxta-anastomotic stenosis.434 This is the RCT of Brooks et al.,434 which included 43 patients with venous stenosis at the pAVF access in the forearm, 19 of which were treated with surgery and 24 with PTA. Those treated with surgery obtained greater median long-term patency (12 months) versus PTA (4 months) (p < 0.01). It is not mentioned whether CVC was needed to perform any of the procedures.
No more recent studies comparing both procedures have been found, although there are several studies in the literature that support PTA use in the treatment of these lesions437 versus surgery167 as it allows proximal venous capital to be preserved, is more widely accepted by the patient and it is difficult to treat proximal anastomoses located on brachial and axillary veins surgically.8 Surgery is reserved for failed PTA treatment and prior to stent placement as well as in cases of recurrence.167,438
Stent use with stenosis recurrence has not improved patency439 in the same way as other technical improvements such as high pressure408 or cutting440 balloons, among others, have. Recently, however, a multicentre controlled study found significantly greater patency in the treatment of anastomotic venous stenosis at 6 months using covered stents versus simple PTA (51% versus 23%).419 The study is limited as long-term follow-up was not performed. In a more recent article424 where a retrospective study was conducted on 41 patients with complex pAVF stenosis (defined as rigid and resistant stenosis, stenosis with recoil or intra-stent stenosis) treated with vascular endoprosthesis (stent graft), good results in primary patency, but elevated restenosis and thrombosis rates, are obtained. Restenosis, however, is not located in the stent graft in place and is only responsible for a few cases of new dysfunctions. The authors conclude that the placement of stent grafts can be used to solve local problems but they do not improve the average patency of the access because it is associated with lesions in other locations.
The use of stents is controversial, though there seems to be agreement on the use of stent graft versus uncovered stents. Several articles in the literature have found an improvement in the primary patency rates of these devices versus PTA and uncovered stents.420,421,441,442 The increase in primary patency, according to some authors, seems to be related to a lower presence or absence of neointimal hyperplasia inside the stent graft.442,443
From evidence to recommendation
In the case of nAVF, and although observational studies report no differences between both techniques, as there are no randomised cost-benefit studies, and despite the advantages and drawbacks of using both (PTA does not deplete the bed, but requires procedures to be repeated, and surgery depletes the vessel, but may still allow cannulation and has better primary patency), studies coincide that there is better patency with surgery, though assisted patency is similar. Surgery, therefore, can be considered the initial indication if it is technically possible, as it requires fewer procedures to maintain patency. However, if surgery requires catheter placement, the endovascular technique should be considered as the first option.
This recommendation was submitted to a vote by GEMAV. The wording of the recommendation was unanimously accepted. However, the number of members of the working group who felt that the recommendation should be strong (one third) was not sufficient to award it this category. The remaining members felt that it was weak, or abstained in the vote.
In pAVF, endovascular therapy is more advantageous as it is less invasive than surgery; it does not deplete the venous bed and does not exclude surgical procedure. Thus, despite its higher cost and lower primary patency rate, it can be considered an equally valid therapeutic option to surgery. However, until the publication of comparative studies with surgery, a degree of evidence cannot be established in favour of either technique.
The use of covered stents (stent graft) to treat early recurrence of venous stenosis in prosthetic fistulae seems to provide an improvement in medium-term survival but more studies and longer-term assessment are needed to recommend use.
Clinical question XV. Recommendations
R 5.1.1) We suggest surgical treatment of juxta-anastomotic stenosis of the native arteriovenous fistula be performed provided a central venous catheter does not need to be placed
R 5.1.2) We suggest venous juxta-anastomotic stenosis of the prosthetic arteriovenous fistula be treated indistinctly by angioplasty or surgical intervention
Treatment of non-perianastomotic stenosis
Non-perianastomotic venous stenosis, i.e. those located proximally to the juxta-anastomotic area, also referred to as the middle segment or needling area, are usually caused by mechanical trauma during AVF cannulation, and can be associated with the aneurysmal degeneration of the vein,444 with risk of skin necrosis, and with bleeding after HD sessions.445 They might not be associated with alterations during HD or to pump flow (QB) problems and, as a result, they may remain undetected if there is no careful clinical assessment or follow-up.
Treatment options include surgical repair by performing a prosthetic bypass or percutaneous repair using PTA. Although there are studies in the literature comparing both techniques, there are no randomised studies and they are unable to establish a better treatment option. Despite showing that the results of surgery are better in terms of patency,446 most support the initial use of percutaneous AVF treatment since they treat dysfunctional AVF less aggressively.
Although endovascular treatment is not a permanent solution, it is effective in increasing patency; it is a relatively non-invasive, repeatable technique that rarely requires CVC placement and preserves vascular bed integrity without compromising subsequent surgical procedures.
Surgical treatment of stenosis in this location includes creating a bypass excluding the stenotic segment; likewise, its placement in the shape of a loop may be considered, thereby lengthening the cannulation area. Surgery in the access cannulation area may cause the need for temporary HD through CVC, which is the main limitation of the technique. In contrast, when stenosis is associated with aneurysmal dilatation with cutaneous disorders, surgery can treat both during the same intervention.
In studies in the literature, results of surgery versus endovascular treatment are better in terms of the primary patency rate,446 but similar in assisted patency. These same studies support the initial use of percutaneous treatment because it is relatively non-invasive, can be performed in an outpatient’s clinic, avoids CVC and preserves the vascular bed, allowing new surgical procedures. However, PTA and surgery should be considered complementary and uncompetitive techniques.
There is no evidence to support the use of stents in the treatment of stenosis and it is recommended they not be used except in early and repeated recurrences after PTA of middle segments of nAVF and in vein ruptures that do not respond to balloon compressions.
It is advisable to keep in mind that heart failure or distal ischaemia after PTA should be prevented in patients at risk, especially if the flow is high.194,447 In these cases it is important not to over-dilate the stenosis, mainly in the first PTA and in those located in the arm, to avoid an excessive increase in QA. In patients at risk, such as diabetics or the elderly, caution should be exercised, and it is advisable to avoid using balloons with a diameter > 7 mm. If there is recurrence and there are no signs of ischaemia, the stenosis may be over-dilated 1 mm more.425 For this reason, it is imperative to know the QA of the AVF to indicate therapy.
If post-HD haemostasis problems are important, stenosis dilation is indicated by under-dilating and assessing risk-benefits. In this respect, a double dilation technique associated with surgical reduction of QA has been proposed.448
To sum up, both surgical and endovascular treatment have proven to be safe techniques in stenosis of the needling segment, with good rates of technical and clinical success. However, although there is a better rate of primary patency with surgery, and although assisted patency is similar, the majority opinion of experts, as well as of GEMAV, suggests that percutaneous treatment should be introduced at the outset because it is less aggressive as a technique. In AVF that require an additional surgical procedure, such as in aneurysmal AVF, which have a large mural thrombus or are associated with trophic lesions, surgery is suggested as the first repair technique. Despite the possibility of using endovascular techniques with endoprosthesis placement, there is no experience that can endorse this indication at the present time.
Treatment of cephalic arch stenosis
The cephalic vein is part of the superficial venous system of the upper limb, and follows an anterolateral subcutaneous trajectory along the arm; proximal to the arm it continues in a superficial position in the deltopectoral groove until it flows into the deep venous system in the axillary vein just before the clavicle. This confluence occurs in the anatomical region known as the cephalic vein arch (CVA), which is the segment in which this vein changes direction through the clavipectoral fascia. It then moves from a superficial position to a deeper level, eventually flowing into the axillary vein, which is the venous drainage trunk of the upper limb.449,450
As it is an anatomical transition segment between the superficial and deep venous systems, its access stenosis presents a series of particular characteristics that force it to be considered separately from stenosis that occurs in the trajectory of the cephalic vein in the arm.
First, it is one of the most common causes of nAVF dysfunction,451-453 and this dysfunction usually presents with significant haemodynamic changes334 and a marked association with arm nAVF (39%) versus nAVF in the forearm (2%).451,452
Likewise, in comparison to stenosis in other locations, these are lesions with a significantly poorer response to treatment using PTA, with greater resistance to dilation (4.8% versus 1.3%), higher rate of vascular rupture (14.9% versus 8.3%) and shorter free interval between angioplasties (10.6 versus 18.3 months).449,451 Finally, a higher rate of thrombosis has been found in patients with stenosis in CVA.334,454
Several possible pathophysiological mechanisms for the development of this type of stenosis have been put forward, such as lack of adaptation to the high-flow situation, presence of valves in the cephalic-axillary confluent, alterations due to the angle of the confluent, absence of elasticity at the level of the clavipectoral fascia or intrinsic alterations in the venous wall due to uraemia.449,454-456 The sequence of mechanisms that leads to the development of stenosis has not been identified to date, leading some authors to consider the possibility that all the indicated agents could be involved in a variable way.453
Therapeutic options of cephalic arch stenosis
As mentioned, the management of this type of stenosis involves greater complexity, given its poor response to treatment and the higher rate of recurrence and complications.
Percutaneous transluminal angioplasty
It is the most widely used therapeutic technique, in many cases due to the unavailability of other technical options in practice. The study by Rajan et al.452 reports a technical success rate of 76%, having required the use of high pressure balloons (> 15 atm) in 58% of cases, with 6% ruptures of the target vessel. Primary patency at 6 months was 42% and 23% at 12 months, with primary assisted patency of 83% and 75% at 6 and 12 months, respectively, requiring an average of 1.6 procedures per year to achieve this, results similar to those later published by Vesely and Siegel.440 Dukkipati et al.,457 in a study on the results of PTA in the CVA, describe an average of 91.5 days between PTA to maintain VA patency.
Thus, results of PTA on CVA stenosis demonstrate a markedly lower effectiveness than in other venous territories, with a higher rate of complications and with lower primary patency than the standards recommended by some clinical practice guidelines.10 However, it is a minimally invasive and widely available treatment mode, justifying its extensive use in clinical practice. Despite this, new treatment options have been proposed in different studies which have been published.
Percutaneous transluminal angioplasty with stent placement
In order to improve the clinical success and patency of the procedure, placement of an intravascular stent has been proposed.449,453 As already mentioned, it is a technique commonly used in clinical practice for the treatment of PTA complications, in cases of vessel rupture, and also for recurrent stenosis, as it is a safe and minimally invasive procedure. On the other hand, it is a technically complex procedure, since it requires placement adjacent to the cephalic-axillary venous confluent, which means there is a risk of compromising axillary vein permeability with stent deployment or with posterior migrations of the stent, thereby limiting the creation of new accesses in the limb.453 In addition, in the case of peripheral veins, stent placement has not been shown to increase VA patency.458
Currently available evidence on stent placement in the CVA comes from two published studies, in comparison to simple PTA457and stent versus the deployment of endoprosthesis,442 with 39% primary patency of the procedure at 6 months. The study of Dukkipati et al.,457 which compares simple PTA versus PTA with stent placement, found an association between stent deployment and an increase in patency, reducing the number of PTA needed after the procedure to maintain it.
Despite providing a modest improvement in primary and primary assisted patency for simple angioplasty, the overall results of stent deployment in the CVA hardly justify the cost/benefit of its use in a systematic way, except in cases of technical complication during PTA.442
Cutting balloon
As this is a stenosis with poor response to simple PTA, the possibility of treatment using PTA with cutting balloon has also been proposed.
Despite the theoretical benefit that this technique might offer, evidence from a prospective randomised study of 340 patients (including stenosis at various sites)440 found no benefit in relation to the primary patency of the procedure, and a higher complication rate (5.2%) was observed versus PTA with conventional balloon. Subsequently, another study459 also failed to find better patency of cutting balloon versus simple PTA.
Endoprosthesis
Placement of a vascular endoprosthesis—stent covered with prosthetic material (polytetrafluoroethylene [ePTFE])—may prevent the development of endothelial hyperplasia present in the recurrence of CVA stenosis. It is, however, a technique with a high medical cost.449
The RCT conducted by Shemesh et al.,442 comparing stent and endoprosthesis placement, describes a technical success rate of 100%, with 82% primary patency at 6 months, significantly greater compared to that reported in uncovered stents. Similar results have been reported by Shawyer et al.,460 who found primary patency at 6 and 12 months of 82% and 73%, respectively, and a secondary one of 91% at 6 months.
Despite the improvement found with this technique, given the high financial cost of the procedure, new studies are needed to confirm the results in order to recommend its widespread use in clinical practice.
Surgical transposition
Given the suboptimal results obtained by PTA in this type of stenosis, the surgical transposition of the cephalic vein into the brachial or basilic vein has been proposed by different authors.449,453 The described technique consists of disconnecting the cephalic vein arch with ligation of the proximal vein and reanastomosis in the basilic or brachial vein in the axillary cavity, by subcutaneous tunnelling, so that drainage to the deep venous system occurs at this level. This surgery is of a moderate technical complexity, and can be performed with locoregional anaesthesia.
The evidence currently available is from several published case studies,461-463 and there are no direct comparisons with other types of treatment. The results show primary procedure patency of 70-79% at 6 months and 60-79% at 12 months, with a complication rate of 8%.453-463
Likewise, significantly better patency has been reported in PTA procedures performed after surgery, so there are authors462 who recommend their use in combination.
Thus, the surgical transposition of the CVA is a safe therapeutic option that offers superior patency results to PTA with or without stent placement, presenting the disadvantage of being an invasive technique of intermediate complexity. Large-scale studies that can confirm its usefulness in clinical practice are therefore necessary.
Other techniques
Given the association between turbulent flow and development of endothelial hyperplasia, the indication of flow reduction techniques has been put forward to reduce this turbulence. In the retrospective study of Miller456 on a group of patients with intervention (minimally invasive limited ligation endoluminal-assisted revision [MILLER]) to reduce the flow for other reasons (distal hypoperfusion syndrome (DHS) or high flow), there is a significant improvement in PTA patency after reducing access flow. However, there are no further studies on the role that flow reduction may play in the treatment of these lesions.
Finally, the possibility of performing a surgical procedure through surgical angioplasty with patch through a direct approach of the CVA has also been proposed.464 While it is a technically relatively complex technique, further studies are necessary to determine its role in clinical practice.
Cephalic arch stenosis: therapeutic management
Only a relatively small number of studies supporting the use of the different treatment methods in a clinical setting are available for CVA stenosis, despite their significant prevalence. Consequently, the available evidence is based, in most cases, on a small number of published cases that do not compare different techniques. Therefore, the recommendations made are essentially based on the opinion of GEMAV members, taken on the basis of currently available studies and criteria for good clinical practice (Table 22).
Treatment of cephalic arch stenosis
| Study | Type of treatment | No. | Primary patency, 6 months (%) | Primary patency, 12 months (%) | Patient re-interventions/year |
|---|---|---|---|---|---|
| Rajan et al., 2003452 | PTA | 26 | 42 | 23 | 1.6 |
| Kian et al., 2008461 | PTA | 13 | 8 | 0 | 3.5 |
| Shemesh et al., 2008442 | PTA + stent | 12 | 39 | 0 | 1.9 |
| Heerwag et al., 2010459 | PTA + cutting balloon | 17 | 81 | 38 | 0.9 |
| Shemesh et al., 2008442 | PTA + endoprosthesis | 13 | 82 | 32 | 0.9 |
| Shawyer et al., 2013464 | PTA + endoprosthesis | 11 | 82 | 73 | NC |
| Chen et al., 2005460 | Surgical transposition | 7 | 80 | 70 | NC |
| Sigala et al., 2014462 | Surgical transposition | 25 | 79 | 79 | 0.1 |
| Kian et al., 2008461 | Surgical transposition + PTA | 13 | 69 | 39 | 1.0 |
| Miller et al., 2010456 | Flow reduction + PTA | 33 | 76 | 57 | 0.9 |
PTA has been the treatment of choice in cases of CVA stenosis, as it is a safe technique, with low complexity and acceptable results in other venous sectors.453 It is, in addition, a widely available procedure in practice that, in many cases, has no feasible therapeutic alternatives. We realise that both expert opinion and studies confirm the suboptimal result, with poor patency and higher rate of complications than in other locations.452,462 Despite this, PTA is still considered as a first-line technique for treating these lesions, given its good cost/benefit ratio, its minimally aggressive nature and the acceptable rates of both assisted patency and number of procedures required to maintain it.
In contrast, the use of stents has not shown a parallel increase in the effectiveness of the technique442; therefore, its use is not justified in a generalised and systematic way given its greater cost. Their placement would be reserved for cases of technical failure in simple PTA (vessel rupture or persistent stenosis).
Along the same lines, incorporating the cutting balloon device, one study has not shown better patency than simple PTA,459 and the results of the largest study to date (340 patients in all sites along the whole AVF segment)440 do not show an improvement in results and even have a higher rate of complications, so its widespread use raises doubts regarding the cost of the procedure and its safety.
The results of studies on endoprosthesis placement have actually shown better results versus simple PTA.442,460 Although this is a recently introduced technique with little available evidence to support it and it is a procedure with far higher costs than the rest, its routine use is determined by evidence that may arise from new studies.
With regard to surgical techniques, although there is relatively limited evidence, transposition of the cephalic vein has also proved to be a useful treatment as it both increases primary patency and decreases the need for angioplasty after surgery.461-462 Therefore, it can also be considered a first-line treatment in the treatment of CVA stenosis.
Finally, it was considered that the very limited evidence on flow reduction techniques and surgical angioplasty do not allow us to make any recommendation regarding use, as future studies must be conducted in order to determine their usefulness in clinical practice.
→ Clinical question XVI Are there any criteria that indicate in which cases, when and how to treat central vein stenosis, assessed in terms of usable arteriovenous fistula patency and/or thrombosis?
(See fact sheet for Clinical question XVI in electronic appendices)
| Summary of evidence | |
| Observational studies find that continuous surveillance without intervention may be sufficient for cases in which adequate development of collateral veins has occurred and there is no severe symptomatology | Low quality |
| Observational studies find that endovascular treatment shows suboptimal results in the medium and long term. They may cause more aggressive hyperplastic lesions in the intima and neoproliferative lesions in restenotic areas than those found in the original lesions | Low quality |
| Although technical success rates are high, 70% to 90%, observational studies find that percutaneous angioplasty achieves primary patency rates at 12 months of between 12% and 50%, and accumulated patency between 13% and 100% | |
| Observational studies found that primary patency at one year after stent placement ranged among studies between 14.3% and 100%, and secondary patency between 33% and 91% | Low quality |
| Several studies have found cases of complications due to stent (such as migration, fracture, neointimal intra-stent hyperplasia, and the occurrence of stenosis that are not related to the initial one | |
| Observational studies found similar patency rates for angioplasty versus stent | Low quality |
Rationale
Central veins are considered to be the subclavian vein, the brachiocephalic vein (also called the innominate vein) and the superior vena cava. The subclavian vein is a continuation of the axillary vein and starts on the lateral edge of the first rib. Due to their intra-thoracic location, i.e. protected by rib arcs, clavicle and sternum, central veins are less accessible to surgery than the peripheral veins of the arm, and are also larger, support more flow and are more elastic.465,466
Stenosis or occlusion in the central veins of an upper limb in which VA has been created may lead to venous hypertension that is symptomatic, secondary to progressive oedema of the arm that may become refractory, VA dysfunction, trophic disorders of the limb and increase in collateral circulation in the neck and thorax. This may appear in 15-20% of patients on HD, often with previous history of handling and cannulation of the central, subclavian or jugular vein.427,428 Regardless of CVC location, the greater the number and duration of the CVC, the greater the risk of developing stenosis. A higher prevalence of stenosis is also described in CVC placed on the left side because of the longer and more tortuous trajectory of the central veins on this side.466 In patients with defibrillators or pacemakers requiring AVF, this should be created in the arm opposite the location of the cardiac device. In the HD patient, central stenosis usually remains asymptomatic until an AVF is made in the ipsilateral limb, at which point, the stenosis becomes symptomatic as QA increases.466
The main cause of central venous stenosis in patients on HD is the development of intimal hyperplasia secondary to chronic trauma caused by a CVC, plus high flow and secondary turbulence in patients with AVF in the arm.467 Ninety percent of patients with stenosis have had a central venous catheter.468 Forty percent of CVC in the subclavian vein and 10% of CVC implanted in the jugular vein cause central vein stenosis.14
When central venous stenosis is suspected, the imaging test of choice is fistulography or venography. If the patient already has an AVF, the study can be performed by direct vein needling of the AVF (outflow segment). As it is impossible to directly view central vessels with US, this imaging test is relegated to a secondary place, although it must be performed before fistulography to rule out stenosis in any other segment of the access that is accessible to ultrasound waves. Fistulography also allows the VA to be treated during the procedure, if indicated.
Other diagnostic means for the study of central veins are Computed Tomography angiography (CT angiography) and Magnetic Resonance angiography (MR angiography). CT angiography has an advantage over MR angiography in that it provides better image resolution, but both techniques have disadvantages (see section “Monitoring and surveillance of arteriovenous fistula”), as they have a high cost and will not prevent fistulography if there is central stenosis. In cases of iodinated contrast allergies, MR angiography may be indicated, although there is a risk of nephrogenic systemic fibrosis.
Evidence synthesis development
The literature unanimously agrees that only symptomatic cases should be treated.14,15,466,469 In the review of Levit et al.469 of asymptomatic patients in HD with central stenosis > 50%, in 28% of them angioplasty was not performed and none developed symptoms later. However, in 8% of patients treated the stenosis worsened and became symptomatic which, according to the author, would be the result of endothelial damage produced by the balloon. Chang et al.470 described similar findings.
The treatment of choice in central vein stenosis is dilation with balloon catheter.14,15,466 PTA in central veins has a high technical success rate ranging from 70% to 90% depending on the studies.466 Buriankova et al.471 obtained a 96% success rate in stenosis and only 50% in occlusions. Results of patency after PTA vary (according to authors) between primary patency of 12% to 50% at one year and secondary patency of 13% to 100%,466 although these results can be improved with the systematic and increasingly widespread use of larger diameter and high pressure balloons.466,471 The most serious complication following central venous PTA is vein rupture, which, although exceptional, should be immediately identified and initially treated by low pressure balloon compression for 6 min, three consecutive times. If it is not possible to stop the bleeding, the other option is to implant a covered stent.465
The different guidelines and recent bibliographic reviews recommend stent implantation in dilation-resistant elastic stenosis and in recurrence under three months following the last PTA.14,15,466 When a stent is placed, it is very important not to occlude areas of venous confluence such as the internal jugular ostium and the contralateral brachiocephalic trunk, to prevent problems during the placement of future VA.
Outcomes of stents, the same as with PTA, vary according to the authors, with primary patency rates at one year which fluctuate between 14.3% and 100% and secondary patency between 33% and 91%.466 In some comparative studies between PTA and stent implants, there appears to be no significant differences in primary and secondary patency.472,473 In the future, the development of new specific stents for veins which have adequate diameters and high radial strength may make these outcomes improve. Covered stents may be another option, and show promising initial results, although there are no prospective and randomised studies.467
Theoretically, covered stents cause less intimal hyperplasia than uncovered ones. As a factor against, as they are covered, they can more easily occlude venous confluence areas that prevent CVC placement in the future. With regard to complications, the most common ones are shortening, fracture and migration of the stent.467 Shortening and migration are less common since nitinol stents are used; due to their thermal memory, these best adapt to tortuous venous areas.418
From evidence to recommendation
If central venous stenosis is clinically suspected during VA follow-up in HD patients, fistulography is required to confirm the diagnosis. Fistulography is the diagnostic method that locates the lesion and prepares the therapeutic approach.
In central stenosis processes where collateral circulation has developed to compensate the stenosis and there is no clinical significance, treatment would not be necessary given there is no positive risk-benefit balance. Therefore, it is only recommended to treat the stenosis with clinical repercussions.
Should stenosis require treatment, the approach of choice would be endovascular treatment by means of balloon PTA, reserving stent placement for cases of stenosis that present resistance to dilation or frequent or early recurrence of the stenosis, within 3 months. While placing the stent, occluding areas of venous confluence should be avoided to prevent problems with future VA.
Clinical question XVI. Recommendations
R 5.1.4) We recommend fistulography be performed if central venous stenosis is clinically suspected
R 5.1.5) We recommend only central vein stenosis that are symptomatic be treated
R 5.1.6) We recommend endovascular therapy be performed using percutaneous transluminal angiography with balloon as the first treatment option for central stenosis
R 5.1.7) We suggest the use of stents be limited to selected cases where there is technical failure of angioplasty and frequent relapse of stenosis, and we recommend they not be used in venous confluents
Recommendations
R 5.2.1) We recommend priority be placed on attempting to restore the patency of potentially recoverable thrombosed arteriovenous fistula, preferably within the first 48 h. In all cases, the priority should be to salvage the arteriovenous fistula and avoid central venous catheter placement
R 5.2.2) We recommend an imaging test be carried out after restoring arteriovenous fistula patency, which should be performed immediately after thrombectomy to detect any possible stenoses requiring treatment
- (•)
NEW R 5.2.3) We initially recommend native arteriovenous fistula with thrombosis secondary to juxta-anastomotic stenosis be treated by surgical treatment, as long as the technique does not require central venous catheter placement
- (•)
NEW R 5.2.4) We recommend the patency of native arteriovenous fistula in thromboses not associated with juxta-anastomotic stenosis be restored by surgical treatment or by endovascular therapy, using mechanical thrombectomy or aspiration devices, if necessary
- (•)
NEW R 5.2.5) We recommend it be attempted to restore the patency of thrombosed prosthetic arteriovenous fistula by surgical or endovascular treatment
- (•)
NEW R 5.2.6) We recommend elective intervention be performed on the dysfunctional arteriovenous fistula with significant stenosis instead of restoring after thrombosis
NEW R 5.2.7) We recommend attempting to restore the patency of thrombosed arteriovenous fistula rather than create a new arteriovenous fistula and place a central venous catheter, because it is associated with lower health costs, lower hospitalisation rate and lower morbimortality
Rationale
Thrombosis is suspected when physical examination fails to detect murmur or thrill through AVF auscultation and palpation, and must be confirmed with an image test.
Thrombosis is the main AVF complication. The main predisposing factor is the presence of venous stenosis and accounts for 80% to 90% of thromboses.291,474 Most stenosis are usually located in the proximal segment of arteriovenous anastomoses in the nAVF and in the venous anastomosis of pAVF.14 Any thrombosed VA should be evaluated urgently, and access patency restored when indicated, within the first 24-48 h after the event. Whether the salvage procedure performed is endovascular or surgical, once the thrombus has been removed, fistulography should be performed to locate the stenosis and, in the same procedure, resolve the underlying cause to prevent episodes of rethrombosis.475,476 Other causes of thrombosis are arterial stenosis and non-anatomical factors such as excessive VA compression after HD, hypotension, elevated levels of haematocrit, hypovolaemia and states of hypercoagulability.477-480
Due to the importance of VA for the patient’s clinical evolution, the morbidity associated with CVC and the anatomical limitation for multiple VA creation, the salvage of every potentially recoverable AVF should be attempted. The only absolute contraindication is the active infection of the VA. Relative contraindications include allergy to iodinated contrast, unstable or life-threatening clinical situation; biochemical or hydroelectrolytic alterations requiring treatment with urgent dialysis such as pulmonary oedema, hyperkalaemia or severe metabolic acidosis; right-to-left heart shunt; severe pulmonary disease and aneurysmal AVF with thrombosis of a great length of the VA.
Thrombosis of the VA for HD should be regarded as a therapeutic emergency requiring immediate solution. Strategies must be established to take this into account so that in each centre, all the professionals involved participate in a multidisciplinary approach to the problem. Urgent restoration of VA patency allows, in the first place, temporary CVC placement to be avoided, with the morbidity that this implies. However, prior to any therapeutic procedure, a clinical assessment of the patient and an analytical study should be performed to rule out situations of potential risk or severity (pulmonary oedema and severe hyperkalaemia). If the patient requires urgent HD, a CVC should be placed, and the thrombectomy procedure delayed. This delay should be less than 48 h after thrombosis occurred.14,481 The thrombi become progressively fixed to the vein wall or the ePTFE prosthesis making thrombectomy more difficult the later the unblocking procedure is attempted.14 However, the “time” factor is not necessarily restricted, given that thrombosed accesses have been salvaged even after several weeks following thrombosis.271
Endovascular thrombectomy
The main objective of the endovascular technique is to re-cannulate the thrombus, using hydrophilic guides, preferably with an angled tip, as they are less traumatic and avoid venous dissection. Thrombus aspiration is performed with manual thromboaspiration systems with negative pressure271,482 with a thick catheter of 7 to 9 Fr or thromboaspiration by suction. To avoid any procedure-related complications, it is advisable to administer sodium heparin. At the end of the procedure there is no standardised indication for pharmacological treatment, although some authors recommend low molecular weight heparin on alternate days to HD to prevent AVF rethrombosis,271 and other anti-aggregants with acetylsalicylic acid or clopidogrel during 72 h post-thrombectomy.475
Wen et al.475 review their results using the AngioJet thrombus aspiration system in 109 patients with nAVF thrombosis. They obtain a technical success score of 76% (80% before three days and 63% after three days) with primary patency rates of 67%, 57% and 39% at 30, 90 and 180 days, respectively. These results are similar to those obtained with other thromboaspiration devices (Arrow-Trerotola, Hydrolyser and thrombectomy with balloon) and pharmacological thrombolysis.475,483 These same authors considered nAVF revascularisation more difficult than in pAVF, since, in their experience, native veins are more susceptible to lesion or breakdown, and have a more complex anatomy with occasional onset of multiple stenosis and/or aneurysmal formations. In conjunction with these data, several authors recommend the use of manual thromboaspiration with catheter in nAVF as the catheters are more flexible, are pre-shaped, are smaller in size than other thrombectomy devices, and are therefore less damaging to the vascular endothelium.271,484
The complications described during the procedure are pulmonary thromboembolism, arterial embolism, rupture or dissection of the vein and haematoma at the needling site, which can become anaemic.271,475 The use of stents in cases of thrombosis is poorly documented but could be useful in aneurysmal dilatations with residual thrombi after thromboaspiration.271
Surgical thrombectomy
Traditionally, nAVF thrombosis has been treated surgically,485,486 and it is still performed in many HD units487 through embolectomy catheter, early surgical review of the access and its afferent and efferent vessels. Intra-operative radiological evaluation is also used to treat the underlying lesions found with good results and at a low cost. Treatment includes repair with the reconstruction or creation of a new anastomosis a few centimetres more proximal, or bypass of the stenotic area by interposing an ePTFE segment. If the thrombosis is located in the area adjacent to the anastomosis of radiocephalic and brachiocephalic AVF, the vein may be preserved and the creation of a new anastomosis is recommended, even if several days have elapsed.291,485 In addition to the surgical technique normally used to perform a proximal reanastomosis, there are authors who have proposed the interposition of an ePTFE segment, in order to avoid depleting the venous pathway inherent to surgery. The results published by these authors show similar patency rates to proximal reanastomosis, although as a drawback, they introduce prosthetic material in the VA.273,488
New surgical techniques that have been proposed via manual thrombus extraction followed by PTA of stenotic lesions show good results (technical success in 87% of procedures). The authors consider that this is a simpler and cheaper procedure than percutaneous thrombectomy or thrombolysis, and also allows acute and chronic thrombus to be eliminated as well as that in aneurysmal segments.489
Finally, one of the indications for review and surgical treatment is in early nAVF thrombosis (first hours or days), which is mainly related to technical problems.
Pharmacomechanical fibrinolysis
Percutaneous pharmacomechanical fibrinolysis is a minimally invasive method that uses thrombolytic drugs and a PTA balloon for the treatment of thrombosis. The commonly used thrombolytic drugs are urokinase and the recombinant tissue plasminogen activator (rt-PA). The procedure combines releasing fibrinolytics locally, which can be performed in several ways, and PTA of the thrombus. The fibrinolytic drug is released after breaking through the thrombus and the stenotic area responsible for the thrombosis with the hydrophilic guidewire; pulse-spray is the most commonly used system.490 After patency is partially restored, a thrombectomy and PTA of the thrombus,491,492 using balloon catheter, and the treatment of lesion(s) responsible for the occlusion are also carried out, in the same procedure.
The literature contains four RCTs493-496 and a retrospective study497 comparing fibrinolysis with urokinase and percutaneous mechanical thromboplasty. No statistically significant differences were observed between either technique in relation to technical success, patency and complications493-495,497 with the exception of a study conducted by Vogel,496 where the authors found a higher percentage of bleeding complications, primarily at the needling site, with the use of fibrinolytics. The impossibility of lysing the entire thrombus should be added to this drawback.
On the other hand, although most studies found the longer procedures to be a disadvantage of fibrinolysis, in the study conducted by Vashchenko in 2010, where 563 procedures were studied comparing fibrinolysis of thrombosed access by the technique of “urokinase injection and wait” versus mechanical thrombectomy with mechanical device,497 they found the lower cost of fibrinolysis to be an advantage, given the high price of mechanical thrombectomy devices. There is no study comparing the financial cost of VA fibrinolysis to thrombectomy with catheter.
In the review conducted by Bush et al. in 2004,498 comparing different techniques of both endovascular and surgical revascularisation, including fibrinolysis, they also found no differences between the different methods used.
Even with its drawbacks, fibrinolysis is a therapeutic tool that may be useful in certain cases when mechanical or aspiration thrombectomy is not sufficient for the complete removal of thrombi.14 Its greatest usefulness is in combination with mechanical thrombectomy, allowing the use of lower dose of fibrinolytic drugs and reducing the systemic complications derived from its use.
5.2.1 Treatment of native arteriovenous fistula thrombosis
→ Clinical question XVII In native arteriovenous fistula thrombosis, what would be the initial indication (percutaneous transluminal angioplasty versus surgery) assessed in terms of patency of the native arteriovenous fistula and/or thrombosis? Does it depend on location?
(See fact sheet for Clinical question XVII in electronic appendices)
| Summary of evidence | |
| There have been no RCTs comparing surgical versus endovascular treatment. There are only published clinical case series that together show better results for surgery in relation to technical success and patency percentages per year | Very low quality |
| Although there are only a few case series, better results are found both in technical success and in AVF patency in those located in the forearm than in the arm, regardless of the repair method used (endovascular or surgical) | |
Evidence synthesis development
So far there have been no RCTs comparing the results obtained with surgical treatment and endovascular therapy. There are, however, recent retrospective studies. Ito et al.476 compare both techniques in a sample of 587 patients of which 25% had nAVF. In this subgroup, a patency of 33.7% at 2 years with endovascular treatment stands out compared to 37.5% with surgical thrombectomy and 59.8% if surgery is performed with an additional graft or a new VA (p = 0.0005).
In the review of Tordoir499 in which there are only observational studies to describe the behaviour of both techniques in the nAVF, surgery maintains better results in primary patency at 1 year (74% versus 40%) and secondary patency (87% versus 72%), with the results being similar in technical success (90% versus 89%).
In forearm nAVF, there is a slight advantage of surgical treatment over PTA when comparing long-term primary and secondary patency. The study does not establish a separation between stenosis in different locations, so the best results in the forearm may be related to the treatment of the juxta-anastomotic stenosis.
Similar findings regarding outcomes by location have been found in two studies analysing endovascular treatment in the arm and forearm500,501 and in one study with patients treated with surgery.502 All of them report a greater primary patency for AVF located in the forearm.
From evidence to recommendation
The results obtained from retrospective studies, and in the absence of RCTs, indicate a moderately better primary patency of surgery versus endovascular treatment. When results are analysed in the thrombosed fistulae secondary to juxta-anastomotic stenosis, better long-term primary and secondary patency rates are found, which allows surgical treatment to be recommended at this location, given the better results in the treatment of this stenosis. This decision should be associated with the priority of avoiding CVC placement, so if surgery does not guarantee it, endovascular procedure can be contemplated.
In the treatment of thromboses not associated with juxta-anastomotic stenosis, both endovascular and surgical treatment has a high clinical success rate, with no evidence currently available to recommend a specific therapeutic alternative. Therefore, the technique of choice should be decided in accordance with the patient’s clinical context, and the avoidance of CVC placement made a priority whenever possible.
In any case, existing evidence on the treatment of thrombosis of nAVF is difficult to interpret since not only are there no studies that directly compare the procedures, but these studies present considerable technical heterogeneity, both in the endovascular and surgical approach. It cannot be ruled out that the use of different devices in different circumstances may play a role in this variability. Therefore, the limited available evidence also allows a partial therapeutic orientation with these recommendations being based on the interpretation of GEMAV.
Clinical question XVII. Recommendations
R 5.2.3) We initially recommend native arteriovenous fistula with thrombosis secondary to juxta-anastomotic stenosis be treated by surgical treatment, as long as the technique does not require central venous catheter placement
R 5.2.4) We recommend the patency of native arteriovenous fistula in thromboses not associated with juxta-anastomotic stenosis be restored by surgical treatment or by endovascular therapy, using mechanical thrombectomy or aspiration devices, if necessary
5.2.2 Treatment of prosthetic arteriovenous fistula thrombosis
Treatment of prosthetic arteriovenous fistula thrombosis
Rationale
Despite having a higher rate of complications than nAVF, pAVF is a good solution for patients with an exhausted venous vascular bed and in elderly patients, with thrombosis being the main complication. In these cases, thrombosis is located mostly in the venous anastomosis.14 and is secondary to intimal hyperplasia derived from haemodynamic mechanisms due to lack of adjustment between the vein and the ePTFE prosthesis.503,504
As in nAVF, urgent assessment with subsequent thrombectomy in recoverable pAVF is indicated, if possible, in the first 24-48 h after the event, in order to avoid CVC placement and associated morbidity. The same strategies mentioned in the previous section are established (section 5.2.1). Imaging studies (DU or fistulography) should be performed after restoring patency to locate stenosis, and in the same procedure to perform treatment of the lesions conditioning episodes of rethrombosis.475,476
Stenosis and thromboses can be treated endovascularly or surgically. Numerous studies have evaluated both methods, concluding that a combination of both can be highly beneficial. At the end of the 1990s, thrombosed prosthetic accesses were primarily treated surgically followed by angiographic assessment to identify the cause of thrombosis and the presence of residual thrombus. The development of new endovascular devices and the lower invasiveness of this type of procedure have resulted in a predominance of the latter. In any case, the aim of both types of treatment in the detection and treatment of underlying stenosis is always to ensure their ongoing long-term patency.
→ Clinical question XVIII In prosthetic arteriovenous fistula thrombosis, what would be the initial indication (percutaneous transluminal angioplasty versus surgery versus fibrinolysis) assessed in terms of patency of the arteriovenous fistula and/or thrombosis? Does it depend on location?
(See fact sheet for Clinical question XVIII in electronic appendices)
| Summary of evidence | |
| The published systematic reviews and RCTs comparing surgery with percutaneous transluminal angioplasty found similar clinical results in the management of thrombosed pAVF | High quality |
| With regard to treatment by thrombectomy versus chemical thrombolysis, three RCTs comparing fibrinolysis with urokinase and three different options for percutaneous mechanical thromboplasty have been identified, and have shown similar clinical results | Moderate quality |
Evidence synthesis development
Traditionally, surgical thrombectomy has been used in pAVF thrombosis, followed by repair with bypass by interposing a graft or associating reanastomosis in a proximal segment of vein without stenosis. Percutaneous treatment of VA thrombosis is a therapeutic option that is less invasive than surgery475 and allows the preservation of proximal venous territory. As a disadvantage, there is a need for a greater number of procedures to maintain pAVF patency.451,474
A meta-analysis by Green et al.474 concluded in 2002 that surgery was superior, both in terms of technical failures and in primary patency. However, in a recent meta-analysis performed by Kuhan et al.,505 analysing 6 RCTs comparing endovascular therapy and surgery in thrombosis of pAVF, the results were comparable between both techniques. Technical success rates were, on average, 74.5% with endovascular treatment versus 80.3% with surgery (p = 0.13); primary patency at 30 days was 64.6% for endovascular therapy and 66.8% for surgery (p = 0.46); at one year, 14.2% with the endovascular approach versus 23.9% with surgery (p = 0.06). Primary assisted patency at one year was analysed in a single study, with 20.5% with endovascular treatment versus 43.9% with surgery (p = 0.03); however, secondary patency at one year, also analysed in a single study, was 86% for endovascular treatment versus 62.5% for surgical (p = 0.14).
Unlike the meta-analysis conducted by Green et al.,474 in which results were clearly favourable for surgery, the study of Kuhan et al.505 placed endovascular therapy on a par with surgical, with the former being less aggressive. Endovascular techniques, using mechanical and aspiration thrombectomy devices, and the incorporation of new angioplasty balloons with more technical features, have levelled the balance between surgery and endovascular treatment, the latter having the advantage of being less invasive. The comparison of percutaneous mechanical techniques with pharmacological fibrinolysis in three RCTs493-495 shows no significant differences in patency results.
In this respect, however, despite its invasiveness, urgent surgery avoiding CVC placement with subsequent assessment and endovascular treatment has recently been reported with very good results.167 The authors obtained a patency rate of 67% at three years and a thrombosis rate of 0.45 events per patient per year.
Finally, the use of uncovered metallic prostheses is highly controversial, and results similar to those described for the treatment of non-thrombosis-related VA stenosis have been reported (section 5.1). With regard to the use of covered metallic prostheses (stent graft), in the study by Nassar et al.505a where the results of 66 patients with thrombosed pAVF are analysed, the authors find poorer outcomes than those observed in other studies referring to treatment of venous stenosis without thrombosis,419-421 with poor primary patency (47% and 21% at 3 and 12 months), similar to that observed following thrombectomy without stenosis treatment.498 The thromboses were not associated with the development of an intra-stent stenosis, and so the authors concluded that there must be other factors that determine VA thrombosis different to stenosis in the venous anastomosis, and do not recommend its use in case of thrombosed pAVF.
From evidence to recommendation
The reviewed studies and clinical trials show no significant differences in patency results between surgery and endovascular treatment, being lower in all cases than those obtained in nAVF. Surgical treatment has better rates of technical success, primary and assisted primary patency (although not significantly), while on the other hand, percutaneous treatment is less aggressive and avoids CVC placement.
Therefore, the approach to thrombosed pAVF can be therapeutically oriented indistinctly, either using the endovascular or surgical approach. The choice of technique should take the patient’s clinical context into consideration, and CVC placement be avoided where possible.
Thrombosis due to stenosis in the axillary vein territory warrants separate consideration, as the technical complexity of exposing a proximal venous segment in surgery makes percutaneous treatment the treatment of first choice.
Finally, similar to nAVF, the heterogeneity, both of the studies and the technical conditions, means that the opinion of GEMAV has contributed to the interpretation of the evidence.
5.2.3 Elective treatment of arteriovenous fistula stenosis versus post-thrombosis
Elective treatment of arteriovenous fistula stenosis versus post-thrombosis
Rationale
As mentioned, thrombosis is the main AVF complication and the main cause of its definitive loss. The main predisposing factor is the presence of venous stenosis which accounts for 80 to 90% of thromboses.291,474 Most stenosis is usually located in the segment proximal to arteriovenous anastomoses in nAVF and in the venous anastomosis in pAVF.14
Irreversible AVF thrombosis will have a series of negative consequences on the prevalent HD patient,269 increasing morbidity and mortality, frequency of hospital admissions and healthcare costs.270 In relation to the access thrombosis, it should be noted:
- •
It is not always possible to restore all thrombosed AVF.271
- •
Several studies indicate that the secondary AVF patency after post-thrombotic restoration is lower than elective AVF stenosis repair prior to thrombosis.272,273
These data suggest that it is appropriate to perform elective stenosis treatment prior to AVF thrombosis and this means it is important to conduct surveillance and monitoring of both nAVF and pAVF.
→ Clinical question XIX In the presence of stenosis in the native arteriovenous fistula, is there a significant difference between elective intervention or performing treatment after thrombosis?
(See fact sheet for Clinical question XIX in electronic appendices)
| Summary of evidence | |
| There is no prospective, randomised or non-randomised study comparing elective surgery to correct AVF stenosis versus the option of waiting and treating once this has thrombosed. Two retrospective studies comparing results of arteriovenous fistula surgery with stenosis but without thrombosis occlusion versus surgery of fistulae with already developed thrombosis have been found273,506 | Low quality |
| One of them, performed in complicated nAVF with stenosis, finds no significant differences between those treated with elective surgery or post-thrombosis surgery regarding restenosis, but does so for VA loss rate, which is lower for elective surgery. The other study finds no differences between the two options regarding primary and/or secondary patency at 12 months | |
| Finally, a prospective study does find significant differences favouring elective intervention on the dysfunctioning nAVF272 | |
Evidence synthesis development
Two retrospective studies compared results of elective surgery of nAVF with stenosis but without occlusion versus thrombosed AVF surgery.273,506
The retrospective study of Lipari et al.273 provided results of 64 patients with forearm AVF stenosis, treated 32 with elective surgery and 32 after thrombosis. It did not find differences in the restenosis rate of access: 0.189 per AVF year, the same for both types of surgery, but there were differences in VA loss: rate of 0.016 per AVF year for the elective surgery group and 0.148 for surgery after thrombosis (p = 0.048). Technical success was 100% for elective surgery and 84% for surgery after thrombosis.
The retrospective study of Cohen et al.506 reports on 43 patients with AVF in arm who had received 48 interventions in stenosed AVF and 15 in already thrombosed AVF. They did not find significant differences in terms of patency of the access at 12 months:
- •
Primary patency of the access at 12 months: 56% for AVF with stenosis and 64% for already thrombosed AVF (p = 0.22).
- •
Secondary patency of the access at 12 months: 64% for AVF with stenosis and 63% for already thrombosed AVF (p = 0.75).
Technical success of the surgery was 95% overall (60 of 63; two failures in thrombolysis and one in the primary surgery for the stenosis).
In one prospective study elective surgery to correct the AVF stenosis versus waiting and operating when thrombosis of the AVF develops272 are compared. Researchers describe a greater patency in AVF salvaged following dysfunction than following thrombosis, both as a whole and when analysed in a disaggregated way by type (native or prosthetic). In this prospective study with a 5-year follow-up, 317 AVF were evaluated (73% nAVF and the rest pAVF [ePTFE]), on 282 patients. 88 thromboses occurred, corresponding to a rate of thrombosis/access/year of 0.06 for nAVF and 0.38 in pAVF. In total, 66.6% of AVF salvage repairs were elective, with emergency surgery in 76% of thromboses. The added patency of all incident AVF repaired after dysfunction was 1062 ± 97 days versus 707 ± 132 in those repaired for thrombosis (p < 0.02). The increased risk for AVF loss in those repaired post-thrombosis versus dysfunction was 4.2 (p < 0.01).
From evidence to recommendation
Despite the lack of randomised studies and their scarcity and methodological limitation, studies analysing the evolution of elective versus post-thrombotic treatment show a preference for elective therapy in their results, with both lower AVF loss and better patency.
At the same time, the outlook of a patient with a thrombosed access must be taken into account regarding elective procedure. The greater likelihood of there being less controllable factors, such as patient clinical situation, extent of the thrombosis or CVC requirement, means that the guarantees of success may be compromised.
Therefore, GEMAV recommends performing an elective or preventive intervention of the stenosis rather than post-thrombosis salvage, following the criteria presented in section 4, associated with the high risk of thrombosis.
5.2.4 Thrombosis: salvage versus new vascular access
Thrombosis: salvage versus new vascular access
Rationale
AVF thrombosis results in a substantial number of hospital admissions, the use of CVC and, consequentially, an increase in healthcare expenditure. In addition to this, CVC-associated morbidity and mortality and anatomical limitation for multiple accesses must be considered, which is why clinical guidelines currently in force consider AVF thrombosis to be a medical emergency.6
When an AVF is thrombosed, the possible options are:
- •
Place a CVC to dialyse the patient and then refer him/her for a new AVF;
- •
Attempt to urgently salvage the AVF for later use, to avoid hospital admission and CVC placement.
Both procedures imply healthcare costs and expenditure, and cost analysis studies should be conducted on these procedures.
With regard to pAVF treatment, there appears to be an agreement in the literature on a major advantage of urgent thrombectomy, either surgical or endovascular, versus a new VA.272,507 That is not the case in native accesses. While nAVF are considered superior to pAVF as VA, they are not problem-free. Over the last decade, thrombosed nAVF have been managed surgically or endovascularly. Despite this, attempts to salvage them have not been widely established. Although the percutaneous management of a thrombosed nAVF is highly successful, repeated interventions are usually required to sustain long-term patency.507 Data published in relation to the healthcare expenditure involved in the surveillance and elective treatment of stenosis to prevent thrombosis of VA are controversial281,508 with few cost-effectiveness studies.
However, there are several studies on the significant healthcare costs caused by VA in prevalent patients undergoing HD.509 The study of Manns et al.510 shows the high cost of HD incident patients with primary failure in their AVF due, in part, to the increase in the number of diagnostic procedures: image and interventional procedures. For health systems which strictly control financial expenditure, this is extremely relevant.
After conducting a financial analysis of expenditure on AVF maintenance, Bittl et al.508 conclude that this is higher than the cost of creating a greater number of nAVF in the prevalent population (with lower percentage of thrombosis and dysfunctions). The article does not refer to what would happen with a prevalent population with a very high percentage of nAVF.
On the other hand, in the study by Coentrao,511 a retrospective analysis of healthcare costs and expenses was conducted comparing the treatment of thrombosed nAVF and the subsequent follow-up with the creation of a new AVF. They observed that percutaneous thrombectomy and treatment of stenosis versus creation of a new VA and waiting for its maturation is associated with a reduction in costs. The group where this procedure was conducted is associated with a higher number of hospital admissions and problems with AVF management (4 times greater), with shorter patency of the new AVF and the consequent comorbidity associated with the CVC.
Finally, in a very recent study where urgent surgical treatment of 268 AVF thrombosis episodes versus scheduled surgery was tested retrospectively and over a period of 11 years,487 the authors obtain a financial saving of €5397 in favour of urgent AVF repair versus creation of a new access. This benefit is derived from the greater hospital expenses associated with creating a new AVF (CVC complications), and the need to perform interventions to achieve maturation. Extrapolation of savings to the entire Spanish population with 23,000 patients undergoing HD would be €9,930,480/year. The study does not analyse, however, the differences between nAVF and pAVF; nor does it include endovascular therapy of the thrombosed AVF.
Therefore, although no prospective studies or clinical trials comparing both of these procedures have been found, the data obtained from the literature seem to suggest that the creation of a new VA results in higher expenditure and morbidity associated with CVC placement than by urgent restoration of the thrombosed AVF.
5.3Management of the non-matured fistulaRecommendations
NEW R 5.3.1) We recommend a clinical check-up be performed at 4-6 weeks to definitively detect delay or absence of arteriovenous fistula maturation from its creation to this moment and elective treatment be proposed. We recommend confirming the suspected lack of maturation by Doppler Ultrasound
NEW R 5.3.2) We suggest early treatment of the non-matured native arteriovenous fistula to favour maturation and to prevent thrombosis and definitive loss
- (•)
NEW R 5.3.3) We recommend percutaneous or surgical techniques not be used systematically to promote maturation of native arteriovenous fistulae
- (•)
NEW R 5.3.4) We suggest surgery as the first treatment option (proximal reanastomosis) in native arteriovenous fistulae with maturation failure associated with juxta-anastomotic stenosis. In cases where this is not possible, endovascular treatment (percutaneous angioplasty) should be proposed
- (•)
NEW R 5.3.5) We suggest significant accessory veins associated with maturation failure be disconnected by percutaneous ligation, surgical ligation or endovascular embolisation with coils. We suggest endovascular treatment be used in the presence of stenosis and surgical treatment when there is no stenosis as the first option, given the lower complexity and healthcare costs
- (•)
NEW R 5.3.6) We recommend angioplasty in cases of non-matured native arteriovenous fistulae with proximal venous stenosis
- (•)
NEW R 5.3.7) We suggest angioplasty of the arterial stenosis when this is the cause of non-maturation of arteriovenous fistula, in cases in which the vascularisation of the limb is not compromised
Rationale
It is estimated that between 28% and 53% of AVF do not mature enough for use in HD.512 In general, QA of 500 mL/min and a diameter of at least 4 mm are required for nAVF to be suitable for dialysis. In successful fistulae, these parameters are met in 4 to 6 weeks. In other cases, from 4–6 months must be waited to conclude that the AVF has failed. In the interval, if HD is needed, a tunnelled CVC is inserted exposing the patient to the morbidity and mortality associated with the use of this VA.
This problem could hypothetically be resolved through the early detection of cases of lack of maturation and treated using surgical or endovascular methods to induce VA maturation.
Two factors, separately or combined, tend to cause most cases of lack of nAVF maturation: venous stenosis and the presence of a significant accessory vein (a venous branch that leaves the primary venous channel which forms the AVF). Both problems can be suspected during clinical check-ups and, after being confirmed with DU, therapeutic intervention could be considered.
The increase in QA and the diameter of the outflow vein occur soon after nAVF creation.513-515 These studies have shown that fistulae that are definitively going to mature do so in the first 2-4 weeks. Thus, good medical practice would advise VA assessment after 4-6 weeks from creation.512,516 The recommendation for early monitoring is based on the fact that most nAVF with delay or no maturation have stenotic lesions in the AVF circuit, which, because vascular stenoses are usually progressive, will lead to thrombosis and VA loss over time.
In most cases, potential patients with non-matured nAVF can be detected through careful physical examination, as indicated in section 4, which can provide orientation on the cause of the dysfunction (Table 23).
Arteriovenous fistula maturity. Physical examination
|
After the presumptive diagnosis, a DU scan will confirm the immature fistula diagnosis (diameter < 0.4 cm and QA < 500 mL/m), and will also allow the cause of the absence of maturation to be detected in most cases. In situations where the DU does not do this, an imaging test (fistulography) may be indicated.10,512,566,517
Different studies have shown the usefulness of early therapy in cases with impaired AVF maturation,512,516,518,519 thereby producing the likelihood of an increase in maturation by 47% in these patients.520 Likewise, the procedures performed (surgical and endovascular) have been shown to be safe, with a low rate of complications.521
→ Clinical question XX Is there a treatment with better outcomes (percutaneous transluminal angioplasty versus surgery or prosthesis interposition) in non-matured arteriovenous fistula management, evaluated on arteriovenous fistula, which enables it to be used in dialysis, patency and/or thrombosis?
(See fact sheet for Clinical question XX in electronic appendices)
| Summary of evidence | |
| A retrospective study, with a small sample of patients, finds better results for surgery in comparison to PTA in relation to AVF patency at one year (AVF valid for HD) | Low quality |
| Numerous clinical series in non-matured AVF treated by PTA find high rates of clinical success (AVF valid for HD) and secondary patency at one year | |
Evidence synthesis development
Treatment of non-matured fistula
- •
Juxta-anastomotic stenosis. The most common cause of maturation failure is the presence of stenosis in the segment of the vein that, in most cases is located in the juxta-anastomotic region. Therapeutic alternatives are surgical treatment (proximal reanastomosis) and PTA.
In general, the same considerations as those for the treatment of juxta-anastomotic stenosis of mature AVF can be applied. Thus, several reviews517,519,521-523 that include different clinical case series, which have analysed the effectiveness and safety of percutaneous angioplasty to treat non-maturing AVF, show good rates of immediate results. However, cumulative patency of the AVF that has undergone one or more interventions to induce maturation has been shown to be significantly lower than of those not requiring such techniques.524 In the only study comparing one technique with the other (reanastomosis versus PTA), results have shown that cumulative patency of the fistula at one year was significantly higher among patients treated by surgery (83%) than in those treated with PTA (40%).525
- •
Accessory veins. The influence of dilated accessory veins in the non-matured fistula has not been fully elucidated.516 Though it is a common finding in these patients (46%), its development has been interpreted by some authors more as a result of proximal stenosis than as a cause of AVF non-maturation.517,526
Some authors have found good outcomes in isolated disconnection,527 although in most studies this was indicated as a complementary treatment of venous stenosis,512,516,517 with the best results being described in cases where the accessory veins were disconnected,526 suggesting they have a certain influence on the lack of AVF maturation.
There are three techniques described in the literature: percutaneous ligation, surgical disconnection or endovascular embolisation using coils. Different publications have shown they are safe and have good results, in isolation or in combination with the treatment of co-existing stenosis, although there are no studies that compare them with each other.516,519,527,528
- •
Proximal stenosis. There are no published case series on isolated cases of proximal stenosis; the main articles and reviews are with joint data from proximal and juxta-anastomotic stenosis, which are all treated endovascularly.
Results describe a high rate of immediate success and safety of the procedure, as well as a high rate of restenosis.517,519,521,522 There are no published case series on surgical treatment of this type of lesion.
- •
Arterial stenosis. The evidence on the isolated treatment of lesions in the afferent artery in nAVF with impaired maturation comes from a single study by interventional radiology.526 The authors describe a high rate of immediate success as well as access maturation, although there was a high incidence of arterial ruptures during the procedure (18%), 7% limb ischaemia after the procedure and an undetermined number during follow-up.
No studies were found on surgical or conservative treatment in cases of arterial lesions proximal to the nAVF.
Medium- and long-term results of interventions to promote access maturation have been evaluated in different studies.519,524,525,529
Lee et al.524 found a significant decrease in cumulative access patency in AVF that required interventions of any type to induce maturation. The tendency to restenosis of these procedures seems to be due, according to most authors, to the mechanical aggression of the angioplasty balloon on the vascular endothelium and the subsequent intimal hyperplasia that it entails.512,530
A subsequent work525 identifies the group of lower cumulative patency in those patients treated by angioplasty, whereas no significant differences are found between the surgery group and the group of AVF that did not require any procedure for their maturation.525 Similar results were described by Long et al.529
From evidence to recommendation
Most of the haemodynamic and morphological changes produced after VA creation take place during the first 2-4 weeks; after that, there is no progression or even a progressive decrease in access flow in cases of immature fistula; this is the reason why early clinical control (4-6 weeks) is recommended to detect cases with alterations in AVF maturation and to use DU on non-matured AVF to confirm the clinical diagnosis and search for associated lesions.
Early treatment of the underlying lesions may increase the likelihood of access maturation by 47%, so it is recommended to act in cases where there is an indication. If we take into consideration the poor prognosis of immature nAVF (diagnosed as such within 4-6 weeks after its creation), on the one hand, and on the other, the worse prognosis for accesses subjected to percutaneous treatment to induce maturation (early restenosis), it could be considered that the best therapeutic option in juxta-anastomotic stenosis is surgery (proximal reanastomosis), since it will allow the access to be salvaged without determining a poorer prognosis in relation to the AVF that have not presented maturation problems. In these cases, percutaneous angioplasty is a safe option with a high rate of success in AVF maturation, although the higher incidence of associated restenosis makes its use advisable in cases where its surgical correction is not indicated.
Some authors defend the choice to perform PTA systematically and at an early stage in all fistulae to induce maturation, but this systematic use is not recommended due to the high incidence of restenosis and the poor access patency associated to these interventions, although more studies are needed to define indications in clinical practice. Therefore, at present maturation inducement techniques can only be recommended in the diagnosed cases of AVF non-maturation.
In cases of immature nAVF associated with significant collaterals, the three therapeutic options described in the literature (percutaneous ligation, surgical disconnection or endovascular embolisation using coils) have proved to be reliable techniques with low morbidity and high rate of immediate success. Therefore, the technique of choice should be indicated by the procedure associated with the surgery, as in the case where venous stenoses coexist. In cases of veins developed with no other lesions, the choice of treatment mode depends on the characteristics of the accessory vein (depth, surgical accessibility, proximity to needling areas, etc.), although the greater technical complexity and higher healthcare cost of percutaneous techniques is recognised.529 As a result, as there are no significant differences regarding success and complications, surgical or percutaneous ligation should be the first choice for treatment.
At present, the limited available evidence on the treatment of lesions located in the outflow vein and in the afferent artery refers to series of PTA-treated nAVF.
Proximal vein stenosis in non-matured nAVF may be treated endovascularly, as it is a safe and effective procedure, although it should be associated with a follow-up protocol due to tendency to restenosis.
Evidence for endovascular treatment of arterial stenosis comes from the case series published by Turmel-Rodrigues et al.,531 which show a high success rate using the procedure, but associated with arterial rupture and an undefined percentage of ischaemia of the limb. As PTA is performed on the artery responsible for the vascularisation of the limb, PTA as well as possible restenosis and arterial thrombosis could adversely affect the natural course of the obliterating disease in these patients and cause ischaemia of the limb, a risk that is not resolved once the AVF is disconnected. Good clinical practice recommends the indication of these techniques only in those patients in which there is a proven compensatory trajectory in the vascularisation of the limb (PTA in radial arteries with proven patency of the ulnar artery and palmar arch).
Clinical question XX. Recommendations
R 5.3.3) We recommend percutaneous or surgical techniques not be systematically used to induce maturation of native arteriovenous fistulae
R 5.3.4) We suggest surgery as the first choice for treatment (proximal reanastomosis) in the native arteriovenous fistula that fails to mature associated with juxta-anastomotic stenosis. In cases where this is not possible, endovascular treatment should be proposed
R 5.3.5) We suggest percutaneous ligation, surgical ligation or endovascular embolisation with coils be used to disconnect significant accessory veins associated with failure to mature. We suggest endovascular treatment for stenosis and, where there is no stenosis, surgical treatment as the first choice for treatment, given their lower complexity and healthcare cost
R 5.3.6) We recommend angioplasty be performed in cases of non-matured native arteriovenous fistulae with proximal venous stenosis
R 5.3.7) We suggest angioplasty of the arterial stenosis when this is the cause of non-maturation of the arteriovenous fistula, in cases in which the vascularisation of the limb is not compromised
Recommendations
NEW R 5.4.1) We recommend the infection of a native arteriovenous fistula be treated with appropriate antibiotics over 4-6 weeks. In cases of fever > 38 °C and/or associated bacteraemia, we suggest initiating intravenous antibiotic treatment
NEW R 5.4.2) We recommend existing collections be surgically debrided in the infection associated with a native arteriovenous fistula. If the anastomosis is affected and in cases of suppurated thrombophlebitis or septic embolism, we recommend the fistula be closed and the anastomosis be resectioned
R 5.4.3) In cases of partial infection of prosthetic arteriovenous fistula, we recommend the affected segment be surgically excised, and appropriate antibiotic therapy be administered. Where possible, an attempt should be made to maintain vascular access patency by replacing the segment through a new trajectory
R 5.4.4) We recommend the prosthetic arteriovenous fistula be totally excised in extensive infections or in cases involving anastomosis, and adequate antibiotic therapy be administered
Rationale
AVF infection is usually due to inadequate application of asepsis measures for VA management. Therefore, the whole protocol of action should be reconsidered and training should be provided for health staff on hygienic preventive measures of VA infection. Knowledge of activities related to hand hygiene and skin disinfection must be reinforced before accessing AVF.
Infection can present as an area with pain, heat and redness or as a small abscess or scar in the needling area. If any sign or symptom denoting the presence of infection appears, the infection control protocol must be started.
If the AVF can still be used, a series of precautions must be taken. The infection site should be isolated to prevent contamination of the skin where the cannulation is to be made and to keep needling as far as possible from the area. The infected area should not be managed or cleansing performed during the HD session.
Microbiology of arteriovenous fistula infections
Staphylococci are unanimously considered the most frequent cause of infection associated with VA in the literature. A close relationship between personal hygiene and S. aureus nasal and/or cutaneous colonisation has been described, as well as a higher incidence of VA infections in patients with nasal S. aureus.97,532
The second most frequent group is gram-negative bacilli, being especially frequent in infections of pAVF in lower limbs. Consequently, empirical antibiotic coverage in cases of infection should be active against gram-positives and gram-negatives. It is important to know the local susceptibility data of the microorganisms in order to define the appropriate empirical treatment in each centre. Once the responsible microorganism is isolated, antibiotic treatment will be adapted to it.
Infection in the native arteriovenous fistula
nAVF-related infections are relatively infrequent, and they are the VA type with the lowest incidence of this complication. Clinical presentation corresponds to skin and soft tissue infections: pain, local erythema, plus drainage of purulent material and appearance of fluctuating masses on the vein trajectory.14,533
Diagnosis is essentially clinical and analytical, and its extent is defined by physical examination.
These infections usually respond adequately to antibiotic treatment, which should be initiated intravenously when there is fever and/or bacteraemia. The treatment will be maintained for 6 weeks adjusted for microorganism susceptibility.
They are most frequently located in the venous pathway, due to previous cannulations, so cannulation in the affected area should also be suspended.
With adequate medical treatment, the vast majority of cases present a good clinical response, which usually allows the AVF to be completely preserved.
In cases where the physical findings suggest the presence of fluid collections, these should be drained through needling or surgery after ultrasound confirmation.
The infection may, on rare occasions, be located in the arteriovenous anastomosis, in which case AVF disconnection is indicated, due to the high risk of bleeding in artery-vein anastomosis.
In cases of infected thrombus and/or septic embolisms, AVF disconnection is also be indicated.
Infection in the prosthetic arteriovenous fistula
pAVF infection is 2 to 3 times more frequent than in nAVF, and it is also more frequent in lower limb pAVF. Known risk factors include lack of hygiene, diabetes mellitus, hypoalbuminemia, advanced age, cannulation difficulties, formation of periprosthetic haematomas, prolonged post-dialysis bleeding and lack of sterility at the needling site.97 Clinical symptoms may include local pain, graft exposure, appearance of a fistulous tract with drainage of purulent material or a fluctuating mass on the prosthetic tract, localised erythema or a combination of the above, with or without the onset of fever or septicemia.14
The diagnosis is primarily clinical and should be complemented with a DU of the VA to rule out or determine the extension of possible periprosthetic collections. In diagnostic doubt or subacute or chronic infections, a leukocyte scintigraphy should be indicated to detect the presence and extent of infection.533
VA patency is not a necessary condition for prosthetic infection, so it can also occur in old non-functioning pAVF, and this possibility should be ruled out in the presence of any fever or sepsis in these patients.
Antibiotic treatment should start empirically until the causative microorganism has been identified, making sure the most frequently involved microorganisms are covered (S. aureus, coagulase-negative staphylococci and gram-negative bacteria).
In disorder management priority should first be placed on complete resolution of the infection process but at the same time an attempt should also be made, where possible, to preserve the VA. This is why an imaging test of pAVF should be done in order to determine the presence and extent of fluid collections and thus to limit the infection area.533
The only definitive treatment for the infected prosthetic area is surgical excision.97,534 Based on this, several surgical possibilities have been described in the literature:
- •
Total prosthesis excision. It is the classic surgical treatment technique in prosthetic infection. The graft is completely excised with closure of the arteriotomy using a patch of autologous material. It involves CVC placement for HD. Indicated in cases of extensive prosthesis involvement. The anatomical area is not usable in future AVF.
- •
Subtotal prosthesis excision. Similar technique to the previous one. The prosthesis is excised preserving the proximal and distal segments, which are ligated. It is performed to avoid dissecting the tissues proximal to the anastomosis and associated morbidity. It is indicated in cases of widespread infection, but perianastomotic territory is preserved. It involves CVC placement for HD.533
- •
Partial prosthesis excision. Indicated in cases of segmental pAVF involvement. The infected segments are re-sectioned preserving those uninfected, and replaced by another prosthetic segment located in a new trajectory through the tissues. CVC placement can be avoided. It is considered the technique of choice in those cases where technically feasible.533,535-537
- •
Excision and replacement with cryopreserved vein graft. The infected pAVF is totally excised and exchanged for a cryopreserved vein graft prosthesis from a deceased donor. The technique is reported by some authors with good initial outcomes,538 but other published studies have found high rates of serious complications such as infection, dilatation and rupture of the pAVF, so they advise against use.539
- •
Prosthesis excision with brachial artery ligation. Indicated in cases of patients with compromised general condition, this technique offers the advantage of eliminating much of the surgical morbidity secondary to arterial repair; by making the ligation distally at the deep brachial artery exit site, patients have a good tolerance to ischaemia.540 It is considered a fall-back technique.
The technique of choice should be discussed on a case-by-case basis, taking into account the patient’s general condition, how widespread the infection is and what VA alternatives are available. In general, the removal of all infected material will be mandatory in all cases, and an effort made to maintain VA patency through a new trajectory and a new prosthesis. The use of cryopreserved grafts cannot be recommended.
Alternatively, the use of prostheses with high resistance to infection (biosynthetic collagen prosthesis on Dacron matrix) has been proposed. Although these have presented good results in the first published studies,541,542 there is still a shortage of broader studies that may determine their role in the treatment of prosthetic infection.
5.5Distal hypoperfusion syndrome (“steal syndrome”)Recommendations
- (•)
NEW R 5.5.1) In distal hypoperfusion syndrome, we recommend a complete angiographic study and Doppler ultrasound be performed before proposing arteriovenous fistula intervention
- (•)
NEW R 5.5.2) We suggest surgical/endovascular treatment be indicated in distal hypoperfusion syndrome with invalidating symptoms or with tissue loss (stages IIb-III-IV)
- (•)
NEW R 5.5.3) We recommend that techniques that preserve the arteriovenous fistula be prioritised over ligation in the presence of distal hypoperfusion syndrome
- (•)
NEW R 5.5.4) In the presence of significant arterial stenosis in the proximal inflow, we suggest it be treated by percutaneous angioplasty
- (•)
NEW R 5.5.5) We suggest each patient’s characteristics, distal hypoperfusion syndrome stage, arterial anastomosis location and arteriovenous fistula blood flow level be taken into account when choosing surgical technique
- (•)
NEW R 5.5.6) If the banding technique is performed, we suggest it be performed in association with intra-operative arteriovenous fistula blood flow check-up, and discourage using it in isolation
Rationale
One of the potentially more serious, but fortunately infrequent, complications is the development of ischaemia in the distal territory of the limb following AVF creation. The incidence of the disease varies from 1% to 20% of all AVF in the upper limbs85,87-87b; it is more common in nAVF in the arm (10-25%), with its incidence being lower in pAVF (4-6%), and is not very common in nAVF located in the forearm (1-2%).87
Pathophysiology87b,88,543
After AVF creation, the presence of a communication between the arterial and venous circuits causes a flow shunt towards the latter, with much lower peripheral resistance, to the detriment of the distal vascular bed of the limb. This effectively produces a phenomenon whereby much of the flow from the brachial artery is ‘stolen’ and shunted to the venous sector of the AVF. This is the reason why limb ischaemia is known as “AVF steal syndrome”.
This short circuit between arterial and venous circulation causes a physiological response in the body in the form of compensatory mechanisms to maintain tissue perfusion in the distal territory of the limb, which is why the vast majority of patients present no ischaemia in this territory. Ischaemia only presents clinically in cases where, due to previous patient conditions, compensation mechanisms are altered.
These mechanisms consist primarily of an increase in size and hypertrophy in the access afferent artery, which allows the increase in arterial flow necessary for the correct development of the AVF. Secondly, circulation develops through collaterals, especially at the expense of the deep brachial artery in arm fistulae and ulnar artery and palmar arch in forearm fistulae. Finally, in response to ischaemia, generalised vasodilation occurs in the vascular bed distal to the AVF, which causes a decrease in the resistances in this territory and an increase in perfusion.
Thus, in addition to haemodynamic “steal” phenomenon, other factors commonly predispose the appearance of DHS: presence of stenosis or occlusion in the proximal arterial territory or an inability of the distal vascular bed to adapt to the new haemodynamic situation created. That is why most authors, as well as the clinical guidelines, prefer the use of the term “distal hypoperfusion syndrome” to “fistula steal” to refer to this disorder.10,14,87,87b,88,543
Risk factors
Diabetes mellitus, use of the brachial artery, peripheral arteriopathy, advanced age, smoking, female gender, previous failed VA in the same limb and a history of DHS in the contralateral limb are considered risk factors for developing ischaemia.88,92,544,545
In contrast, authors do not agree on anastomosis diameter as an isolated risk factor,87b since although there appears to be a direct relationship between the diameter of the anastomosis and the flow in small-sized AVF, that relationship disappears from a given diameter (75% of the donor artery).544
Clinical presentation87b,545
Symptomatology can present acutely (after the intervention), subacutely (in the first days), or chronically (one month after creation). The acute form, while less common, tends to occur in pAVF while the chronic version is usually progressive over time and is related to nAVF at brachial artery level.546
Clinical presentation is superposable to that developed in other territories with ischaemia, pain, paraesthesia, paralysis, loss of distal pulse, coldness and pallor.545 In more severe cases, it can lead to necrosis and irreversible tissue loss.
In clinical practice, severity of the disease is determined by the analogous classification proposed by Fontaine et al.547 for chronic ischaemia of the lower limbs87,87b,548,549 (Table 24).
Clinical classification of distal hypoperfusion syndrome87b
| Stage I | Paleness and/or coldness of the hand without the presence of pain or sensory/motor disturbances |
| Stage IIa | Tolerable pain during exercise and/or HD |
| Stage IIb | Intolerable pain during exercise and/or HD |
| Stage III | Pain at rest or motor deficit |
| Stage IVa | Limited tissue loss |
| Stage IVb | Significant tissue loss that irreversibly affects hand functionality |
HD, haemodialysis.
Diagnosis
The disorder is diagnosed on the basis of anamnesis (history of previous VA) and on the presence of the previously mentioned symptomatology.
Although DHS diagnosis is essentially clinical, it can be confirmed by means of vascular laboratory testing. Of all the tests proposed, the one that has proved most useful in practice is the Digital Pressure Index (DPI), which consists of measuring the ratio between the digital pressure of one limb and the contralateral brachial artery550. Other useful tests in practice are the calculation of the systolic pressure index between the two limbs, photoplethysmography and oxygen saturation.87,87b,551
Differential diagnosis. Ischaemic monomelic neuropathy
The symptoms, together with access creation history, do not usually pose any diagnostic doubts, and differential diagnosis is proposed with few illnesses: carpal tunnel syndrome, nerve injury associated with surgery and destructive cases of arthropathy, in which a detailed anamnesis and physical examination plus electromyogram usually allow the diagnosis.87,87b
Of particular importance is the differential diagnosis of the entity known as ischaemic monomelic neuropathy (IMN). IMN appears acutely after VA creation surgery, and is an exclusive pathology of diabetic patients and of brachial artery accesses.87b
This disorder is considered to be related to a selective ischaemia of the nervous tissue in the antecubital fossa and has a global effect on the three main nervous trunks of the forearm (radial, ulnar and median nerves). It presents clinically immediately after surgery as refractory pain and motor deficit, coinciding with a physical examination showing no signs of ischaemia and laboratory tests that rule out significant ischaemia. In diagnostic uncertainty, electromyography will typically show the joint involvement of the three nerves mentioned.552
The main risk of the condition lies in the irreversible sensory and motor deficit it can cause; therefore, in these cases, immediate ligation of the AVF is indicated to minimise such sequelae.87b,552
Prevention of distal hypoperfusion syndrome
Once ischaemia has developed, despite proper medical and surgical management, there is a high risk of access loss. For this reason, the ideal approach to adopt should be to detect cases that have a high risk of ischaemia in order to create an AVF with a low risk of DHS.
Firstly, this condition may present depending on the number of ischaemia risk factors in the patient,88,546 so some authors consider the presence of two or more of these factors to identify patients at high risk of DHS.87b
Correct pre-operative assessment should also identify this group of patients. This assessment should include systolic blood pressure determination in both limbs, palpation of peripheral pulses and Allen test. The presence of pressure differences > 20 mmHg between the two limbs, the lack of peripheral pulses or a pathological Allen test are signs indicating a high risk of presenting ischaemia after VA creation.87b
Finally, alterations in pre-operative haemodynamic tests are also suggestive of a high risk of ischaemia, especially alterations in the DPI and the reactive hyperaemia test.60,87b,553,554
Although authors agree it is important to detect patients at risk of developing ischaemia after the VA is created, there is little published literature on the approach to be followed in these cases. Thus, in a given patient, it is not possible to determine if DHS will present.87b,88 Also, the progressive increase in age of the patient in HD leads to the presence of multiple risk factors for ischaemia in the majority of AVF candidates.555
In spite of this, the clinical importance of DHS means that it is necessary to adopt all the measures aimed at minimising the possible presentation of ischaemia in the limb after identifying the patient at risk87b,88,113 (see section 1).
Thus, for a patient with a high risk of ischaemia, the authors recommend the use of the proximal radial artery (PRA) for AVF in the forearm, given the lower incidence of DHS in this procedure.87b,113 The use of the PRA for the AVF in the antecubital fossa has been shown to be a safe technique with no additional morbidity, presenting a lower risk of ischaemia,113,119 with less technical complexity than the other techniques described. Thus, it is considered to be the technique of choice for the prevention of DHS in those cases where technically feasible.87b
Treatment objective
There is general agreement in stating that the goal of therapy should be twofold: to relieve ischaemia and to preserve the access. The different surgical techniques used in treatment are reviewed.
→ Clinical question XXI What is the approach to native or prosthetic arteriovenous fistula diagnosed with steal syndrome?
(See fact sheet for Clinical question XXI in electronic appendices)
| Summary of evidence | |
| The available evidence comes from expert opinions, based on their experience and clinical case series. They indicate that the choice of surgical treatment should be based on patient characteristics, clinical condition and prognosis, stage of the disease, location of the arterial anastomosis, and QA level within the access | Low quality |
Evidence synthesis development
As mentioned, DHS is a potentially serious complication after VA creation. This makes the early detection of symptomatology and the need to act in an appropriate way to prevent irreversible lesions important. Also, given the growing evidence available on surgical techniques that have haemodynamic repercussions and preserve the access, today the aim of treatment should be considered twofold: to improve ischaemia and preserve the VA.87b,88,543,545,549
Symptom management must be appropriate for the clinical stage and severity of the symptoms. In mild cases (stage I and IIa), therefore, in which the intensity of symptomatology does not incapacitate the patient nor represent a risk for limb viability, medical treatment (pentoxifylline, naftidrofuryl, cilostazol, etc.), physical measures (protection and warmth of the limb) and clinical follow-up should be indicated and initiated. In situations where symptoms are incapacitating or involve the risk of tissue loss (stages IIb-IVa), a surgical intervention should be indicated to resolve ischaemia. Finally, in cases of irreversible widespread necrosis (stage IVb) or when it presents acutely, VA closure should be prioritised as treatment of choice87b,88,545,549 (Table 25).
Once the disorder has been identified, when the clinical stage indicates surgery, DU should be performed routinely on the VA and an angiographic study of the limb vascularisation conducted.
Angiography should be performed in all cases in which surgical treatment is considered, and the proximal arterial trunks from the thoracic portion must be examined, since up to 50% of patients with DHS may have significant lesions associated with VA inflow.543,556
Likewise, the access must be studied using DU, since it will provide essential information on QA in the AVF, which is necessary in order to indicate the procedure to be performed.87b
After studying each case individually, surgical correction is indicated, and several techniques have been described in the literature.87b,545
Closure of the access
This is the surgical disconnection of the created AVF in order to reverse the haemodynamic situation and make the ischaemic symptoms disappear. Since it does not fulfil the objective of preserving the access, it is a fall-back technique, indicated only where other techniques fail, in cases of high surgical risk, in acute ischaemia, IMN or where there are lesions with important associated tissue loss87b,549,552 (Table 26).
Endovascular treatment. Percutaneous transluminal angioplasty
In significant stenosis of the arterial inflow associated with DHS, treatment using PTA should be indicated with or without stent placement, which can be performed during diagnosis. It is a safe technique with a high rate of immediate clinical success, resolves symptomatology, and is indicated in cases of arterial lesions in the feeding artery.359,556
Banding
The banding technique consists of restricting the flow in the AVF by limiting the diameter in the anastomosis or in the segment of the juxta-anastomotic vein. There are a number of techniques described, which can be performed by a ligature of non-reabsorbable material, by surgical plication in the outflow vein, interposing a segment of prosthetic material (ePTFE, Dacron), or by placing an external band of the aforesaid prosthetic material.545,549 The aim of banding is to restrict QA through the access, improving perfusion of the distal territory. Therefore, it is indicated exclusively in AVF with high QA, and is especially recommended in cases of AVF with very high output that require significant QA reduction.88
The main limitation of this technique lies in its capability to determine the degree of flow restriction that must be made to improve ischaemia symptomatology without compromising access viability. Therefore, several methods of intra-operative monitoring have been proposed to serve as a guide during surgical intervention: photoplethysmography monitoring, QA control in the AVF, clinical control—radial pulse recovery—, determination of the Doppler curve in the radial artery, monitoring by pulse oximetry and improvement of the patient’s symptomatology.87b,545 Likewise, results are controversial in the medium and long term, and high QA recurrence rates of 52% per year have been described.557
Banding is the first DHS treatment technique described, so it is extensively documented in the literature; the best available evidence comes from the review published by Scheltinga et al.,558 based on 39 clinical case series corresponding to a total of 226 cases. This author finds significant differences between the 16 case series in which there was no intra-operative monitoring or only radial pulse control, with a clinical success rate (recovery of ischaemic symptoms) of 60%, and of access patency of 53%, in comparison to the case series in which some of the monitoring methods described were used. Among these, there was a clinical success rate of 89% and a rate of VA patency of 97%, after an average follow-up of 17 months.
With reference to QA, which should be highlighted as the main objective of banding, flow differs slightly according to the authors, with a value of 400-600mL/min in nAVF and 700-800 mL/min for pAVF being widely recommended, and there is an increased risk of thrombosis in pAVF with flows < 700 mL/min.87b,545,559
Distal revascularisation and interval ligation
First described by Schanzer et al. in 1988560, the acronym DRIL (distal revascularisation and interval ligation) refers to the surgical procedure consisting of 2 combined techniques:
- •
Distal Revascularisation (DR): interposition of a bypass from the proximal to the distal artery to the VA, in order to ensure the perfusion of the distal territory.
- •
Interval Ligation (IL): Ligation of the distal artery to the VA anastomosis, in order to prevent the phenomenon of haemodynamic steal (retrograde flow in the artery distal to the AVF).
Thus, the overall effect sought is to prevent steal phenomenon in the access while favouring the distal perfusion of the limb by means of a bypass of lesser peripheral resistance than the original arterial circuit.
Since this technique was described, it has been used by a large number of groups, and good results have been described in the treatment of DHS.561
Reviews of the case series published87b,561 offer a clinical success rate of 78% to 90% (disappearance of clinical symptoms of ischaemia), maintaining a VA patency of 73% to 100%.549
The main disadvantage of this technique is, firstly, an axial artery has to be ligated, which means that, despite the excellent rates of patency published,87b in case of occlusion, a more severe case of ischaemia than the previous one can be caused. Secondly, some studies find that the degree of clinical improvement is QA-dependent and is less effective as the QA increases in the VA.562 For this reason, it is mainly indicated in the treatment of DHS in AVF with normal or decreased QA.87b
Technical variants
To minimise the risk of ligation in the axial artery, several authors have proposed performing the procedure without ligating the interval, i.e., performing only DR.563 At the same time, in order to increase distal perfusion and the effectiveness of the technique, it has been proposed that proximal anastomosis of the bypass be performed in the most proximal arterial sector, increasing the separation between the anastomosis and the AVF.87b,562
These technical variations are based on the findings of theoretical and experimental models562,564; however, confirmation of their clinical usefulness is necessary in studies with sufficient evidence in order to recommend their systematic use.
Proximalisation of arterial inflow
In this technique, proximalisation of the arterial inflow (PAI), first described by Zanow et al.,565 the AVF in the anastomosis is ligated and this AVF is vascularised by a bypass of prosthetic material between the axillary or proximal brachial artery and the AVF outflow vein. It is applied to accesses located in the arm, improving ischaemia by a combination of several haemodynamic mechanisms: firstly, when a proximal vessel is used as feeding artery, the pressure drop in the distal bed caused by the access decreases; secondly, retrograde flow in the distal artery to the AVF (haemodynamic steal) is minimised or completely suppressed; and thirdly, when a small prosthetic graft (4-5 mm) is implanted, a flow limiting effect is achieved, as described in banding.562,566
As this is a relatively new technique, there is limited evidence regarding clinical outcomes.566 There are only two studies published in the literature with a total of 70 cases, with clinical success (disappearance of symptoms of ischaemia) being described in 84% to 90% of cases, with a primary patency 62-87% at two years.565,567
PAI has several advantages. As it is a technique that causes an increase in access flow, it can be performed in the AVF with a decreased flow and DHS. It also has an advantage over DRIL as it does not require ligation of an axial artery, which means it does not cause ischaemia in cases of occlusion of the procedure. However, as a drawback, it transforms nAVF into pAVF with the increase in associated infectious complications and thrombosis. There is also limited evidence currently available on its outcomes.87b,545,562,566
Revision using distal inflow
A technique initially described by Andrade et al.568and by Minion et al.569 (revision using distal inflow [RUDI]). This consists of disconnecting the VA anastomosis and transposing it distally using a retrograde bypass—prosthetic or autologous—from a distal arterial trunk (radial or ulnar arteries) to the AVF outflow vein.
When a smaller artery is used for the inflow of the VA, AVF flow is reduced, so it is indicated in DHS associated with a high-flow AVF.87b,557
As it is a relatively recent technique, the available evidence is based on case series; in the review published by Vaes et al.,570 only 51 cases have been identified to date, and symptomatology was improved in all cases, with a thrombosis rate of the access of 20%. These authors also describe a reduction in the flow of 60% in the access, together with the potential advantage versus banding of being a more durable technique over time, because flow shows no tendency to progressively increase in the AVF after surgery, unlike banding.570
Distal radial artery ligation
When DHS is caused by an AVF at the wrist, it is frequently associated with hypertrophy of the palmar arch with inverted flow at the level of the radial artery distal to VA anastomosis.571 In these cases, after verifying palmar arch patency as well as retrograde flow in the distal radial artery by angiography and DU, disconnection of the radial artery distal to the AVF can resolve ischaemia (distal radial arterial ligation [DRAL]).87b,88,549
This disconnection may be performed through endovascular intervention, by inserting coils or through minimally invasive surgery.
This technique is limited to rare cases of DHS associated with radiocephalic AVF, and is considered to be a technical variant of DRIL, in which distal vascularisation depends on the ulnar artery together with the palmar arch. For this reason, there is scarce evidence in the literature.87b Miller et al.571 describe a case series (15 patients) in which it is shown to be a safe technique that achieves clinical improvement in a large number of these patients.
Endovascular banding
A technique described by Beathard et al.518 and Goel et al.,572 which consists of performing minimally invasive banding (minimally invasive limited ligation endoluminal-assisted revision [MILLER]): an angioplasty balloon is percutaneously inserted in the VA anastomosis (balloon of 3 to 5 mm in diameter), then inflated in order to later perform banding through a skin incision, maintaining the balloon inflated in the vessel.573
Technically and haemodynamically, it is a variant of the previously described banding technique, but it is less aggressive surgically and is more precise in determining the diameter of the residual lumen. Its main drawback is that morphological parameters (residual diameter of the vessel) rather than haemodynamic (QA in the AVF) are used for monitoring.87b
The available evidence refers to two published case series,572,573 with an immediate clinical success rate of 89% and primary patency of 75% at 6 months, and a secondary patency of the access of 77% at 36 months.573
Proximal radial artery ligation
Bourquelot et al.574 describes this technique, consisting of the ligation of the PRA (proximal radial artery ligation [PRAL]) adjacent to the anastomosis, as a method of limiting QA rate in radiocephalic AVF with high flow. This procedure significantly reduces flow in the access and maintains vascularisation of the hand and of the AVF through the ulnar artery via the palmar arch and collaterals of the interosseous artery.
Initially proposed as a treatment for cases of high-flow radiocephalic AVF, the author describes the resolution of any associated ischaemic condition.574 There is no further evidence published on this technique.
Therapeutic management of distal hypoperfusion syndrome
Given the abundance of treatment techniques described for DHS, most of them of a reconstructive nature (maintaining VA patency), several authors have published proposals on the therapeutic decision of choice in ischaemia treatment, depending on the characteristics presented by each technique.87b,88,543,545,549,556,566
As mentioned earlier, the degree of severity of the symptoms should be established in DHS diagnosis, with stages I-IIa being susceptible to medical management and follow-up; in contrast, stages IIb-IV should be diagnosed and surgical correction proposed.
The authors unanimously indicate access reconstruction in preference to its disconnection, except in the cases mentioned above87b,88,549,566 (Table 26).
Thus, in stages IIb-IV a diagnostic study must be carried out in order to propose the best therapeutic option. This study must necessarily include an angiographic assessment of limb vascularisation and a DU study of the VA.87b,545
Arteriography is necessary to rule out the presence of stenosis or occlusion in any sector of the vascular tree, and must include the assessment of both the proximal (brachycephalic trunk, subclavian, axillary and brachial artery) and distal arteries. The AVF must be compressed to allow evaluation of the distal trunks and the patency and development of the palmar arch.87b,543,556 Likewise, the precise topography of the vascular tree is considered necessary to propose any type of reconstructive VA surgery.545
DU examination, as well as haemodynamic assessment of the access (inversion of flow in the distal artery, presence of accelerations, calculation of resistive indices, diameter of the anastomosis), should include the calculation of QA in the VA, which is essential information required to propose the appropriate treatment in each case.87b,88
Arterial pathology
If the presence of significant arterial lesions is diagnosed in the segment proximal to the AVF, the authors agree to recommend percutaneous treatment, usually during the same diagnostic procedure.87b,543,545,556 The resolution of the ischaemic condition has been described in most patients treated with this type of lesion.556,575
Distal hypoperfusion syndrome in the high-flow vascular access
DHS associated with a VA with high QA (> 800 mL/m for nAVF and > 1000 mL/m for pAVF) indicates the prevailing presence of haemodynamic steal phenomenon, due to the short circuit created when connecting the high pressure and high resistance arterial system to the venous system, of low peripheral resistance. In these cases, the logical proposal is one which reduces AVF flow, an option proposed by most authors.87b,543,549,558,559
Thus, the techniques posited for the treatment of DHS in these patients are banding with flow monitoring, endovascular banding (MILLER) and revascularisation using distal inflow (RUDI). The three have proven to be safe techniques with a high percentage of technical and clinical success,87b,545 although no publications determine differences in effectiveness between them. Consequently, recommendations of the different authors are primarily based on personal experience. However, it has been suggested that the reduction of QA in the VA of banding with monitoring is more effective in the cases of AVF with very high output, thanks to the intra-operative monitoring of the technique, which is why it is especially recommended in these cases.87b
Distal hypoperfusion syndrome in low flow vascular access
The pathophysiology of cases of ischaemia associated with medium and low QA accesses (< 800 mL/min in nAVF and < 1000 mL/m in pAVF) is not considered to have direct relation to the existing vascular short circuit, but depends primarily on a failure in the physiological compensatory processes that maintain distal tissue perfusion in this type of patients.87b,88 For this reason, the main objective in these cases is not to effectively reduce the access flow, but improve perfusion pressure in the distal vascular bed.
The techniques used to do this are PAI and distal revascularisation with interval ligation (DRIL) or without ligation of the arterial interval (DR).
In this case, there are also no published studies in the literature comparing the effectiveness of these techniques, so the available evidence is based on case series and expert opinion.
The most widespread technique, DRIL, was shown to be a safe technique with good outcomes,87,561 besides being the technique that provides a greater increase in the perfusion pressure in the distal territory in experimental models.564 Its main drawback is the need for autologous material for revascularisation and, secondly, disconnection on an axial artery. As a result, some authors have suggested not ligating the interval if the bypass anastomosis is proximalised.87b
PAI is also a safe technique with good outcomes, and is recommended by several authors87b,565 as it does not require ligation of the artery. It has, however, the disadvantage of introducing prosthetic material in nAVF.
In spite of the above, the current level of evidence for these techniques makes it necessary to conduct further studies to help define their suitability in clinical practice.
Distal hypoperfusion syndrome in the distal accesses
The presentation of DHS in distal accesses (forearm and wrist) is uncommon87b,545 because, in the first place, the smaller diameter of the radial artery predisposes development of high QA in the VA to a lesser extent; secondly, the ulnar and interosseous arteries have excellent collaterality that compensates steal phenomenon in these patients. Due to its low incidence, special emphasis must be placed on differential diagnosis, in order to rule out the presence of other conditions, especially neurological (carpal tunnel syndrome, post-surgical neuropathy). Likewise, the degree of impact of ischaemia is usually mild in most cases, so treatment is only required in a few cases.545
Two techniques are essentially described for treatment: DRAL and PRAL. In both, available evidence in the literature is limited. The best available evidence in the case of DRAL is the study of 15 cases published by Miller et al.,571 which describes a clinical success rate at 9 months of 87%, without any loss of the access in any case. In PRAL, the best evidence comes from Bourquelot et al.,574 consisting of a case series where the technique is only used in 2 cases due to DHS symptomatology; it is mainly indicated by the existence of high-flow syndrome.
From evidence to recommendation
DHS is a condition with multifactorial aetiology and complex haemodynamics, triggered by AVF creation in the limb, with a consequent short circuit between the arterial and venous systems. Although inverted flow is detected in the distal artery in most patients with AVF, only in some cases does a clinically relevant ischaemia develop.
Therapeutic management of distal hypoperfusion syndrome
When DHS develops, there is no difference between authors in relation to recommending surgical/interventional treatment in cases of severe ischaemia, with invalidating symptomatology or that jeopardises tissue viability, an opinion based on good clinical practice. Likewise, the indication of conservative treatment and evolutionary control in cases with mild non-disabling symptoms is also widely accepted, since in most patients with mild clinical symptoms after access creation, the condition progressively improves, with a tendency to spontaneous resolution.87b
The indication of technique of choice in each case must be determined by severity of the condition, QA of the access, anatomical characteristics and location of the VA. Several of these techniques are documented as safe techniques with low morbidity, which is why good clinical practice currently recommends the priority reconstruction of access prior to ligature, a technique restricted to the cases shown (Figure 6).
The evidence surrounding surgical techniques available for reconstructing the access, as mentioned, is based on case series and expert opinion, but there are no studies comparing the different techniques with each other.
Arterial pathology
Based on experience from case series, most authors first recommend angiographic assessment of the arterial tree and percutaneous management of the significant stenosis present. It has been decided to adopt this recommendation given the clinical evidence showing improvement in ischaemic symptoms after PTA of the significant stenosis, its minimal invasiveness, high technical success rate and lack of evidence on surgery in the treatment of this condition.
Distal hypoperfusion syndrome in the high-flow arteriovenous fistula
In high-QA AVF, most authors recommend the implementation of a technique that prioritises reducing AVF flow. Thus, the techniques of choice are banding with flow monitoring, MILLER and RUDI. Surgical banding (with QA monitoring) is the most broadly documented, but at present there are no studies comparing techniques, so a recommendation cannot be made on the technique of choice based on the available evidence.
When performing banding, GEMAV considers that the available evidence advises against its use in isolation (without QA monitoring), because it has low VA patency versus other techniques. Thus we recommend this intervention always be associated with intra-operative QA monitoring of VA.
Distal hypoperfusion syndrome in the low flow arteriovenous fistula
When DHS is present in a VA with normal or low flow, treatment must aim to increase distal perfusion pressure. Among the techniques described (DRIL, PAI and DR), DRIL is the technique with the highest degree of evidence, where it has proved to be a safe technique with a high index of clinical success and VA patency. PAI results are similar to DRIL although there are few published case series, while DR has to date been poorly represented in the literature. As there is no evidence from studies comparing results between these procedures, the GEMAV considers that although there is sufficient evidence to justify the use of both techniques (DRIL and PAI), a firm recommendation on the technique of choice in these cases cannot be made at present, and further studies are needed.
Distal hypoperfusion syndrome in distal accesses
As previously mentioned, DHS very rarely develops in these cases and in most cases only presents with light intensity. Consequently, available evidence does not allow any recommendation on the technique of choice to be proposed to treat the condition. Having said this, DRAL is the most widely documented technique in the scarce bibliography, and shows good outcomes in terms of safety, clinical success and VA patency.
Clinical question XXI. Recommendations
R 5.5.1) In distal hypoperfusion syndrome, we recommend a complete angiographic study and Doppler ultrasound be performed before proposing arteriovenous fistula intervention
R 5.5.2) We suggest surgical/endovascular treatment be indicated in distal hypoperfusion syndrome with invalidating symptoms or with tissue loss (stages IIb-III-IV)
R 5.5.3) We recommend that techniques that preserve the arteriovenous fistula be prioritised over ligation in the presence of distal hypoperfusion syndrome
R 5.5.4) In the presence of significant arterial stenosis in the proximal inflow, we suggest it be treated by percutaneous angioplasty
R 5.5.5) We suggest each patient’s characteristics, distal hypoperfusion syndrome stage, arterial anastomosis location and arteriovenous fistula blood flow level be taken into account when choosing surgical technique
R 5.5.6) If the banding technique is performed, we suggest it be performed in association with intra-operative arteriovenous fistula blood flow check-up, and discourage using it in isolation
Recommendations
R 5.6.1) We recommend true arterial aneurysms be surgically resectioned and the artery reconstructed
R 5.6.2) We suggest surgical treatment be indicated for venous aneurysms if they are associated with significant stenosis, necrosis or cutaneous disorders with risk of aneurysm rupture
- (•)
NEW R 5.6.3) We suggest external manual compression, guided by Doppler ultrasound, be first tried in patients with pseudoaneurysm in the needling segment of native arteriovenous fistula before resorting to surgical or percutaneous treatment
- (•)
NEW R 5.6.4) We suggest percutaneous methods (ultrasound-guided injection of thrombin) be used to treat pseudoaneurysms in the needling segment of native arteriovenous fistulae which do not respond to treatment by external compression, and surgical treatment be reserved for cases of failure of the other techniques
- (•)
NEW R 5.6.5) In patients with uncomplicated prosthetic pseudoaneurysms that are small in size, we recommend needling be avoided and clinical stability be monitored by means of Doppler ultrasound
- (•)
NEW R 5.6.6) In a prosthetic pseudoaneurysm with complication criteria, we suggest the affected segment be surgically removed, preserving the patency of the access if technically feasible. We suggest that the possibility of vascular endoprosthesis placement be studied on a case-by-case basis
- (•)
NEW R 5.6.7) We suggest surgical review be done in patients with pseudoaneurysms affecting the anastomosis of the arteriovenous fistula, and the case be considered as an infection of the vascular access
Rationale
The formation of aneurysmal dilatations and pseudoaneurysms is a potentially serious complication that can develop in any AVF. True aneurysms are defined as dilatations or ectasias in vessels in the fistula territory that maintain the entire structure of the venous or arterial wall. In contrast, pseudoaneurysms or false aneurysms are known to be expandable dilatations caused by persistent bleeding through a loss of wall continuity in the nAVF and pAVF, which can be located at the needling site or in anastomosis.
5.6.1 True aneurysms
The dilatation of a vessel above its normal size is known as true aneurysm. Depending on morphology, these may be saccular (eccentric dilatation) or fusiform (concentric dilatation), the latter being almost exclusively related to the VA, and may develop both in the arterial territory of the feeding artery and in the drainage vein.
Definition and incidence
Following AVF creation, normal physiological response comprises an increase in size, both of the artery and the venous pathway. The increase in the venous system may frequently lack uniformity, but have alternating segments of variable diameter. It is therefore difficult to define the term.
There are definitions based on the absolute value of the vessel diameter (> 20-30 mm),576,577 on the increase in size versus the preceding segment (increments of 2-3 times the previous diameter),576,578 on the sum of longitudinal and transverse diameters of the dilatation,579 and even on vessel volume.580 Finally, other authors recommend a wide acceptance of the term, defining it as an “abnormal” dilatation of the vessel.581
Given the different criteria used in its definition, incidence varies between 5% and 60%, depending on the studies published.581
Venous aneurysms
As discussed above, following AVF creation, dilation of the drainage veins is a physiological and necessary response for the VA to function correctly.
There are, however, certain circumstances that can cause anomalous and excessive dilatation of the vein. It can occur, firstly, due to a weakness in the vessel wall, as in patients with renal polycystic disease and in Alport’s syndrome, or because of an increase in endoluminal vessel pressure, as occurs when stenosis develops in a proximal venous segment and in AVF with long-term evolution.579,581,582
Repeated cannulation of the same vein segment may also cause a weakness in the wall that predisposes to ectasia, a phenomenon known as unipuncturitis (1-site-itis), and is usually detected in clinical practice.10
A possible protective effect of diabetes mellitus on the formation of aneurysms has been reported in several published studies, probably in relation to the arterial system’s lower capacity to cause high flow, which occurs in these patients.579 In contrast, the mechanisms by which the use of the buttonhole technique appears to prevent the occurrence of aneurysms is unknown.240,581
The diagnosis is essentially clinical, and scanning with DU is useful to determine the diameter and presence of endoluminal thrombus.
The presence of one or several venous dilatations in the cannulation trajectory does not usually require any intervention, given the benign and stationary nature of the process, which is usually stable for a long time.581
Treatment is indicated when cutaneous changes can be seen, such as signs of cutaneous atrophy, erosions, appearance of inflammation or presence of eschars, which are signs that predict the risk of bleeding. AVF bleeding is the main complication of venous aneurysms; bleeding can be massive, putting the patient’s life at risk in the short term. Other indications of treatment include aneurysm thrombosis, venous hypertension, high flow, and cosmetic reasons.576,583
Bleeding due to VA breakage is a life-threatening emergency, so emergency surgery is indicated. The priority must be to control the bleeding, and, if possible, to preserve the VA.581 On remaining occasions, the main purpose of surgical correction should be to preserve the correct VA function, except in cases where the access is not in use, in which case ligation is indicated.576
A wide variety of surgical techniques has been described for the treatment of venous aneurysms.581 All of them are described in published case series, and there are currently no studies comparing them to each other. The technique of choice, therefore, is determined by the patient’s individual characteristics and by the anatomy of each VA.
These techniques include exclusion of the aneurysm (with or without excision of the aneurysm) with autologous or prosthetic graft interposition,576,584 excision with direct end-to-side anastomosis,585 partial resection of the aneurysm584,586,587 as well as different types of aneurysmorrhaphy.585,588-590
The percutaneous treatment of venous aneurysm consists of the placement of a covered stent (endoprosthesis) in the involved segment.581,591 It offers the possibility of treating associated stenoses in the same act, without the need for CVC placement. In contrast, its drawbacks include possible difficulty in needling the stent-bearing segment and it is also often necessary to associate a partial aneurysm excision procedure or an aneurismorrhaphy to allow vessel cannulation. Despite the good results reported in published case series,592 at present the degree of evidence on the use of these devices does not allow recommending their systematic use, and further studies are needed to determine the indications of this technique.
Arterial aneurysms
Aneurysmal degeneration in the afferent AVF artery is a rare complication after the access is created, with an estimated incidence of approximately 4.5% of all accesses. Its preferred site is in the distal segment of the brachial artery.593
Its appearance is triggered by high QA in the AVF, which is also directly related to the time taken for the access to develop. Finally, several studies have reported a higher frequency in patients with renal transplantation, related to the possible effect of immunosuppressive drugs on the vessel wall. The progressive dilation of the artery has also been observed in these patients, even after the ligature of the access.577,594
It may present clinically as an asymptomatic pulsatile tumour in a third of cases, whereas, on other occasions, symptomatology may comprise symptoms derived from the compression of the median nerve, in the form of neuropathic pain and/or paraesthesia, compression pain from other neighbouring structures, oedema or ischaemia symptoms associated with distal embolisation. Contrary to what happens in other locations, rupture of an aneurysm is a rare complication.577,593,594
Diagnosis of suspicion is based on physical examination, while a DU confirms the diagnosis, offering information on the diameter, length and presence of intraluminal thrombus.
Surgery is indicated by the presence of associated complications and in large aneurysms (> 30 mm) in cases that are technically feasible.577
As it is rare and in many cases is asymptomatic, the evidence in the literature regarding treatment is scarce, restricted to case series with a limited number of patients.
The surgical technique of choice, according to most authors, is the resection of the aneurysm maintaining arterial continuity through direct suture between the proximal and distal artery segments to the ectasia, thus avoiding the interposition of autologous or prosthetic material. If this option is technically not feasible, the use of autologous material (internal saphenous vein or veins of the affected limb) is recommended to revascularise the arterial tree, while the possibility of using prosthetic material (ePTFE) is usually reserved as a last option due to risk of infection and lower patency. The published results of the explained techniques are excellent in terms of patency and clinical success, and symptomatology has been resolved in all cases described.577,593-595
5.6.2 Pseudoaneurysms or false aneurysms
The denomination of pseudoaneurysm refers to the presence of a haematoma which communicates with the lumen of the vessel. It differs from true aneurysm in that the wall of the dilatation is not composed of the usual layers that can be found in the vessel; it is a wall of fibrous tissue and organised haematoma created around a cavity with flow present.596 This is why they are also commonly called false aneurysms or pulsatile haematomas, which are synonymous terms.
In the genesis of the false aneurysm, there is always a loss of integrity in the vessel wall or in the anastomosis, which leads to a leakage of flow to the adjacent tissue, a leak contained by the presence of the haematoma and the fibrous tissue mentioned, thereby determining the possibility of a rapid and expansive growth.583
They are usually caused by traumatic needling in the venous pathway or to repeated needling in the same area in pAVF. When it presents in arteriovenous anastomosis, following VA creation, it is usually caused by a lack of sealing in the anastomosis, whereas late presentation is usually due to active infection in the VA.581
Diagnosis of suspicion is clinical (presence of a rapidly growing pulsatile tumour with presence of haematoma/ecchymosis in the adjacent skin), while DU exploration confirms the diagnosis, and also allows the size of the pseudoaneurysm to be measured.
→ Clinical question XXII In native and prosthetic arteriovenous fistula pseudoaneurysm, when is surgery versus percutaneous versus conservative management indicated, assessed in terms of severe bleeding complications or death?
(See fact sheet for Clinical question XXII in electronic appendices)
| Summary of evidence | |
| A clinical study with three patients showed that external manual compression, guided by ultrasound, can be effective in the treatment of pseudoaneurysms, in order to achieve complete VA patency and functionality, without recurrences in follow-up | Very low quality |
| Different clinical studies separately analysing surgery and endovascular intervention, with stents, show that they are effective treatment techniques in a high percentage of patients in order to recover VA patency and functionality | Very low quality |
Evidence synthesis development
False aneurysms or pseudoaneurysms make up 2%-10% of pAVF. They may or may not be infectious, and can be located in an anastomosis or in repeated cannulation sites and where prosthetic material has deteriorated.
No study was found comparing different treatment approaches to pseudoaneurysm in nAVF and pAVF (surgery versus percutaneous versus conservative management). The available studies are of very low quality, as they are only based on case series that analyse the effect of a single mode of treatment, without a comparison group.
Likewise, the results obtained according to AVF type (nAVF or pAVF) and the location of the pseudoaneurysm (needling or anastomotic area) are not disaggregated in most published series.
Treatment of post-cannulation pseudoaneurysm in the native arteriovenous fistula
Conservative management: external manual compression guided by ultrasound
Ultrasound-guided compression is routinely used in the treatment of post-cannulation arterial pseudoaneurysms, and its usefulness has been widely reported in the published literature.597
Although the technique is widely used to treat pseudoaneurysms in autologous VA, there is very little evidence currently available, with reference to the publication of case series.597,598 In these series, it is described as a safe and effective non-invasive technique that should be tried before resorting to surgical or endovascular treatment, with successful outcomes in 64% to 90% of patients.
Surgery
The surgical technique of choice should be decided on a case-by-case basis, although in most pseudoaneurysms requiring surgery, it consists of manual drainage of the haematoma and direct suture of the leakage point, and may be performed with or without placement of a proximal tourniquet (surgery with ischaemia tourniquet).599
There are no case series published with data from post-cannulation pseudoaneurysms in nAVF treated exclusively with surgery; all of them bring together cases of post-cannulation pseudoaneurysms, anastomotic and pAVF, in addition to reporting various surgical techniques.585,599-601 Thus, the study of Zheng et al.600 describes surgery results in 20 pseudoaneurysms in AVF, with technical success in all cases and primary patency of 95%, leading the authors to consider surgery as the best option to repair pseudoaneurysms in fistulae. Georgiadis et al.601 evaluate surgery in 28 pseudoaneurysms in nAVF and pAVF, with primary patency of 75% at 6 months. In the study of Belli et al.,585 the results of the different processes are also not disaggregated. However, throughout the literature, regarding the outcomes of post-cannulation pseudoaneurysm, surgery offers a technical success rate of 100%.
Percutaneous treatment
As in the other treatment modes, there is scant evidence of percutaneous ultrasound-guided treatment with thrombin injection for pseudoaneurysms in nAVF, which mostly refers to the treatment of pseudoaneurysms in other locations. With a technical success of 80%, Ghersin et al.602 recommend this treatment mode in anatomically favourable cases, based on minimal invasiveness and good technical outcome.
Endovascular treatment
The endovascular treatment described consists of the placement of a stent or endoprosthesis at the point of leakage to seal it.603 As with the other therapeutic options, there is little evidence currently published, in series dealing with a very limited number of cases, with only 17 cases reported.603-605
These studies describe a technical success of 90-100%, with a primary patency of 70% to 90% at 6 months, without the availability of disaggregated statistics of the infection rate.603-605
Treatment of post-cannulation pseudoaneurysm in the prosthetic arteriovenous fistula
Repeated cannulation of a vascular prosthesis causes persistent structural damage in the wall of the ePTFE structure. This damage accumulates in space and time (accumulation of cannulations in the same segment, in prostheses with prolonged periods of use) and can lead to loss of structural integrity in the prosthetic wall.606
It is for this reason that in clinical practice, pseudoaneurysms associated with repeated cannulation of a vascular prosthesis, and with or without infection of this prosthesis, can appear; these are subject to the same complication possibilities as nAVF (expansive growth, compression by neighbouring structures, spontaneous rupture).10
Sometimes the diagnosis is a chance finding, as is the case of small pseudoaneurysms that can remain stable over time. In this case, conservative management can be carried out by ultrasound controls, avoiding the cannulation of the affected area in all circumstances.585
In contrast, when the pseudoaneurysm presents a risk of developing potential complications, both clinical guidelines and expert opinion recommend its treatment. Table 27 shows the main indications for treatment of prosthetic pseudoaneurysms.10,585,607
Prosthetic pseudoaneurysms. Indications for treatment10,585,607
| Rapid growth |
| Size more than double the diameter of the prosthesis |
| Presence of trophic skin disorders |
| Signs of infection |
| Significant shortening of the cannulation segment |
Because of the underlying disruption in the prosthesis wall, treatment must correct it. Both surgical and endovascular treatments have been described.
Surgical treatment
The technique consists of excluding the affected segment, maintaining continuity of the circuit by performing a prosthetic bypass between the sectors proximal and distal to the lesion, through a new subcutaneous bed independent of the previous one.585
Despite being the first standardised technique in the treatment of prosthetic pseudoaneurysm, the existing literature is scarce, and its evidence is limited to case series.585,601 Georgiadis et al.601 describes primary patency of 78% at 6 months in the absence of significant technical complications.
Endovascular treatment
The endovascular treatment of choice consists of the percutaneous deployment of a vascular endoprosthesis in order to seal the pathological prosthetic segment. Some authors recommend proceeding later with the drainage of the pseudoaneurysm thrombus by percutaneous puncture or surgical approach.605,608 Contraindications are associated trophic skin lesions and the presence or suspicion of infection.607
Characteristically, it is advantageous as it does not require surgical approach, and maintains the prosthesis functional and intact from the moment the procedure is performed, while the main drawback lies in the relatively high rate of associated infections (up to 42%).609
Different studies support its clinical usefulness,603-605,608-612 with a technical success rate of 85-100%, primary patency of 20-36% at 6 months, and secondary patency of 54-76%, slightly lower than those of surgical treatment.607 Prosthetic infection rate related to the procedure ranges from 23% to 42%. This high incidence is believed to be due, in most cases, to the presence of a prior subclinical infection associated with pseudoaneurysm.607,609
Treatment of anastomotic pseudoaneurysm
The presence of a pseudoaneurysm in the arteriovenous anastomosis of the AVF is due to the lack of sealing of the suture line. It can occur in two types of circumstances, depending on when it develops. Firstly, anastomotic pseudoaneurysm that appears after the intervention (hours or days after the access is created) is related to surgical technique, whereas after the post-operative period a leak in the anastomosis usually means the presence of a highly aggressive infection with colonisation of the suture line.581
Repair of the pseudoaneurysm is indicated in both cases and must be done through surgical intervention. Placement of an endoprosthesis is contraindicated because of the high risk of infection.581 If it occurs in the post-operative period, surgery must be indicated with haemostasis of the leakage point, whereas if it occurs in relation to VA infection, the infected material must be removed and the AVF reconstructed if technically feasible,607 in accordance with the recommendations made in the section on VA infection treatment.
In the case series published by Shojaiefard et al.599 on 8 patients with surgically treated anastomotic pseudoaneurysms, a technical success of 88% is reported with primary patency of 88% at 15 months. In the absence of complications, the procedure is considered viable, safe and cost-effective.
From evidence to recommendation
As discussed, the currently available evidence on the different therapeutic modes in false aneurysms is based on published case series of the different techniques, albeit without comparative studies. This makes it difficult to establish a criterion based exclusively on this evidence regarding which treatment option to recommend in each case. Recommendations have therefore unanimously been adopted on the basis of good practice by the members of GEMAV. Since these techniques have a good clinical success rate, the most important factor in determining their use has been the degree of procedural invasiveness, and the use of less aggressive techniques is firstly suggested.
Treatment of post-cannulation pseudoaneurysm in the native arteriovenous fistula
Manual external compression guided by ultrasound
This technique, widely used in clinical practice, is the least complex option and can be applied immediately, while the diagnosis is being made by DU. Despite being one of the most widespread therapeutic options, the available evidence on its use is paradoxically scarce. However, for all of the above reasons, and in particular because it is the simplest and least invasive technique, it has been decided to suggest its use in the first instance, where technically feasible.
Percutaneous treatment
The ultrasound-guided injection of thrombin in the pseudoaneurysm cavity is also a minimally invasive technique widely used in practice. Despite the limited published evidence on its use, it has been shown to be a safe technique with a high technical success rate, and has therefore been included as a second therapeutic option after manual compression.
Surgical treatment
As it is the first type of treatment described, there is a greater number of published case series in the literature than in the previous cases. It is safe and has good outcomes in terms of technical success and patency of the procedure. Its main drawback is that it is a technique with a higher degree of invasiveness, so its indication is suggested when previous procedures are not technically feasible or after their failure.
Endovascular treatment
Placement of intravascular stents and/or endoprostheses is another method that has proved useful in the treatment of AVF pseudoaneurysms. It is a minimally invasive technique and has good technical success rates; its disadvantage lies in the greater complexity in contrast to thrombin injection, lower patency in comparison to surgical treatment, as well as in the possibility of infection of the implanted prosthetic material. Finally, the greatest limitation for the placement of an endoprosthesis in AVF is due to the need for a favourable anatomy to achieve correct deployment, which restricts its use in clinical practice. As a result, its systematic use cannot be recommended for treatment in these cases.
Treatment of post-cannulation pseudoaneurysm in the prosthetic arteriovenous fistula
Surgical treatment
Surgical treatment, despite the scarce existing literature, has traditionally been the only therapeutic option available, offering a high clinical success rate, without affecting the prognosis of the pAVF in terms of patency, with a low complication rate. Also, by excluding the affected segment and creating a new subcutaneous tunnel, it is possible to effectively resolve cases in which there is an undetected component of infection, so it remains the technique of choice in these cases. When the prosthetic segment that remains in situ is insufficient to allow correct cannulation, an immediate cannulation prosthesis should be put in place in order to avoid CVC placement.
Endovascular treatment
The deployment of an endoprosthesis to seal the structural defect of the wall is a more recently introduced technique, despite which there are several published case series. This is a minimally invasive procedure, with a high rate of technical success and acceptable patency. In addition, the structural characteristics of the prostheses allow effective deployment in most cases.
The presence of an active infection contraindicates use and makes it obligatory to assess risk/benefit placement on other occasions where infection has not been ruled out.
Treatment of anastomotic pseudoaneurysm
Currently, the only viable therapeutic option in anastomotic pseudoaneurysms is surgery. Since this is a process that indicates active infection, it is recommended the intervention be proposed accordingly, as previously recommended in the section corresponding to the treatment of AVF infections.
Clinical question XXII. Recommendations
R 5.6.3) We suggest external manual compression, guided by Doppler ultrasound, be first tried in patients with pseudoaneurysm in the needling segment of native arteriovenous fistula before resorting to surgical or percutaneous treatment
R 5.6.4) We suggest percutaneous methods (ultrasound-guided injection of thrombin) be used to treat pseudoaneurysms in the needling segment of native arteriovenous fistulae which do not respond to treatment by external compression, and surgical treatment be reserved for cases of failure of the other techniques
R 5.6.5) In patients with uncomplicated prosthetic pseudoaneurysms that are small in size, we recommend needling be avoided and clinical stability be monitored by means of Doppler ultrasound
R 5.6.6) In a prosthetic pseudoaneurysm with complication criteria, we suggest the affected segment be surgically removed, preserving the patency of the access if technically feasible. We suggest that the possibility of vascular endoprosthesis placement be studied on a case-by-case basis
R 5.6.7) We suggest surgical review be done in patients with pseudoaneurysms affecting the anastomosis of the arteriovenous fistula, and the case be considered as an infection of the vascular access
Cannulation in the apical area of venous aneurysms
The skin in areas above aneurysms is more prone to losing elasticity properties, healing power and barrier effect against infections. Therefore, it is more advisable to cannulate in areas of non-damaged skin and, if cannulation is needed in the aneurysm, it should be performed at its base. This avoids complications such as bleeding risk, both when cannulating and in haemostasis, poor healing with risk of scarring or necrosis, and infections.
5.7 High-flow syndromeRecommendations
- (•)
NEW R 5.7.1) We suggest arteriovenous fistula flow be reduced through surgery in patients without clinical improvement following medical management and with blood flow > 2000 mL/min and/or blood flow/cardiac output > 30%
- (•)
NEW R 5.7.2) In patients with a high-flow fistula and heart failure attributed to the arteriovenous fistula, we suggest intervention using banding or RUDI
Rationale
Heart failure is the most common cardiovascular disease associated with CKD613 and is present in one third of patients undergoing HD,614 which involves a high risk of cardiovascular mortality for these patients.615 At the same time, up to 75% of patients with advanced chronic kidney disease have left ventricular hypertrophy at the beginning of dialysis, which is also a predictive variable of mortality.616 Heart failure in the HD patient differs from that of the non-uraemic patient due to several factors; among these, there stand out volume overload and QA of the VA, which could contribute to the development of heart failure.
Cardiovascular consequences of the arteriovenous fistula
Several mechanisms have been proposed that could lead to the generation of cardiac pathology following AVF creation. Once created, there is a persistent reduction in blood pressure, arterial stiffness and peripheral resistance, which increases sympathetic nervous activity. This, in turn, increases cardiac frequency and contractility in order to maintain blood pressure, with the consequent increase in the ejection fraction and therefore cardiac output (CO), which can be increased by 10-25%.617-620 Within days or weeks, blood volume and left ventricular end-diastolic volume and pressures increase. A greater increase in the CO can develop in about 3 months, with an increase in the mass and left ventricular size, as well as in the atrial size.621 A systolic and diastolic dysfunction, ventricular dilatation and reduction of the ejection fraction with an increase in pulmonary flow and subsequent pulmonary hypertension may progressively appear.194,622 In fact, the incidence of pulmonary hypertension of up to 40% in the patient on HD with AVF has been described,623 in the context of high QA. However, it has been suggested that there may be an underlying dysfunction in pulmonary vascularisation in a uraemic environment which would cause AVF to precipitate the decompensation of the pulmonary circuit by causing a decrease in vasodilation.624
This whole process would begin with cardiac remodelling at the expense of an eccentric left ventricular hypertrophy, in relation to volume overload, with a relatively normal wall thickening unlike concentric hypertrophy due to pressure overload.625 Hypertrophy and dilatation of the left ventricle, as adaptive phenomena in response to increased pressure and volume loading, usually occur in athletes, pregnant women, and in the growth period from childhood to adulthood. Volume overload produces an increase in systolic afterload which is associated with radial wall stress in the systolic phase, resulting in the addition of sarcomeres to the myocardial fibres predominantly with a serial pattern rather than in parallel. This myofibrillar elongation contributes to the enlargement of ventricular lumen and to eccentric rather than concentric hypertrophy.626,627 But although ventricular dilatation may initially be adaptive, according to the Frank-Starling mechanism, progressive increase in ventricular volume, concomitant myocardial fibrosis, and relative myocardial ischaemia (even in the absence of coronary disease) may eventually result in an affectation of systolic contractility and to lead, in time, to cardiac failure.628 This ventricular remodelling has been associated with poor long-term prognosis in chronic renal failure.629
The risk is potentially higher during the nAVF maturation period due to the haemodynamic changes that occur secondary to the large increase in QA caused by the nAVF,193 as well as during the first 120 days after starting HD, since the mortality rate is maximum within this period.630
Arteriovenous fistula flow and cardiac output
High cardiac output in adults has been defined when it is > 8 L/min or a cardiac index > 3.9 L/min/m2.631 The increase in CO is proportional to QA, which is usually between 1 and 2 L/min, in order to maintain adequate peripheral perfusion. If myocardial contractility is impaired, excess volume caused by QA in combination with inadequate peripheral compensatory vasoconstriction to maintain systemic blood pressure may lead to the onset of heart failure.632 Cases of patients with high symptomatic CO with QA 3-4 L/min and CO 7-10 L/min have been reported360,633 where this relationship is evident. However, there are no clear criteria for defining a high-flow AVF, since the description of heart failure associated with chronic renal failure in high CO is limited and confined to case series.360,634
In a prospective study with 96 patients to describe the relationship between QA and CO, Basile et al.194 observed greater cardiac failure in the proximal AVF, describing a third-order polynomial regression as the best model to explain this relation, in which high-output heart failure could occur from values > 2 L/min. All 10 subjects who developed heart failure had QA of 2.3± 0.3 L/min, while in all other patients it was 1.0 ± 0.4 L/min. Other authors suggest that the QA/CO ratio may provide an estimate of the contribution of VA to CO, and if it is > 0.3, it may increase the risk of developing high output heart failure,635 or more specifically, if it is > 40%.636 Although it has not been confirmed with prospective studies and despite the scarce sample, it is suggested that it can be reasonably assumed from 2.0 L/min there is a predictive power of high CO heart failure, as well as a QA/CO ratio > 0.3. This could be a decompensatory factor for pre-existing cardiac failure and even lower flows could also decompensate for heart failure in patients with poor cardiac reserve.637-639
But this relationship of QA and CO is not demonstrated linearly from the clinical point of view. Wijnen et al.,640 like Basile et al.,194 noted that in patients without heart failure, CO is significantly higher among proximal AVF compared to distal AVF. However, only a small percentage of these proximal AVF are in a risk area for the development of high-output heart failure. At the same time, there are studies that demonstrate a low frequency of heart failure due to high QA in AVF (3.7%).641 Thus, the cause of evolution from left ventricular hypertrophy due to overload at heart failure is not clear. Therefore, some authors suggest, on the one hand, the participation of underlying heart disease625 and, on the other, possible participation of a high end-diastolic volume in the left ventricle.628 Indeed, it has been observed that QA > 2 L/min presents this greater tendency to a greater left ventricular end-diastolic volume642 and that flows < 2.2 L have no impact on CO.194 The causes of this behaviour are not known, but it can be hypothesised on the existence of some type of myocardial reserve that can allow the adaptation capable of supporting increases in QA in the long term without precipitating the occurrence of heart failure.637 For this reason, the aim would be to identify the patient with underlying heart disease with a higher risk of suffering the repercussions of a high flow on cardiac function in order to intervene.639,643
In this respect, although the relationship between AVF QA and CO is proven635 and there are studies that show AVF creation as the most determining factor for developing heart failure,193 no increase in mortality has been shown from the epidemiological point of view in relation to flow.644 There are even studies in which higher QA has been associated with less heart damage,645 and a reduction in peripheral resistance and blood pressure, with a parallel increase in ejection fraction which may be potentially beneficial.620 In this context, in an observational study of 4854 patients,646 the long-term association of the AVF with lower cardiovascular mortality of any type was demonstrated when compared to CVC use (p < 0.004), regardless of the comorbidity of both groups. This confirms the controversy surrounding the extent to which cardiac function is altered after AVF creation, given the presence of multiple confounding factors in these patients. In other words, whether the AVF contributes to the onset of heart failure, but from a limit, or it is an underlying heart disease that is decompensated by the AVF.647
Ligation of the arteriovenous fistula in the kidney transplant patient
There is evidence to support the fact that there is a regression in the cardiac indexes after ligation or reduction in AVF QA. This has been demonstrated in transplanted patients who have undergone AVF ligation and have presented a regression in dilatation and in left ventricular mass648,649 or a significant improvement in the ejection fraction.650 In addition, when comparing the effects of nAVF and pAVF, there are no differences in the increase in left ventricular measurements, suggesting that flow, rather than VA type, influences the development of high QA.651 These favourable results, however, have not been confirmed with clinical trials, so AVF ligation cannot be recommended in a standardised way in the asymptomatic transplanted patient.
Strategies to manage heart failure in relation to high flow of the arteriovenous fistula
Management of symptomatic heart failure should be directed primarily to treat excess volume and symptoms medically, such as correction of anaemia and other treatable factors. In the absence of success, an attempt should be made to correct the cause of the high output. In this case, it would be necessary to reduce AVF flow, trying to preserve the VA. The same surgical techniques as those used to treat DHS in high-flow AVF reviewed in the previous section would apply. They would mainly include, on the one hand, banding or variants such as MILLER and, on the other, a new distal anastomosis (RUDI).559,572,573,650,652-655 The aim, as in DHS, is to preserve AVF use and reduce heart failure, but bearing in mind, in the last instance, that AVF ligation should be performed when this reduction cannot be achieved.
Choosing arteriovenous type of in patients with cardiac pathology
When planning AVF creation, it must be remembered that proximal AVF presents a higher QA. Thus, the risk must be weighed up in patients with underlying heart failure as they are more likely to present a worsening cardiac function with this type of access than in those who have a distal VA.640 This forces the choice of the most appropriate VA for each patient with heart failure, and should assess the risk of heart failure decompensation after AVF creation. In this respect, it has been suggested that patients with heart failure classified according to the New York Heart Association (NYHA) as class I-II could start HD through a distal nAVF (wrist or anatomical snuffbox)646,656; in patients with class III, the decision on the creation of a distal nAVF versus a tunnelled CVC placement or the transition to another dialysis technique, namely peritoneal, would have to be decided on a case-by-case basis according to the degree of cardiac affectation; and, finally, patients with heart failure and significant reduction in systolic function or class IV would be subject to CVC placement to initiate HD treatment or the choice of another dialysis technique.646,656
→ Clinical question XXIII In the high-flow arteriovenous fistula, what therapeutic approach should be taken and what are the criteria (risk factors)?
(See fact sheet for Clinical question XXIII in electronic appendices)
Evidence synthesis development
Intervention criteria in the high-flow arteriovenous fistula
Ideal HD AVF should work with a QA needed to prevent thrombosis while providing maximum efficiency for HD.657 Flows in the range of 600 to 1500 mL/min have been considered as optimum, with high-flow fistulae being classified as having flows between 1500 and 4000 mL/min.657
Other authors658 consider that a flow between 400-600 mL/min in an arteriovenous fistula is generally sufficient to maintain effective HD. On the other hand, it is pointed out that although there is no consensus definition about when a flow can be considered high, a cut-off of 2000 mL/min is normally used since, as seen, some studies have found that heart failure is more frequent in HD patients with a QA of the VA above this threshold.
The existence of a hyperfunctioning fistula with high QA has been associated with several potential problems: cardiac overload, cardiopulmonary recirculation, rapid access growth with formation of aneurysms, or recurrent venous stenosis resulting in VA failure.657 As already mentioned in previous sections, it may also cause distal hypoperfusion syndrome, as well as venous hypertension in the absence of central venous stenosis. After diagnosis of any of these situations, intervention should be performed to solve or mitigate the problem, while at the same time try to preserve VA.
High QA is often detected by chance in a routine measurement658 that, if confirmed on repeated occasions, raises the question of whether to proceed to a flow reduction intervention. However, the decision to treat is controversial due to a lack of absolute criteria for starting it.
No studies have been found comparing the clinical evolution of patients with high QA fistula, depending on whether they have been treated to reduce QA or not. The evidence available comes from expert opinions and case series, thus are of low quality.
Recent reviews consider that the therapeutic approach should depend on each patient’s history and clinical condition.657,658 For example, it makes sense for a patient with high QA in the AVF and with compromised cardiac function to undergo an intervention to reduce QA in the AVF, given that if this patient doesn’t, she/he may sooner or later develop an additional cardiac event. But it also seems a sensible decision not to intervene if a high QA is detected in an AVF in a young patient with normal cardiac function who is on the waiting list for a kidney transplant.
It must not be forgotten that, in addition to cases related to distal hypoperfusion syndrome or cardiological repercussions of AVF, intervention may be required in patients who present aneurysms or with exaggerated AVF development, in cases of central venous stenosis, or when the difference between inflow and outflow causes inflammation in the arm and AVF dysfunction.657,658
Treatment options
As already comprehensively reviewed in the section of DHS, the main techniques that have been developed to reduce high QA in AVF are banding or one of its variants and RUDI.
Banding
In the study published by Miller et al.,573 already discussed, with 183 patients treated with banding, in addition to the complete remission of symptoms in 109 of the 114 patients who had DHS, they also managed to achieve remission in 69 patients with high flow with diseases such as congestive heart failure, aneurysms and high venous pressure. The primary patency of the intervention at six months was 75% and 85%, respectively, for DHS and high flow. The secondary patency of the access at 24 months was 90% and 89% and thrombosis rates were 0.21, 0.10 and 0.92 per year with the access for nAVF of arm, forearm and pAVF, respectively.
Moreover, two case series analyse the Miller banding technique in patients with central venous stenosis. Jennings et al.659 used banding in 22 patients with high flow and central venous occlusion with clinical repercussion in terms of inflammation of the limb. Inflammation disappeared immediately in 20 patients and showed sufficient improvement in the other two. The mean flow dropped from 1640 mL/min to 820 mL/min after the intervention (p < 0.01). Two of the AVF failed, one at 8 months and the other at 13 months.
Miller et al.456 also analysed the effect of banding in 33 patients with stenosis of the brachiocephalic trunk followed up for a mean of 14.5 months. The reduction of the flow was from 2226 mL/min to 1225 mL/min, with a mean of 42%. Patency at 3, 6 and 12 months was 91%, 76% and 57%, respectively. The rate of interventions on the brachiocephalic trunk dropped from 3.34 to 0.9 per year of access.
Schneider et al.660 describe a different mode of banding, T-banding, which aims to avoid possible movements of the graft, by a prosthesis that surrounds the vein in the post-anastomotic and the anastomotic zone. In a case series of 22 patients, 20 of them with heart failure, 6 of these patients also had DHS and two only DHS, a mean flow reduction of 44% (range 27-71%) was achieved, from a mean flow of 1956 mL/min to 983 mL/min one month following surgery. 72% of the patients showed complete improvement in symptoms and four, who presented partial improvement, required another intervention to achieve complete improvement. The procedure was successful in 95% (19/20) of patients with heart failure and in 83% of those with DHS (5/6). The access continued to be used in all patients, with primary patency of 90% and secondary patency of 100% at 1 month and 3 months.
Revascularisation using distal outflow
Like the banding technique, RUDI can also be used for AVF with high flow, as mentioned above. Chemla describes a case series of 17 patients with symptoms of heart failure (15 nAVF and 2 pAVF)641 with QA > 1600 mL/min, on which the technique is performed, achieving a reduction in the flow from 3135 ± 692 to 1025 ± 551 mL/min (p = 0.0001). The decrease in CO was from 8 ± 3.1 to 5.6 ± 1.7 L/min (p = 0.001) achieving a resolution of symptoms. 7 stenosis or thrombosis were developed, of which 3 were submitted to surgical review.
Proximal radial artery ligation
In a prospective study, Bourquelot et al.574 included 37 patients (8 children and 29 adults) who underwent PRAL to treat high flow in radiocephalic fistulae: 2 for ischaemia, 14 with aneurysmal degeneration of the vein, 7 for heart failure and 14 for the prevention of cardiac overload. The pre-operative QA in children of 1316 mL/min and 1736 mL/min in adults decreased by 50% and 53%, respectively. Primary patency rates at 1 and 2 years were 88% and 74%, and secondary patency, 88% and 78%, respectively.
Transposition of the radial artery
Another study by Bourquelot et al.,448 in 47 patients with an AVF created on the brachial artery, transposed the distal radial artery to the elbow area, where it is anastomosed to the AVF, previously disconnected from the brachial artery in order to achieve a reduction in flow. Indications for treatment were hand ischaemia (4), heart failure (13), concern about future cardiac dysfunction (23) and chronic venous hypertension resulting in aneurysmal degeneration of the vein (7). Technical success was achieved in 91%. The mean reduction of flow was 66%, from an initial mean flow of 1681 mL/min. Clinical success in symptomatic patients was 75%. The fistula, however, had to be ligated in three cases of heart failure due to insufficient clinical improvement. Primary patency rates at 1 and 3 years were 61% and 40%, and secondary patency at 1 and 3 years were 89% and 70%.
Ultrasound-guided flow reduction surgery
Tellioglu et al.661 analysed the role of QA reduction surgery by monitoring the flow by DU in 30 patients with high-flow AVF, 25 nAVF and 5 pAVF. The indications for the operation were heart failure (n = 18) or DHS (n = 12). The preoperative measurements of nAVF, pAVF and the diameter of the anastomosis were: 2663 mL/min (1856-3440 mL/min); 2751 mL/min (2140-3584 mL/min) and 7.3 mm (6.1-8.5 mm), respectively. The flow was reduced to 615 mL/min (552 - 810 mL/min) for nAVF and 805 mL/min (745-980 mL/min) for pAVF. The mean diameter of anastomosis was reduced to 4 mm (3.5-4.3 mm). There were no re-interventions. After a median of 1 year of follow-up, patency rates were 100% for nAVF and 80% for pAVF. Cardiac output rate decreased from 8.5 L/min to 6.1 L/min (p < 0.01).
From evidence to recommendation
VA impact is proportional to QA, while the development of heart failure symptomatology and high CO depends on both QA and adequate cardiac compensation capacity.
In the event of heart failure, it should be suspected that the AVF is at least partly responsible when the patient’s heart symptoms worsen after the AVF creation, especially in VA with high QA, usually associated with proximal AVF. High values of QA should be considered when they are > 2 L/min and a QA/CO ratio > 0.3. In the asymptomatic patient, the risk of developing high-output cardiac failure may increase in the presence of these values, which is why these patients should be closely monitored.
Likewise, anaemia, dry weight, and additional factors that may cause similar symptoms in this type of patient should also be monitored. Therefore, in the first place, the therapeutic approach should be based on medical management and treatment of volume excess in order to aim for surgical reduction of QA at a later stage and, in case of refractoriness, to AVF ligature.
Although there is a limited number of case series, the main techniques that have demonstrated acceptable success in the reduction of flow, clinical improvement and VA patency are those based on banding or its variants and on RUDI.
The patient’s underlying cardiovascular status should be taken into consideration before AVF is created. In NYHA class III patients, distal AVF should preferably be indicated on a case-by-case basis if peritoneal dialysis cannot be done, and CVC placement can be assessed; and in NYHA class IV, patients would need CVC and another dialysis technique.
Although routine post-transplant ligation has shown good outcomes in the regression of cardiac affectation rates, it is not standardised. Thus, despite its favourable outcomes, clinical trials are needed before performing routine ligation in the stable transplanted patient.
Clinical question XXIII. Recommendations
R 5.7.1) We suggest arteriovenous fistula flow be reduced through surgery in patients without clinical improvement following medical management and with blood flow > 2000 mL/min and/or blood flow/cardiac output > 30%
R 5.7.2) In patients with a high-flow fistula and heart failure attributed to the arteriovenous fistula, we suggest intervention using banding or RUDI
CONTENTS
- 6.1.
Indications
- 6.2.
Catheter selection
- 6.3.
Catheter insertion
- 6.4.
Catheterisation control
- 6.5.
Catheter handling
- 6.6.
Catheter follow-up
- 6.7.
Catheter-related complications
- 6.8.
Catheter dysfunction
- 6.9.
Catheter-related infections
Preamble
The use of central venous catheters (CVC) has risen progressively in patients undergoing haemodialysis (HD). However, the indications for their use should be limited because of increased complications, both thrombotic and infectious.
Despite morbidity and mortality, CVC continue to be an essential vascular access (VA) for all Nephrology Departments. On the one hand, they allow immediate use after insertion, which makes it possible to perform HD in emergency situations when patients present serious clinical conditions, such as severe hyperkalaemia or acute pulmonary oedema. On the other hand, they provide definitive access in patients whose vascular bed is exhausted.
6.1. IndicationsRecommendations
R 6.1.1) We recommend a central venous catheter be used in patients with acute or acute-on-chronic renal failure who require urgent vascular access for haemodialysis
R 6.1.2) We recommend a central venous catheter be placed after a non-recoverable thrombosis of an arteriovenous fistula until a new arteriovenous fistula is created
- (•)
NEW R 6.1.3) In patients who cannot undergo native arteriovenous fistula creation, we recommend a prosthetic arteriovenous fistula be created prior to placing a central venous catheter
R 6.1.4) We suggest a central venous catheter be used as vascular access for haemodialysis in certain special circumstances: life expectancy less than 6 months, cardiovascular condition contraindicating arteriovenous fistula, kidney transplant from living donor and expressed desire of the patient
Rationale
The use of CVC is an alternative to arteriovenous fistula (AVF) and, although the use of CVC is inadequate, there is no doubt that they play an important role in managing patients requiring HD. The first reason for this is that they can be used in theoretically any patient; secondly, they are placed easily and are available for use immediately after insertion. Two types of CVC are used in routine clinical practice: a) non-tunnelled central venous catheter (NTCVC), used primarily in acute situations, and b) tunnelled central venous catheter (TCVC), commonly used for long-term or permanent VA. NTCVC afford the following advantages: they are easy and rapid to place, can be inserted at the patient bedside using sterile Seldinger technique, do not require tunnelling, and there is minimal trauma. Although they provide a lower flow, they quickly access the vascular bed and do not require an image, which makes them very useful in emergency situations. TCVC were developed in 1987 as an alternative to NTCVC.662,663 They are more complex to place and require imaging techniques to ensure tip location and absence of kinking. However, they present a lower rate of complications and reach higher flows, so they are the preferred choice for prolonged periods of time.
However, despite their considerable advantages, CVC are also associated with a high cost in morbidity. For this reason, it is important to set clear indications of use and be familiar with related complications and treatment. They must be used only in those patients who cannot carry a native AVF (nAVF) or a prosthetic AVF (pAVF), either because AVF cannot be created (absence of arteries with an adequate flow or venous bed occlusion) or is pending maturation; and with contraindication for peritoneal dialysis; in the case of acute renal failure, or in special circumstances: reversible renal function deterioration requiring temporary HD, life expectancy of less than 6 months, cardiovascular condition that would contraindicate VA creation or the patient’s express wishes.
According to various published clinical guidelines,10,14 CVC should, in most cases, be considered after nAVF and pAVF when selecting the appropriate VA to start a chronic HD programme. In addition, some guidelines distinguish between TCVC and NTCVC as “third option” and “choice of necessity”, respectively.13 If this order of priority is followed, the situation is not optimal in most developed countries.32,664-667 The multinational European study conducted by Noordzij et al.,666 from the data of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry on 13,044 patients in HD, showed that CVC use to start a HD programme significantly increased from 58% in 2005 to 68% in 2009. In Spain, recent data from the Registre de Malalts Renals de Catalunya Registry (RMRC-Catalan Registry of Kidney Patients), referring to almost 10,000 incident patients in HD, have shown that approximately 50% of patients with advanced chronic kidney disease (ACKD) started HD each year in Catalonia through a CVC during 2000-2011.667 The various factors involved in the excessive number of CVC, both in incident and in prevalent HD patients, have been previously analysed665 and some of these could be neutralised by improving organisation.278,667 For example, the current rate of CVC may be reduced by introducing the figure of vascular access coordinator and/or prioritising the surgical waiting list.668,669
Why should TCVC not be considered as the first choice for VA for most patients? The answer is very clear: because of its greater associated comorbidity.95,630,670,671 By applying a multivariate competitive risk model, it has recently been demonstrated that the risk of all-cause mortality in comparison with nAVF over the years is 55% and 43% higher for patients who start HD through TCVC and NTCVC, respectively.630 During the period of maximum mortality among these patients (the first 120 days), the risk of all-cause, as well as cardiac and infectious, mortality is significantly higher for both TCVC and NTCVC versus nAVF.630
In recent years, there has been a change in the type of CVC used to start the chronic HD programme. In this respect, from 2002 onwards, TCVC use in the first HD session has increased gradually in Catalonia while NTCVC has decreased.667 Similarly, the proportion of incident patients in HD in Australia with a TCVC increased from 39% in 2008 to 42% in 2011 and, in contrast, the percentage of patients who started HD through NTCVC decreased from 22% in 2008 to 12% in 2011.672 This change in the type of CVC used can be attributed, on the one hand, to the generalisation of the tunnelling CVC procedure and, on the other hand, to the demonstration of a significantly increased risk of infection in NTCVC versus TCVC a few days after use, due to a lack of tunnel and anchorage (cuff) to the subcutaneous tissue.667,673
Moreover, CVC has specific indications for use as initial VA. Patients requiring TCVC use are those with total arterial/venous exhaustion or where AVF creation is absolutely impossible, with severe peripheral arterial disease, chronic arterial hypotension because of its association with recurrent AVF thrombosis (mainly pAVF), life expectancy of less than 6 months and severe cardiomyopathy with depressed left ventricular function.390,674,675 In the latter case, after several weeks of HD through TCVC, cardiac function should be reassessed to identify those patients whose heart condition has improved and can benefit from AVF construction.675 In addition, TCVC has also been used as a “bridge” VA to allow time for the nAVF to mature. Occasionally, due to the existing haste, TCVC placement has been unavoidable and HD has been performed when the incident patient had previously chosen peritoneal dialysis technique or was awaiting a living donor transplant.
NTCVC should always be placed transitorily in patients with ACKD,676 only when HD is required without delay in incident patients without AVF or with maturing AVF, or in prevalent patients presenting AVF thrombosis which cannot undergo immediate salvage.
It is important to understand and make clear that CVC is inferior to AVF and is not a substitute. Early nAVF creation is the best way to prevent complications caused by CVC.
→ Clinical question XXIV In patients who cannot undergo native arteriovenous fistula creation, is the central venous catheter the vascular access of choice versus prosthetic arteriovenous fistula?
(See fact sheet for Clinical question XXIV in electronic appendices)
Evidence synthesis development
The systematic review of Ravani et al.96 shows the evidence on the outcome of different types of VA published up to 2012; however, there are no clinical trials directly comparing the different types.
This review discusses a meta-analysis of observational studies showing that CVC-bearing patients have poorer outcomes than those with pAVF in terms of:
- •
All-cause mortality: 15 cohorts from 13 studies, 394,992 patients (relative risk [RR]: 1.38; 95% confidence interval [CI], 1.25-1.52).
- •
Fatal infection: 11 cohorts from 10 studies, 235,176 patients (RR: 1.49; 95% CI, 1.15-1.93).
- •
Non-fatal infection: 17 cohorts from 17 studies, 13,121 patients (RR: 2.78; 95% CI, 1.80-4.29).
- •
Severe cardiovascular event: 8 cohorts from 7 studies, 234,819 patients (RR: 1.26; 95% CI, 1.11-1.43).
- •
Hospitalisation: 4 cohorts from 4 studies, 56,734 patients (RR: 1.51; 95% CI, 1.30-1.75).
Some authors highlight that sometimes the VA is not chosen on the basis of clinical criteria but on the experience of professionals and the availability of expert vascular surgeons and/or radiological teams, thereby increasing the number of CVC in incident and prevalent patients.677
Randomised studies comparing clinical outcomes and costs between pAVF and CVC are needed.678
Use of resources and costs
James et al.678 found that average costs of placing and maintaining VA in HD incident patients in Canada were $13,543 for pAVF and $10,638 for CVC. VA maintenance costs in prevalent patients were $5866 and $3842, respectively.
From evidence to recommendation
Though there are no clinical trials comparing results of CVC and pAVF use, both the work of Ravani et al. and the literature reviewed indicate that CVC should be used as the last VA choice for patients with CKD due to poorer outcomes in all the morbidity/mortality-associated variables. For this reason, when it is impossible to create nAVF, GEMAV recommends constructing pAVF to avoid TCVC placement.
6.2. Selection of catheterRecommendations
NEW R.6.2.1) We recommend that tunnelled central venous catheters placed in the upper central veins be long enough to place the tip in the right atrium and, in the lower central veins, to place the tip at least inside the inferior vena cava
- (•)
NEW R. 6.2.2) We recommend a non-tunnelled central venous catheter be used in situations in which the catheter has to remain in place for under two weeks. For longer periods we recommend tunnelled central venous catheters be used
- (•)
NEW R. 6.2.3) We cannot recommend using any model or type of tunnelled central venous catheter for haemodialysis as a priority over another
Rationale
The function of CVC for HD is to access the bloodstream so as to provide enough continuous blood flow to appropriately dialyse the patient. In recent decades, there have been new developments in designs in CVC making placement and subcutaneous tunnelling easier, obtaining higher blood flows and improving adaptation for use in longer periods.
Currently, CVC for HD are usually classified as NTCVC, indicated for use under 2 weeks, and TCVC, with the same indications for prolonged periods. The reason for this division is based on the greater number of infectious and non-infectious complications found in NTCVC.673,679-681 NTCVC is reserved for patients who require HD with anticipated use of under two weeks, after which the incidence of infections increases.673 It is suggested that TCVC should preferably be used versus NTCVC in cases of acute renal failure, as there is greater efficacy for HD, fewer complications and a lower number of replacements,682 and NTCVC should be reserved for patients with suspected sepsis.
In recent years, it has also been suggested that different placement and care strategies for NTCVC may reduce the number of complications.683
NTCVC are usually made of materials such as polyvinyl, polyethylene or polyurethane. These materials have a relatively hard consistency at room temperature, so can be easily tunnelled in the subcutaneous tissue, and make it easier to insert with the help of a metal guide without requiring a sheath introducer. At the same time, they soften and become more flexible at body temperature, thereby minimising the risk of damage to the vessel wall.684 A smaller number are composed of silicone, a material which makes them less stiff but more difficult to place. NTCVC length usually ranges from 15 to 25 cm, with a double-lumen design and conical tip (proximal lumen of the arterial branch, located 2 to 3 cm from the distal lumen of the venous branch to decrease recirculation)685 and can optimally deliver blood flows > 300 mL/min with venous pressures < 200 mmHg. It can be straight or pre-curved in shape to reduce the risk of kinking at the exit site in the skin; catheter extensions are straight or curved depending on the vein to be cannulated (curved for jugular and subclavian and straight for femoral). As an advantage, these CVC can be placed while the patient is in the hospital bed and be used immediately.
TCVC are usually made of silicone or thermoplastic polyurethane and its derivatives, such as Bio-Flex or carbothane (copolymer), which has become more widespread in use. Length depends on the vein being cannulated and catheter type. They usually have a Dacron or polyester cuff in the extravascular section, which aims to cause fibrosis to prevent the passage of infectious agents and act as anchorage in the subcutaneous tissue. These CVC are softer and more flexible and minimise damage to the intima of the veins, biocompatible, non-thrombogenic and resistant to chemical changes, which increases longevity and decreases the number of complications. The new polyurethane copolymers provide flexibility, maintaining the strength of the walls, which allows for greater internal lumen and greater flow than silicone CVC without needing to increase their calibre.686
It is important to understand what material is used to manufacture the CVC, as certain antibiotics or antiseptic solutions currently in use are incompatible with this material. Alcohol, polyethylene glycol, which mupirocin cream contains, and povidone iodine interfere with polyurethane and can rupture the CVC, while copolymers like carbothane are resistant to alcohol and iodine. Povidone iodine also interferes with silicone, causing degradation and rupture.682,687
Some CVC are coated with anticoagulant, antiseptic or antibiotic products, which aims to minimise risks of thrombosis and infection. Experiences have been reported which show the effectiveness of this strategy, but only in NTCVC used in critical patients and for limited periods of time. There is no evidence to support its routine use in populations of HD with long-term TCVC.688
CVC length for HD depends on the vein to be cannulated and the patient’s clinical condition. NTCVC to be placed in the right internal jugular or subclavian vein usually measure between 15 and 20 cm, and 20 to 24 cm in left internal jugular or subclavian vein. Shorter lengths could cause risk of injury when the CVC lies against the superior vena cava walls. If used in the femoral vein, the CVC should be long enough, between 20 and 24 cm, to avoid significant recirculation and place the tip in the inferior vena cava.687
TCVC are usually longer in length, ranging from 20-50 cm depending on the vein to be cannulated, due to the subcutaneous portion. Length must be appropriate to place the tip in the right atrium (RA), in TCVC inserted through the superior central veins (it has also been suggested that the tip be placed in this location in silicone NTCVC)689 and in those inserted through the femoral vein, at least inside the inferior vena cava.685
CVC can be designed with double lumen (with both lumens symmetrical in a double D or double O or with circular arterial lumen and the venous lumen in a crescent shape); double-lumen but divided distally; two separate single catheters; double with a common anchor; and implantable devices with subcutaneous reservoirs.690 The advantage of circular section lumens is that they do not collapse on kinking or when pressures are highly negative. As a disadvantage, the internal calibre is usually lower for the same external calibre. A double D lumen configuration would allow the best flows with less resistance by contact surface area.682
Currently, different pre-curved CVC with different lengths are available on the market, allowing adaptation to the patient size. Improvement in and ease of placement, with a reduction in intervention time, are advantages to take into consideration, as is the decrease in the amount of kinking and improvement in function.
Other design characteristics are related to CVC tip. They may have a double O (shotgun tip), coaxial, separate tips (split tip), step tip or spiral Z-tip, all with and without side holes.686,690-692
Extensions and hubs should be designed and made of highly resistant material to prevent erosion or rupture caused by the clamps. Depending on CVC type, there are connection accessories, both for insertion and replacement in case of damage.
Although there are multiple comparative studies between different types of catheters that assess the material and design of the tip, they have not succeeded in proving significant differences among them to suggest the use of one model over another.685,691,693-696
Patients with a central venous obstruction that prevents the creation of a VA in the upper limb may benefit from a hybrid prosthesis-tunnelled catheter device (haemodialysis reliable outflow -HeRO- device). This is a VA created in a mixed way. On the one hand, it is a permanent catheter that is implanted through a central venous obstruction or stenosis, connected to a polytetrafluoroethylene (PTFE) prosthesis anastomosed at the level of the brachial artery. The cannulation area is the prosthesis which is tunnelled subcutaneously and drains distally, directly into the atrium (see section 2.4.2).
Observational studies have shown lower rates of infection than TCVC, with primary and secondary patencies similar to those observed in pAVF, but as it is a relatively recent technique there are no randomised clinical trials (RCTs) to endorse its usefulness and safety. From this, it can be deduced that its use is limited to a very small number of patients who are unable to undergo nAVF or pAVF creation in upper limbs and with recoverable central stenosis and/or occlusions, and preserves the venous vascular bed of the lower limbs.143,177,697-700
→ Clinical question XXV Are there differences in the indication to use non-tunnelled catheters versus tunnelled catheters?
(See fact sheet for Clinical question XXV in electronic appendices)
| Summary of evidence | |
| TCVC and NTCVC use varies substantially, depending on the clinical condition of patients. This makes it difficult to correctly design studies comparing the outcome of both types of CVC. Observational analyses show higher rates of complications with the use of NTCVC versus TCVC, primarily from infections and early withdrawals. In one of the broader studies, Weijmer compared 37 TCVC with 235 NTCVC; CVC withdrawal for any complication and infection rate was higher in NTCVC, with CVC patency rate and infection-free patency being better in TCVC from the first 2 weeks of placement | Low quality |
Evidence synthesis development
The observational study of Weijmer et al.673 analysed the results of 272 CVC (37 TCVC and 235 NTCVC) in 149 patients, and with a duration of use of 11,612 catheter days.
Patients with NTCVC presented acute renal failure (40% versus 8% of TCVC, p < 0.001) and hospital admission rates were higher (54% versus 14%, p < 0.001) as a differential characteristic. Results of the comparison were:
- •
CVC withdrawal for any complication: 45.5% (107/235) of NTCVC were withdrawn versus 28.7% (11/37) TCVC (p < 0.001, log-rank analysis). The rates were 1.80 per 1000 catheter days for TCVC and 19.48 for NTCVC (RR: 10.83; 95% CI, 5.82-20.15; p < 0.0000001).
- •
TCVC survival: Actuarial survival analysis was better for both TCVC (95% at 14 days, 95% at 21 days and 95% at 28 days) than for femoral NTCVC (42% at 14 days, 37% at 21 days and 32% at 28 days, p < 0.001 for all periods) and for jugular NTCVC (75% at 14 days, 69% at 21 days and 58% at 28 days; p < 0.05 for all periods).
- •
Infection:
- –
Bacteraemia. Rates per 1000 catheter days: 1.6 for TCVC and 4.6 for NTCVC (RR: 2.67; 95% CI, 1.28-5.59; p = 0.006).
- –
Infections in catheter exit site. Rates per 1000 catheter days: 1.3 for TCVC and 8.2 for NTCVC (RR: 6.26; 95% CI, 3.04-14.22; p < 0.000001).
- –
Survival of infection-free catheter. It was better for TCVC (91% at 14 days, 89% at 21 days and 89% at 28 days; p < 0.05 for all periods) than for jugular NTCVC.
- –
After adjusting for different patient clinical characteristics, the most important risk factor for catheter withdrawal (RR: 9.69; p < 0.001) and for infection (RR: 3.76; p < 0.001) was indwelling NTCVC.
They conclude that, in accordance with these results, TCVC should be used whenever the need for CVC for HD is foreseen for more than 14 days.
The review of Frankel679 on NTCVC has an impact on the indications in which immediate access to the bloodstream is required:
- •
Patients with reversible deterioration of renal function requiring temporary HD.
- •
Patients whose end-stage renal failure has not been previously diagnosed and require urgent HD or are waiting for creation or maturation of a permanent vascular access.
- •
Patients in transition when access has failed, whether AVF or peritoneal dialysis.
Frankel points out that the use of TCVC has a significantly lower rate of infection than NTCVC (8.42 cases versus 11.98 cases per 100 catheter months, respectively) and should be the preferred means of providing temporary VA for periods of more than 2 weeks.
The study of Kukavica et al.680 compared 16 patients treated with a TCVC with 15 patients treated with NTCVC (36-month follow-up) and found the need to replace 24 NTCVC due to thrombosis versus only 2 in patients with TCVC. He also observed that the mean flow rate in patients with TCVC was significantly higher (296 mL/min) compared to NTCVC (226 mL/min) (p < 0.001).
From evidence to recommendation
Although there are no randomised studies directly comparing the results of both types of catheters, observational analyses show higher complication rates with NTCVC versus TCVC. Despite weak evidence, GEMAV has decided to recommend the use of NTCVC for periods of time no longer than 2 weeks and TCVC for longer periods.
→ Clinical question XXVI What is the best material and design for a tunnelled central venous catheter?
(See fact sheet for Clinical question XXVI in electronic appendices)
| Summary of evidence | |
| The main biomaterials used to build TCVC are currently silicone, polyurethane and their copolymers, such as carbothane. They usually have a Dacron cuff for subcutaneous anchoring and have different designs of lumens and tips, with and without side holes. The general reviews of Tal and Ni686 and Ash691 describe the different materials and designs and possible combinations of lumens and tips. Some RCTs have been identified comparing different types of CVC with each other, as well as any kind of CVC versus a special access system (LifeSite). However, as the evidence available is from few RCTs and a small number of patients, no CVC can be recommended in preference to another, nor can it be concluded that a material, specific CVC or brand is superior to others | Low quality |
Evidence synthesis development
The RCT of Hwang et al.701 compared CVC with a “Z” spiral tip design (Palindrome = 47) versus a CVC with step design (step-tip = 50) in 97 patients with 2-month follow-up. Results showed:
- •
CVC dysfunction-free survival rate was significantly higher for Palindrome CVC than for step-tip CVC (78.9% versus 54.4% at 2 months; p = 0.008).
- •
Overall CVC survival rate was also higher for Palindrome CVC than for step-tip CVC (90.6% versus 68.8% to 2 months; p = 0.015).
There were no cases of bacteraemia during the study.
The authors conclude both CVC are equally effective at making HD flow adequate and have low recirculation.
The RCT of Tretorola et al.693 compared two polyurethane CVC, one with divided tip design or split-tip (AshSplit) and the other with step-tip design (OptiFlow), on 132 patients with 6-month follow-up. Results showed:
- •
CVC survival at 6 months was higher for AshSplit (22 of 64 = 34.4%) than for Optiflow (16/68 = 23.5%), which is a significant difference (log-rank test p = 0.02).
- •
CVC-related infections were lower for AshSplit (9 of 64 = 14.1%) than for Optiflow (15 of 68 = 22.1%), but the difference was not significant (RR: 0.64; 95% CI, 0.30-1.36; p = 0.24). Infection rates per 100 catheter days were 0.12 and 0.22, respectively.
- •
Infections that caused CVC withdrawal were lower for AshSplit (6 of 64 = 9.4%) than for Optiflow (11 of 68 = 16.2%), but the difference was not significant (RR: 0.58; 95% CI, 0.23-1.47; p = 0.26).
Both CVC had flows within the acceptable range indicated by the Dialysis Outcomes Quality Initiative—DOQI— (300 mL/min).
The RCT of Tretorola et al.695 compared a silicone step-tip CVC (Hickman 13.5 F) with a polyurethane split-tip CVC (AshSplit 14.5 F) in 24 patients with a 6-week follow-up, of which only 19 completed the study. Results showed that the split-tip catheter obtained higher blood flows and lower recirculation, though both CVC attained flows within the acceptable range indicated by DOQI (300 mL/min) and mean recirculation < 6%. Complications from insertion were limited to the AshSplit CVC group.
The RCT of Atherikul et al.702 compared three CVC with different lumen design: dual-lumen with double-barrel shotgun configuration (PermCath = 22), two separate individual lumens (Tesio = 24) and individual circular lumen separated by a central septum in a double D (VasCath Soft Cell = 18) in 64 patients. Mean blood flow, percentage of HD sessions with blood flow ≥ 350 mL/min in 30 consecutive treatments, adequacy of sessions and recirculation were studied. Results showed:
- •
Mean blood flows: (PermCath, 383.6 mL/min; Tesio, 396.3 mL/min; VasCath, 320.4 mL/min). PermCath and Tesio had similar flows that were significantly greater than VasCath (p < 0.005).
- •
Percentage of sessions with flow ≥ 350 mL/min: Perm-Cath, 86.9%; Tesio, 81.6%; VasCath, 42.3%. PermCath and Tesio had similar results that were significantly greater than VasCath (p < 0.005).
- •
Recirculation and adequacy (Kt/V: urea plasma clearance [K] during HD session time [t] divided by urea distribution volume [V]) with optimised adequacy for the 3: PermCath, 3.7% and 1.42; Tesio, 3.9% and 1.39; VasCath, 4% and 1.32.
No data are provided on infection or CVC dysfunction.
The authors conclude that all 3 CVC types provide appropriate haemodialysis, obtain the same percentage of sessions with 300 mL/min blood flow and acceptable adequacy and recirculation.
LifeSite implantable haemodialysis subcutaneous access system versus Tesio-Cath catheter
The RCT of Rosenblatt et al.694 compared the implantable HD subcutaneous access system (LifeSite) versus CVC with two separate individual lumens (Tesio), in 68 patients followed up for 1 year. Results showed:
- •
CVC survival at 1 year: 74% for LifeSite system versus 48% for Tesio CVC, no significant difference (Log-rank test p = 0.062). After adjusting for different covariates, the difference became significant (p = 0.039).
- •
Infection rates per 1000 catheter days: 3.1 for LifeSite system versus 6.6 for Tesio catheter (p = 0.008).
- •
Device-associated bacteraemia rate per 1000 catheter days: 1.9 for LifeSite system versus 3.4 for Tesio CVC (p = 0.013).
The RCT of Schwab et al.696 compared the implantable HD subcutaneous access system (LifeSite) versus Tesio catheter in 70 patients followed up for 6 months. Results showed:
- •
CVC survival at 6 months: Lower with LifeSite (64.8%) than Tesio (69.1%), after stratifying by diabetes and adjusting for age.
- •
Device-associated bacteraemia rate per 1000 catheter days: 3.4 for LifeSite system versus 3.3 for Tesio CVC.
Blood flow was slightly higher with LifeSite than with Tesio CVC (358.7 versus 331.8 mL/min).
From evidence to recommendation
The available evidence, from comparisons between CVC models, with few RCTs and few patients, is not sufficient to recommend which model or specific type of TCVC should preferably be used for HD.
6.3. Catheter insertionThe insertion of a CVC for HD is a technique that can involve risks. The frequency with which complications appear is highly variable between different units and depends mainly on the experience and, to a lesser extent, the environmental conditions in which the CVC is implanted. Ultrasound use for central venous cannulation has resulted in a decrease in complications.
Recommendations
R 6.3.1) We recommend the central venous catheter placement procedure be performed by qualified and experienced medical staff who are familiar with the technique
R 6.3.2) We recommend tunnelled central venous catheters be placed under strict aseptic conditions and in a room with imaging control (real-time fluoroscopy and ultrasound) and radiological image be used to confirm final position
R 6.3.3) We recommend the right internal jugular vein be used as the site of first choice to place a tunnelled central venous catheter
R 6.3.4) We suggest the common femoral vein be used as the site of first choice to place a non-tunnelled central venous catheter in an emergency
R 6.3.5) We recommend subclavian veins not be cannulated in patients who may need an arteriovenous fistula
R 6.3.6) We suggest central venous catheters not be placed in the jugular or subclavian veins ipsilateral to the member where there is a maturing arteriovenous fistula
- (•)
NEW R 6.3.7) We recommend central venous catheters for haemodialysis be placed using ultrasound guidance
Rationale
Staff
CVC should be implanted by qualified medical staff who have demonstrated a sufficient level of experience. Some authors estimate this experience as at least 50 catheterisations during training and 30 procedures per year to maintain competence.703-705 As radiologists have become progressively involved in this field and the use of imaging techniques has increased,706 there have been good results, albeit limited to countries where CVC had previously been placed by surgeons.707-712 The best results obtained in these studies are attributed to the different implementation conditions and technical means. Placement by cannulation and not by dissection, ultrasound use for venous cannulation and the use of real-time imaging to control CVC tip positioning and the absence of kinking are the aspects that can make that difference.713,714
Site of the procedure
The correct positioning of TCVC has improved with the use of image-guided placement. Cannulation of the chosen vein, as well as the precise positioning of the CVC in its intravascular segment, can be confirmed. Therefore, it is essential to have highly qualified fluoroscopic imaging equipment not only to establish the exact location using the image, but also to emit as little radiation as possible for both the patient and the professionals involved in the process. The room where the digital fluoroscopy device is located must have a technologically adequate ultrasound Doppler device to safely and accurately reach the chosen vein to be cannulated.713,714
TCVC should be inserted in a surgical environment (operating room or room with similar aseptic conditions) to minimise the risk of infection.
In addition to the aforementioned requirements, since this type of procedure also requires prior preparation of the patient, the following must also be on hand:
- •
An appropriate area adjacent to the room/operating room with digital scopia where the patient is prepared before and monitored after the procedure. This area must have appropriate staff and equipment to resolve any of the possible acute complications that occur after the procedure.
- •
Immediate access to emergency resuscitation equipment, including drugs. This equipment must be checked periodically to make sure that it is complete and updated.
- •
Appropriate medication for the treatment of possible acute complications.
- •
Equipment for the treatment of pneumothorax.
- •
Support from a surgical team in case of severe acute complications in a reasonable amount of time.
In those procedures where drugs are administered or sedation is included, equipment to monitor heart rate, oxygen saturation and blood pressure must be available. Medical gases, intubation and ventilation equipment, defibrillators, and emergency resuscitation equipment and drugs must also be provided.
Femoral NTCVC placement should be avoided whenever possible, using the bed where the patient is lying, both because of asepsis and the technical difficulties arising from poor stiffness of the mattress and poor posture of the doctor who performs the insertion. It should be reserved for emergency situations in which no adequately equipped room is available.
Location
Veins are generally cannulated in TCVC in this order: right and left internal jugular veins, right and left femoral veins and right and left subclavian veins. In exceptional cases, the following have also been used: inferior vena cava,715 collateral tirocervicales,716 external jugular vein,717,718 saphenous vein and the artery aorta by translumbar puncture.719 Other veins that can be used are the suprahepatic and gonadal veins.
In CKD, the subclavian vein should only be cannulated when the remaining vessels have been exhausted, as it is associated with increased incidence of stenosis.685,717,720 The incidence of stenosis in the subclavian vein secondary to catheter placement has been mentioned by several authors as between 42% and 50%. In contrast, the percentage of stenosis in the innominate trunk following the use of the internal jugular vein is reported to be 0-10%.717,721 In cases where AVF is to be created in a particular arm, the jugular vein (and even more so, the subclavian) of that side should be avoided as much as possible.
NTCVC insertion may also be inserted in the internal jugular vein or in the common femoral vein. Both sites have potential benefits and risks, including lower risk of infection in the jugular area or lower risk of complications during placement in the femoral territory. It has been suggested that the common femoral vein be used first, mainly due to the minor complications it presents when implanted versus the internal jugular vein. In a controlled clinical trial in 750 patients with severe disease, who were bed-bound and required placement of a first NTCVC, it was observed that the catheterisation of the internal jugular vein does not appear to reduce the risk of infection versus the femoral access, except in adults with a high body mass index (BMI). It also presents a similar risk of catheter-related complications (thrombosis, fibrin sheaths) and a higher proportion of complications during cannulation, such as hematoma formation.722
Moreover, with regard to these recommendations, there are other aspects that should be taken into account prior to the choice of access. The review conducted by Clark and Barsuk,683 in a case series of patients from different sources including kidney patients, establishes the factors favouring the different insertion sites:
Right internal jugular vein:
- •
Critical and bed-bound patient with BMI > 28.
- •
Post-operative recovery of aortic aneurysm repair.
- •
Outpatient/necessity of movement for rehabilitation.
Femoral veins:
- •
Critical and bed-bound patient with BMI < 24.
- •
Patients with tracheostomy or the need to perform one.
- •
Urgent need for dialysis together with operator inexperience or lack of control by ultrasound.
Left internal jugular vein:
- •
Contraindications for access in right internal jugular and femoral veins.
Subclavian veins:
- •
Contraindication for jugular and femoral access.
- •
Preferably use the right side.
Therefore, in the patient suffering severe conditions or in an emergency situation, the option of the femoral approach for NTCVC placement could be considered.
The internal jugular vein is the vein chosen most frequently for TCVC insertion due to ease of access and lower number of complications.703,723,724 The second choice is subject to controversy and is related to the patient’s anatomy and functional characteristics, although many authors consider the common femoral vein as an alternative when it cannot be placed in jugular veins, even though its primary patency is lower (44% per month) and it has greater infection rates (6.3/1000 catheter days).723,725 Prior phlebography and/or ultrasound examination are highly advisable in cases in which catheters have been previously placed or AVF have been created.695,703,704,726
To prevent kinking in TCVC and discomfort when moving the neck in NTCVC, it is advisable to approach the lowest part of the jugular, posterior to the sternocleidomastoid or through the gap between sternal and clavicular insertions of this muscle.
When should the central venous catheter be placed?
NTCVC must be placed on the same day they will be used for HD. TCVC may be placed immediately before use, but it appears to be prudent to do so 24-48 h before.685,687,690,712,727 Polyurethane CVC used immediately after placement often have difficulty in attaining adequate flow, but this disappears spontaneously 24 h later.
Tunnelling technique
In TCVC, the technique employed is usually similar depending on the vein to be cannulated although it differs according to the CVC to be used. Once the vein has been located and identified, and after applying antiseptics for at least 3 min on the surgical area (chlorhexidine 0.5% as skin preparation in clean surgery is associated with a lower rate of infections compared to povidone iodine; RR: 0.47; 95% CI, 0.27-0.82),728 the skin and the surrounding tissue is anaesthetised. Shaving the skin is not recommended because of the trauma caused, and only skin tissue in areas of jugular cannulation and in the incision prior to tunnel creation should be anaesthetised. Anaesthesia is not usually required on subcutaneous cellular tissue as there are no nerve endings.
The ultrasound probe, introduced in a sterile bag, locates the vein to be cannulated. Ultrasound is highly recommended due to the lower rate of complications during cannulation, the higher success rate when assessing vein situation and size, when assessing patency, and when confirming the position of the needle tip at all times.729-731
The vein will be cannulated preferably with a micro-puncture set and, if the internal jugular vein is chosen, in close proximity to the clavicle. Cannulation above this favours CVC kinking, and is very uncomfortable for the patient.
Once the position of the needle has been verified via aspiration and the microguide (0.021”) is advanced, it is replaced by the introducer system. If the distance between the cannulation and RA needs to be measured before choosing the CVC, the microguide may be used, once the tip has been properly positioned in the chosen location. It must be taken into consideration that the position must be at least 2 cm below the ideal site as the CVC rises when the patient stands or sits.714
Once the tunnel entry site has been chosen on the chest wall (the Dacron cuff should be located at least 3 cm from the incision; the entry curve into the vascular system must be gentle to prevent kinking), and a small entry incision for the tunnel has been made, the catheter is then tunnelled, having previously connected the CVC to the end of the tunneller, and pushed in gently until the CVC is properly positioned.
A 0.035” guidewire is inserted into the introducer, the latter is removed and a peel-away introducer of the appropriate size for the chosen CVC is placed after dilating the inlet path. Many peel-away introducers incorporate a valve at the insertion site that prevents air from entering, and therefore gas embolism, so its use is recommended.
After the guidewire is withdrawn, the CVC tip is inserted into the introducer advancing it until it is placed in RA, and the peel-away introducer is then removed. Tip position and the absence of kinking should be confirmed by fluoroscopy and correct operation, by the suction of both lumens. After washing, both lumens must be purged and filled with the chosen lock solution (heparin, citrate, etc.). The procedure finishes after the catheter is fixed to the skin with non-reabsorbable suture and a sterile dressing is placed.
→ Clinical question XXVII Should ultrasound be used as a reference standard for the placement of central venous catheters?
(See fact sheet for Clinical question XXVII in electronic appendices)
Rationale
CVC can be inserted using anatomical landmarks or with the help of ultrasound. It has been suggested that ultrasound-guided placement reduces immediate complications, especially those related to arterial puncture or pneumothorax.
Some authors have shown 27% of anatomical variations of the internal jugular vein versus the carotid artery732,733 and others have reflected absence of or total thrombosis of the internal jugular vein in 18% of patients on dialysis when they have been examined with ultrasound.685 Therefore, image-guided placement of central accesses (ultrasound, fluoroscopy, etc.) is highly recommended.
| Summary of evidence | |
| A meta-analysis of seven RCTs shows that ultrasound-guided CVC placement has better results than insertion based solely on anatomical landmarks, regarding the number of successfully inserted CVC on the first attempt, reduced risk of arterial needling and haematoma and less time needed for successful vein cannulation | High quality |
Evidence synthesis development
The Cochrane review with meta-analysis of Rabindranath et al.730 identified 7 RCTs that included 767 patients with 830 CVC insertions, 5 of them performed in jugular territory, one in femoral and one in both. 3 of the 7 studies described the method used to generate the random sequence, none described allocation concealment and blinding of participants and staff was not possible.
The main results are shown below, and demonstrate that ultrasound-guided placement is technically and clinically better than insertion based only on anatomical landmarks for all the variables analysed; the differences are statistically significant in all cases except for the risk of pneumothorax or haemothorax.
- •
Overall failure risk of CVC placement: 7 studies, 830 catheters (RR: 0.11; 95% CI, 0.03 to 0.35).
- •
Failure risk of CVC placement on first attempt: 5 studies, 705 catheters (RR: 0.40; 95% CI, 0.03 to 0.52).
- •
Risk of arterial perforation: 6 studies, 785 CVC (RR: 0.22; 95% CI, 0.06 to 0.81).
- •
Risk of haematoma: 4 studies, 323 CVC (RR: 0.27: 95% CI, 0.08 to 0.88).
- •
Risk of pneumothorax or haemothorax: 5 studies, 675 CVC (RR: 0.23; 95% CI, 0.04 to 1.38).
- •
Time needed for successful cannulation: 1 study, 73 CVC (mean difference –1.40 min; 95% CI, –2.17 to –0.63).
- •
Insertion attempts/CVC: 1 study, 110 CVC (mean difference –0.35; 95% CI, –0.54 to –0.16).
From evidence to recommendation
Available evidence from systematic reviews with meta-analyses has shown greater effectiveness and safety in ultrasound-guided CVC placement compared to the use of anatomical landmarks. The RCTs analysed include placements in both the jugular and femoral territories, so the recommendation of ultrasound use would be applicable to both territories.
Position of tunnelled catheter tip
Rationale
The length should be adequate to position the CVC tip in RA (in TCVC). The intravascular sections should be 20-24 cm in the right jugular vein and 24-28 cm in the left jugular vein. Placement in RA reduces the risk of trauma-associated venous stenosis on the vein caused by continued CVC tapping during HD and minimises the formation/progression of fibrin sheath.682,789 For femoral CVC, the specified length would be longer, up to 40-50 cm. Although there are currently no articles in the literature that justify this location or endorse the advantages of positioning the CVC tip in the atrium versus the inferior vena cava, the justifications would be similar to those mentioned in the insertion via jugular vein. The availability of devices with larger-sized lumens allows high flows despite the increase in their length.
6.4. Catheterisation controlRecommendations
NEW R 6.4.1) We suggest a post-insertion chest X-ray not be systematically performed to confirm correct placement and positioning of the catheter, unless incorrect placement or suspected complications occur during the procedure
R 6.4.2) We recommend the positioning of the catheter tip be checked by fluoroscopy or radiography in cases where there is dysfunction during use
Rationale
Ultrasound-guided CVC placement, as discussed above, has better results than insertion based solely on anatomical landmarks. The number of CVC successfully inserted on the first attempt is greater, and the number of complications is lower, reducing the risk of arterial puncture and haematoma. The use of ultrasound decreases insertion time by reducing the time for successful vein cannulation.722,729
This means that in acute cases, where urgent placement of a CVC is required because the patient’s life is in danger and there are no imaging techniques available, a temporary catheter is preferably placed in a common femoral vein. Placement in the jugular vein, of second choice, should be performed at least with ultrasound confirmation of patency and, if possible, with knowledge of the size and location of the vein to detect anatomical variables.
If TCVC is going to be placed, fluoroscopy should be used to verify CVC tip location and absence of kinking. It is necessary to check correct location in forced inspiration and to place it 2 cm below the ideal site, as a catheter moves upwards when the patient stands or sits,714 varying the position and causing catheter dysfunction. When there are two catheters (Tesio, Twin), the venous catheter tip should be located in RA and the arterial catheter tip must never be located in the cava, but at most in the junction of the superior vena cava with RA and enough distance must be left between the ends of the catheters to avoid recirculation.685,727,734,735 Some authors recommend that both catheter tips be properly inserted into RA in obese patients or patients with large breasts.735 Arterial catheter placement in the inferior vena cava next to the exit of the suprahepatic vein is an interesting option in obese or patients with chronic obstructive pulmonary disease in order to ensure better flow. When NTCVC are used, it is recommended that the catheter tip be placed in such a way as to reach at most the junction between the superior vena cava and RA because, as the composition of the material makes them extremely rigid, some CVC may perforate the atrium.685
Performing a post-insertion chest X-ray to confirm correct CVC placement and positioning and to evaluate the possibility of complications is normally a routine procedure, especially in the case of NTCVC. However, its need has been questioned over the last few years.
In the field of interventional radiology, a study of 489 patients with placement using fluoroscopy, with no complications in the procedure, the subsequent X-ray showed only 1% with poor tip placement.736 From a nephrological point of view, in a retrospective series of 460 NTCVC with and without Doppler ultrasound (DU) guided placement, of 370 cases in which no clinical complication was suspected, poor placement was only found in 1% of placement procedures using confirmatory X-ray. In studies where DU was used, no complications were observed in cannulation itself, and in those that presented haematoma, the X-ray was normal.737 In a case series published in the field of anaesthesia, in which 173 CVC were placed without ultrasound guidance, only 2 complications were recorded that were cases of either suspected or it was a high-risk case, predicting a correct placement in 97%.738
This results in the consideration that if would be sufficient to take into account the need to perform radiological control after CVC placement if poor placement is suspected or complications present during procedure.
6.5. Catheter handlingRecommendations
R 6.5.1) We recommend that central venous catheters for haemodialysis only be used for haemodialysis sessions
R 6.5.2) We recommend that central venous catheter connections and disconnections be performed only by the specialised nursing staff from the dialysis units, and that two people are required, one of them a nurse
R 6.5.3) We recommend that any central venous catheter handling be performed under strict aseptic conditions
NEW R 6.5.4) We recommend the exit site of the central venous catheter and the skin of the peri-catheter area be covered to preserve integrity and keep it dry
NEW R 6.5.5) We recommend central venous catheter exit site dressing be assessed at each haemodialysis session and changed whenever wet, stained, detached or presents any signs of infection. If any of these conditions are not present, we recommend it be changed once a week
NEW R 6.5.6) We recommend that all haemodialysis units have a follow-up record of infection episodes for the central venous catheter
NEW R 6.5.7) We recommend that all haemodialysis units have specific protocols for handling central venous catheters for haemodialysis and for management of infectious episodes
Rationale
Regardless of the cause, location or type of CVC, care is essential for maintenance, to minimise risk factors and to prevent potential complications; therefore, they must be handled by specialised personnel. Their use should be restricted to the treatment of HD and strict aseptic measures should always be maintained when handling.739,740
Care should be directed to the CVC insertion point or exit site and peri-catheter skin area. Care should also focus on catheter handling and will include patient education in self-care. The aim of all this is to avoid catheter-related infections, either local (exit site, subcutaneous tunnel) or bacteraemias.
During handling, care must be taken to avoid drafts and polluting activities in the environment of the room.
The connection, disconnection and management procedure due to dysfunction, preferably performed by two people,741 must be protocolised in each unit742,743 and include the following steps:
- •
Checking CVC patency and flow.
- •
Monitoring CVC status (extensions and visible part).
- •
Safety measures to prevent endoluminal contamination (avoid leaving CVC lumens in the open air).
- •
Troubleshooting (washes in case of dysfunction).
- •
CVC lock.
- •
Intradialysis and interdialysis CVC protection measures.
The CVC must be handled with strict aseptic measures.744-746 These measures involve the professional managing the CVC, the people around during management and the patient himself. Barrier measures should be maximised in management caused by dysfunction. This consists of both patient and staff using a mask,744 hygienic hand washing and use of gloves and sterile field. The ends of the HD lines to be connected to the CVC should be handled with the utmost precaution to avoid contamination.739,741,745
Weekly cleansing of the exit site is recommended to minimise skin irritation and entry of external agents,746-748 unless the patient arrives at the HD session with an incomplete or stained dressing. According to the results of the systematic review that McCann and Moore749 conducted for Cochrane, there was no significant reduction in exit-site infection or CVC-associated bacteraemia when using transparent polyurethane dressing versus dry gauze, so there is insufficient evidence to recommend one type of dressing (transparent polyurethane or dry gauze). Non-breathable dressings should be avoided. Several authors and guidelines suggest the use of dressings impregnated with chlorhexidine, preferably transparent,746,748,750-753 but there is still not enough evidence to make any recommendations in this regard.750-753
It is necessary to assess whether to avoid the use of alcohol-based antiseptics or other types that may damage the material used to manufacture the CVC, so it is advisable to consult the technical data sheet. The antiseptic recommended is chlorhexidine, and its concentration can be between 0.5 and 2%.728 It is best to use a double dressing for the exit site and for the catheter extensions, clamps and caps. In the first days after placement, lifting the dressing, as well as making abrupt manoeuvres, should be avoided to help fix the Dacron cuff.
At present, there are specific HD barrier connections (bioconnectors) that are used to prevent endoluminal contamination in the connection and disconnection process, although there is insufficient evidence to make any recommendation about their use.754-757 During handling, care must be taken to avoid drafts and polluting activities in the environment of the room.
With respect to self-care education, the patient must be given instructions on:
- •
The need for good hygiene and how to wash daily.
- •
The use of appropriate clothing, avoiding elements that can cause kinking in the CVC or rub the subcutaneous tunnel (braces, chains, etc.).
- •
How to cleanse the exit site, if it is necessary to do so.
- •
Refrain from doing risky activities such as immersion baths, traction on the CVC or the use of sharp objects near the catheter.
- •
They will be given information on possible complications, their causes and the actions that must be performed to try to avoid them.
- •
They should know that whenever they are to be dialysed, they must inform the nursing staff of any incident, and contact the centre if there is any serious event (bleeding, accidental withdrawal, fever, etc.).
Different kinds of recording can be made,758 but at least the following must be included:
- •
Name of professional handling the CVC.
- •
Date of placement and location.
- •
Type of CVC, length and lock volume.
- •
Its functioning (checking the patency of the lumens, need for fibrinolysis, flow and pressures).
- •
Complications (dysfunction, signs of infection).
- •
Number of CVC and causes of replacement.
Recommendations
R 6.6.1) We recommend clinical and functional follow-up of the catheter be performed in each dialysis session and of its evolution over time
R 6.6.2) We recommend the flow provided by the central venous catheter and its recirculation be taken into account in the event of insufficient dialytic efficacy
R 6.6.3) We recommend routine cultures not be made unless there are signs of infection
Rationale
CVC for HD are intended as an access to the bloodstream to allow for effective dialysis with the lowest number of complications. Follow-up is aimed at detecting possible complications as soon as possible and, in this sense, clinical and functional follow-up should be emphasised.
Clinical follow-up, which warns of complications in the patient, should be performed in each HD session and must be recorded in the nursing records. It must be based on the search for symptoms or physical signs that cause suspicion:
- •
Infection: onset of fever, inflammatory signs at the exit site or in the tunnel. The integrity of the dressing, which should be clean, dry and without secretion, must be examined in each HD session.759
- •
Oedema in upper limbs or face that would make suspect central vein thrombosis.708,709
- •
Pain in the shoulder or neck that may indicate CVC rupture or abrupt changes in the patient’s clinical condition that would suggest a serious complication.726
- •
Alterations in skin integrity: dermatitis caused by allergies to the material used, excessive cleansing or dressings that induce maceration of the skin and decubitus ulcers produced by the Dacron cuff or by the CVC itself at the exit site.
- •
Periodic control of the length of the outer part of the CVC. This will allow the determination of possible movements of the CVC and, therefore, tip location.760
The purpose of functional follow-up is to detect alterations that prevent effective HD, in other words, to obtain adequate Kt/V. This follow-up is carried out in each session and evolution assessed over time, since each patient is their own control. These include the following determinations:
- •
Blood flow. Currently, monitors provide a reading of real flow. Flow depends on CVC structure (calibre, materials, etc.) and tip situation (atrium or superior/inferior vena cava). The recommended flow is > 300 mL/min.
- •
Pressures from the circuit. Only dynamic pressures are determined in CVC.
- •
Determination of mean clearance. Determination of mean clearance measured by ionic dialysance (K) helps to monitor HD adequacy by using Kt. Currently, most HD monitors provide this information, which is useful for understanding CVC function and performance. Any changes in Kt should be taken into account, as it may indicate a functional impairment of the CVC.761,762
- •
Recirculation. It is practically minimal in catheters placed in jugular and subclavian veins (there is no cardiopulmonary recirculation unlike AVF), so any recirculation > 5-10% is suggestive of alterations in the catheter: change in tip position, clot in the lumen, fibrin sheath or peri-catheter thrombosis.763 Recirculation is determined by the structure and location of the tip and, in some cases, by inversion of the lines.693,701,764
Recommendations
Rationale
Complications following CVC placement for HD can be classified as acute or early and late.
Acute
These occur within 30 days of the procedure. They are uncommon703,726,765 and are related to venous cannulation or insertion. They can be subdivided into those that are intimately related to the procedure or immediate, which are defined as those that occur within 24 h following the intervention and those that occur after this period of time. Procedural complications usually consist of damage to underlying vital structures and poor catheter positioning. The most commonly associated complications are listed in Table 28.
Immediate complications after central venous catheter placement
| Common |
|
| Exceptional |
|
These complications vary depending on the vein to be cannulated, the physician’s experience, the use (or otherwise) of ultrasound for venous cannulation729-731 and imaging,714 as well as the patient’s clinical condition (immunosuppression, coagulation disorder, obesity, etc.).
It is advisable to strictly monitor during the first hours post-insertion to try to identify complications and proceed with the corresponding treatment immediately, as they can be potentially fatal.
Among the most common, albeit less serious, complications is bleeding through the cutaneous entry site. In most cases, bleeding comes from the venous cannulation site due to high jugular venous pressure. A common mistake is to compress the exit site when there is bleeding; the point that should be compressed is the venous cannulation zone, although the most effective way is to avoid decubitus by placing the patient in the sitting position in order to reduce pressure in the jugular vein.
As the introducer is very thick, gas embolism is not a very uncommon complication. The risk can be decreased by placing the patient’s head in the lowest possible position to increase venous pressure, carefully replacing the introducer dilator by the CVC, and, more recently, using haemostatic valved peel-away introducers.
Perforation of a central vessel is a potentially life-threatening complication caused by traversing the vessel wall with the dilators during the insertion procedure.726 Careful insertion of the dilator through the guidewire, without forcing its advance to prevent kinking, and the use of real-time fluoroscopic imaging techniques makes this complication very unlikely.
Another serious complication, although exceptional, is carotid-jugular fistula. It has been reported in the literature associated with cerebral infarctions.766
Late
This groups the set of complications that occur 30 days after the procedure has been performed. Late complications are usually related to catheter care and function and can be deferred in time following the insertion of the catheter. They are not usually as severe as acute complications but one of their consequences is the withdrawal of the CVC and therefore, the loss of VA for HD. The complications most frequently mentioned in the literature include685,726,767:
- 1.
Infection. It is the most common complication of CVC. The germs involved are usually coagulase-negative staphylococci and Staphylococcus aureus. It may be found at three levels: exit site, tunnel or bacteraemia.
- 2.
CVC-related thrombosis. These are classified as:
- •
Extrinsic:
- –
Mural thrombosis.
- –
Central venous thrombosis.
- –
Atrial thrombosis.
- –
- •
Intrinsic:
- –
Formation of fibrin sheath.
- –
Thrombus in the catheter tip.
- –
Intraluminal.
- –
- •
- 3.
Central venous stenosis. CVC placement is associated with venous stenosis. These are more common when inserted through the subclavian (stenosis between 42% and 50%)685,717,720 than through the jugular (0% to 10%)717,721 and much greater with NTCVC than with TCVC. Although they are usually asymptomatic, they are sometimes accompanied with oedema of the ipsilateral upper limb and may compromise future AVF development in that member.
- 4.
Haemothorax, atrial perforation and cardiac tamponade. Exceptional complications associated with inadequate and prolonged use of NTCVC.685 The rigidity of the poorly positioned CVC causes erosion and subsequent vascular perforation. They are associated with poor tip position (leaning against RA) or proximal migration from the superior vena cava.
- 5.
Pinching and rupture of CVC with migration to cardiac cavities or pulmonary arteries. The passage of the CVC inserted via the subclavian vein through the costoclavicular clamp is usually responsible for this rupture. Although this complication can occur in CVC implanted for chemotherapy or antibiotherapy (mainly catheter reservoirs), it is exceptional in CVC for HD, since the subclavian vein is not used as an access vein.
- 6.
Accidental or voluntary ruptures or disconnections of the catheter or plugs usually produce gas embolism and, on rare occasions, bleeding (in catheters with intrathoracic tip). Extension clamps do not guarantee closure, so the plugs must be secure (threaded). Care should be taken that the clamps act on the same area repeatedly so as not to break the lines. Some equipment usually leave clamps open for this reason, using them only for connection manoeuvres to HD.
- 7.
Difficulty withdrawing TCVC is an uncommon complication (1%)768 due to the formation of a capsule of fibrin, collagen and endothelial cells, which in some patients causes the vein wall and CVC to merge. As a result, the CVC is strongly fixed causing a high degree of resistance to withdrawal.769,770 It has been suggested that CVC retention could be in direct relation to the length of time in place (range between 12 and 120 months) and narrow calibre of the veins used.771-773 Most TCVC can also be removed using local manoeuvres that involve dissection and release of adhesions at the cutaneous exit site or at the level of the subcutaneous cuff, later exerting moderate traction to extract the TCVC. If this is not effective, it is advisable to review the entry point in the blood vessel (base of the neck for jugular vein placements), in order to perform a more exhaustive dissection of the peri-catheter fibrous capsule and release the catheter. When the TCVC is retained, excessive traction to withdraw it may have serious consequences like vascular lesions, rupture and fragmentation of the CVC, and embolisation in RA or pulmonary artery.770,773,774 Currently we do not have evidence on retained catheter management. If it is decided not to remove it, the extravascular portion can be extracted, the proximal part fixed and the intravascular portion retained left in situ. In these cases there will be a potential risk of thrombosis, embolism or infection, but there is no consensus on whether to recommend anticoagulant or antibiotic prophylaxis.771,773 If withdrawal is decided, one option is open surgery, although recently new techniques have been described for the extraction of retained TCVC by interventional radiology. This technique consists of dilating using angioplasty balloon through the TCVC lumens, in order to detach the adhesions between the wall of the vessel and the catheter, and thus be able to release it to enable extraction or replacement.775-777
- 8.
Other complications. Ophthalmoplegia and exophthalmos, oesophageal variceal bleeding, ruptured CVC lumen, and embolisations.
However, thrombotic and infectious complications are the most frequent late complications, and are documented in the following sections referring to TCVC.
6.8. Catheter dysfunctionRecommendations
NEW R 6.8.1) We suggest central venous catheter dysfunction be suspected if it is not possible to obtain or maintain adequate extracorporeal blood flow (blood pump flow < 300 mL/min) to perform a haemodialysis session.
R 6.8.2) We suggest kinking or poor positioning of the central venous catheter tip be suspected when early dysfunction occurs, and intraluminal or peri-catheter thrombosis be suspected when late dysfunction occurs
R 6.8.3) We recommend intraluminal infusion of fibrinolytics be used to initiate treatment of thrombosed or dysfunctional tunnelled central venous catheter
- (•)
NEW R 6.8.4) We suggest that, should the tunnelled central venous catheter need to be replaced due to failure of fibrinolytic treatment, it be replaced using a guidewire, provided there is no tunnel infection or catheter-related sepsis. We suggest fibrin sheath angioplasty be associated before placing a new catheter
- (•)
NEW R. 6.8.5) We suggest fibrin sheath not be treated bystrippingwhere there is tunnelled central venous catheter dysfunction
- (•)
NEW R 6.8.6) We suggest the tunnelled central venous catheter be locked with heparin, citrate, or tissue plasminogen activator alternated with heparin during periods between haemodialysis sessions
Rationale
Together with infections, CVC dysfunction is one of the most important causes that lead to withdrawal. From the first studies published describing this problem727 to date, it is still one of the biggest problems associated with CVC survival.
CVC dysfunction is defined as the inability to obtain or maintain adequate extracorporeal blood flow during the first 60 min of an HD session, despite having made at least one attempt to improve the flow. The KDOQI guidelines established a value of not less than 300 mL/min10; however, current designs of CVC for HD, which provide higher flows (> 400 mL/min) without increasing pump pressures, make it necessary to detect the dysfunction before a drop in the flow to 300 mL/min. In addition to decreasing flow, a decrease in Kt/V, a more negative arterial pressure (Pa) than 250 mmHg and/or a venous pressure of > 250 mmHg, or a decrease in conductance (QB/Pa) > 10% in successive controls may imply an alert.10,778
The causes of dysfunction can be classified as early or late. Early dysfunction occurs the first time dialysis is performed through the CVC. It is usually intimately related to the insertion process, in particular with poor tip position or kinking.714,778
Poor CVC tip position happens when it is situated in the superior vena cava or when the arterial lumen is not placed in the right atrium, and it is more common in the obese, where the change of position from decubitus to standing makes the tip travel from the atrium to the vena cava,735,779,780 and in placements that use the left internal jugular vein as point of entry. The movement from RA to the cavoatrial junction or superior vena cava is more frequent in CVC located in left central veins. Some authors have suggested causes inherent to the mediastinal anatomy (elongation of the venous trunks)781 as reasons why it occurs. One possible solution would be to replace the CVC using fluoroscopy over a rigid guide or to replace it with a longer CVC if it is not corrected.
Kinking occurs at the time of tunnelling. If there is a lack of flow or resistance to aspiration with a syringe after insertion, it is appropriate to introduce a metal guide and replace the CVC.782 Sometimes it is not possible due to defective tunnelling in relation to venipuncture and it must be replaced through a new subcutaneous tunnel. It is recommended that the main curve of the CVC lean on the clavicle.
Late dysfunction is generally due to thrombosis. Its presence, whether intraluminal or because of fibrin sheath formation, represents a major percentage of CVC dysfunction. The fibrin sheath begins to form 24 h after placement in response to the aggression suffered by the vessel. It extends along the CVC surface after the first days of placement,778,783,784 and is the main cause of late dysfunction. Dysfunction tends to appear several weeks after placement,709 although it may even occur at 24 h.778,785 Thromboses have been classified as extrinsic and intrinsic.782 Extrinsic thromboses are secondary to the formation of a mural thrombus which may be located in the superior vena cava or RA. They are usually serious as they require systemic anticoagulation and CVC withdrawal.685,709 Intrinsic thromboses are often the cause of flow deficit through the CVC and are usually associated with fibrin sheath formation, a thrombus at the catheter tip, or an intraluminal thrombus. This more classical classification refers to both CVC dysfunction and to the complications resulting there-from. Currently, in reference to dysfunction, the classification established by Besarab and Pandey778 is more useful (Table 29).
Dysfunction types of the central venous catheter and related complications
| Type | Findings | Symptoms |
|---|---|---|
| Fibrin tail or flap | The fibrin extends from the catheter tip acting as a valve | Possibility of infusing but not of withdrawing blood |
| Fibrin sheath | The fibrin adheres to the entire length and outer surface of the catheter. This allows the presence of thrombus between the sheath and the tip | Impossibility to infuse and/or withdraw blood |
| Mural thrombus | The fibrin from the damaged vascular wall attaches to the fibrin that covers the catheter, increasing the risk of venous thrombosis | Output of liquid injected by the catheter insertion point, oedema, pain, vascular dilatation |
| Intraluminal thrombus | The fibrin sheath is formed within the lumen of the catheter causing partial or total occlusion | Impossibility to infuse and/or withdraw blood |
Clinical relevance of fibrin sheath
The fibrin sheath, initially composed of fibrinogen, albumin, lipoproteins and coagulation factors, appears 24 h after CVC placement as vessel response to an injury.
It then extends along the CVC surface over the following days, and is the leading cause of late dysfunction.778 The emergence of this sheath is intimately linked to the presence of biofilm. This biofilm is defined as a sessile community, characterised by cells that adhere to a substrate or each other, and are protected by a polymeric extracellular matrix of self-producing substances.786 The main complication of the biofilm, in addition to the infection itself, is the development of fibrin sheath. The pathophysiology of biofilm production is unclear, but it has been hypothesised that it may occur due to the initial contact of free bacteria with a foreign surface, the CVC. This may happen between day 1 and 14. This adherence of the cell can generate molecular signalling and proliferate to form microcolonies, thus generating a coating of exopolysaccharides that will adhere to the surface thanks to a glycocalyx matrix, and surround the community of bacterial microcolonies. The presence of the biofilm does not necessarily cause infection; however, the most significant non-infectious complication is fibrin sheath development.786
It is not clear in what order fibrin sheath, biofilm and their interrelationship develop,787 but current evidence suggests that biofilm evolves into fibrin sheath within days or months. In addition to fibrin, other ingredients are added: laminin, fibronectin, collagen, or smooth muscle and endothelial cells. The fibrin sheath begins at the point of contact between the catheter and vessel wall and advances until it covers the whole length of the CVC, thereby creating a possible dysfunction. Although the incidence of sheath formation is 100%, its condition may remain subclinical; however, in symptomatic patients it may be due to thrombus formation and/or infection.786,788 In a retrospective study conducted in 2007789 the authors established an intimate relationship between the fibrin sheath and CVC dysfunctions or complications (infectious and lack of flow), which was present in 76% of the venograms performed to assess dysfunction. Thus, fibrin sheath and associated thrombosis favour bacterial overgrowth and have a clearly unfavourable effect on the patency and durability of the catheter.790 This association between fibrin sheath and thrombosis, dysfunction and infections make their early diagnosis and management necessary.
Clinical and radiological assessment of dysfunctional catheter
The assessment sequence starts by performing a chest X-ray to assess the CVC position and rule out kinking. Once this is ruled out, CVC patency should be evaluated by infusing 10 mL of saline solution followed by aspirating blood. The inability to aspirate blood, to infuse serum or both forms the diagnosis of suspected fibrin sheath (see Table 29).
The study by digital subtraction angiography with contrast is the technique of choice for diagnosis. The angiographic findings in relation to the fibrin sheath are filling defects, contrast reflux towards the proximal end of the CVC with exit through holes in the sheath, excessive output jet through side holes in the CVC, and the absence of a gentle and adequate flow from the tip of the CVC towards RA.783 Peripheral venography is also used for diagnosis after vein cannulation on the back of the hand or foot. Retrograde venography has also been described through the CVC, but once withdrawn and positioned proximal to the entry zone in the central vein.789 Retrograde venography allows the diagnosis of fibrin sheath or the presence of thrombus during CVC replacement procedure and percutaneous transluminal angioplasty (PTA) of the fibrin sheath before insertion of a new CVC
After detecting the dysfunction, the problem must be immediately identified and treated, since delaying the solution predisposes the patient to inadequate dialysis and increased handling, which results in an increased risk of infection.782
Treatment of dysfunctional catheter (Figure 7)
Recommendations of the National Kidney Foundation10 for the treatment of dysfunction of tunnelled CVC include:
- •
Radiological assessment. A radiological study is essential to diagnose CVC dysfunction and to document vessel (central vein) conditions. Venography with contrast through the catheter should be included.
- •
Establish the appropriate treatment.
- –
CVC repositioning.
- –
Treatment of thrombus and/or fibrin sheath using mechanical measures or infusion of fibrinolytic drugs.
- –
Replacement of a dysfunctional catheter.
- –
CVC stripping.
- –
PTA of the vessel and/or of fibrin sheath.
- –
In the event of poor position, kinking or migration of the CVC, the problem should be solved initially by using guides and, if no results are obtained, the CVC must be replaced. Many authors replace the CVC over a guide using the same segment of the tunnel and central venous access.783,789,791 CVC replacement using a guide reduces procedural time and preserves venous access, although the exchange of CVC at the same site could produce a higher rate of infection than placing the new CVC at a different location, as shown in studies in patients without renal failure, with catheter infection and immune-suppressed, in which it is not necessary to preserve potential future insertion sites792. These authors conclude, however, that the change over a guide may be an acceptable option in a patient on HD with bacteraemia or CVC dysfunction, provided adequate antibiotic treatment has been established,792 an option already found by other authors in kidney patients,793 and in fact already proposed at the clinical guideline level.10
Techniques to be applied to central venous catheter dysfunction
- •
Vigorous flushing with saline solution. It must be performed under aseptic conditions to avoid infectious complications.794
A 10 mL syringe should be used. If the problem is not solved after 3 attempts and the flow deficit persists, the procedure for initiating fibrinolytic therapy is started.782
- •
Trans-catheter mechanical therapy. It consists of extracting the thrombus using a guide, a Fogarty catheter or a ureteral biopsy brush inserted via the lumen. It does not cause systemic alterations but has very little effect when thrombosis is secondary to a fibrin sheath.782
- •
Intraluminal fibrinolytic therapy (Figure 7). The infusion of thrombolytic agents through the CVC lumens has been widely used to restore patency and eliminate the fibrin sheath and thrombus, and has been recommended by several clinical practice guidelines.6,10 Several protocols have been described in the literature, for both systemic fibrinolytics at high doses and intraluminal use at low doses. There are no RCTs attesting to the use of one fibrinolytic agent over another, but there are trials in the literature comparing low-dose thrombolytic treatment. In the systematic review conducted by Hilleman and Cambell with diferent rt-PA (recombinant tissue plasminogen activator), there was a greater patency of reteplase (88 ± 4%) versus alteplase (81 ± 37%) and very low patency for tenecteplase (41 ± 5%).795 Reteplase also has the advantage of being lower in cost. The use of low-dose alteplase was evaluated in a prospective clinical trial involving 1064 patients from 80 centres (COOL and COOL1 studies).796,797 Administration of 2 mg/2 mL of alteplase for each catheter lumen, with maintenance of the fibrinolytic in situ for a period of 2 h followed by a similar dose if there was no response, obtained very good patency results (92.4% for double-lumen CVC) with very few side effects (0.4% sepsis, 0.3% gastrointestinal bleeding and 0.3% venous thrombosis). More recent studies obtained similar results with the use of low-dose intraluminal alteplase and with few, or practically no, side effects,795,798,799 without considering it necessary to prolong alteplase permanence in the catheter lumen to obtain similar patency rates. Purging the lumens with 1 mg/1 mL alteplase solution, with alteplase push by saline solution in small quantities (0.3 mL) every 10 min, allows maintaining the drug active in the tip and reducing the period of treatment to 30 min.799 Patency results are similar to those performed with prolonged permanence, but does not increase the risk of bleeding or the cost of the procedures as no high doses of fibrinolytic are used. The use of low doses of urokinase (UK) (5000 and 9000 IU per lumen) obtains poor results on the patency of CVC.800 A clinical trial to assess the efficacy of high dose randomised 2 groups into 25,000 and 100,000 IU per lumen, respectively. The first group required an additional dose (50,000 IU per lumen) in 86% of cases to achieve satisfactory patency and had to be repeated (with 75,000 IU) in the following HD session. The use of high doses of UK (100,000 IU) per lumen from the outset in the second group achieved very good results (100% patency), requiring a second dose of the same amount of UK in 33% of cases. It must be highlighted that both groups had preventive treatment with warfarin. No bleeding complications were recorded.801 Since 1999 UK has been withdrawn from the United States (U.S.) market802, because of its human origin, thus there are no recent comparative U.S. studies between UK and alteplase. In the review conducted by the Cochrane803 there were also no differences that support the use of UK versus rt-PA, so it is considered that both protocols could be useful, although with little evidence, in the treatment of thrombosed CVC.
- •
Systemic fibrinolytic therapy (Figure 7). To try to improve the percentages of patency after UK infusion, in 1998 Twardowski proposed the use of UK at high doses and in continuous infusion, 250,000 IU for 3 h.800 With this treatment regimen, resolutions of 81% were obtained after the first infusion and 99% after the third, much higher than that obtained with local infusions at low doses. However, their administration needs to be strictly monitored and there are a greater number of contraindications and complications, as well as high costs derived from hospitalisation time and monitoring by the skilled health personnel.800 The systemic use of rt-PA factor has been reported in the literature (2.5 mL in 50 mL of saline in 3 h of HD) with 100% immediate responses and 67% at 30 days.804,805 Results are similar to those observed with infusion in short periods, both in patency and in the absence of complications, but it supposes a greater use of time for the performance of the treatment. Preventive treatment with anticoagulants has demonstrated some effects on reducing thrombus formation in patients with CVC.806,807 The patency of CVC in anticoagulated patients is 47.1% versus 8.1% in non-anticoagulated patients (p = 0.01). However, the risk of bleeding and the need for monitoring of levels fail to make it an ideal therapy for routine use in patients in HD.808
- •
Extraluminal mechanical therapy and CVC replacement. Faced with the failure of fibrinolysis to achieve catheter patency, several more aggressive techniques have been designed, all with the purpose of eliminating fibrin sheath without or with CVC replacement.
Percutaneous stripping of the fibrin sheath782 which surrounds the CVC has been performed with acceptable success in terms of CVC patency.782,809 The technique, although effective, implies morbidity associated with an invasive procedure, requires qualified staff to perform the procedure and is more expensive than fibrinolytics. These data are supported by more recent studies comparing fibrinolytic treatment at high doses with fibrin sheath stripping,810 in which better results are obtained for the former in initial patency (97% versus 89%) and primary (86% versus 75% at 15 days).
As regards the use of stripping, there is also the RCT conducted by Merport et al.811 comparing the efficacy of stripping against replacements of CVC over a metal guidewire. The replaced catheters achieved better patency for HD over four months (23% versus 0%, p = 0.05) at a lower cost ($2586 versus $3022; p < 0.01).
In a study conducted with a sample of 66 patients,812 comparing several invasive techniques (thrombectomy of the sheath by stripping, replacement of catheter and replacement associated with PTA of the fibrin sheath), no significant differences were found between the three techniques, nor in immediate results, complications and durability of the catheter. Despite this, most of the studies found in the literature conclude that stripping should not be construed as routine therapy for catheter dysfunction and, if there is no response to fibrinolytic therapy, the catheter should be replaced over a guide.811
Replacement of the CVC used for HD must be performed using a guide and maintain the same central VA to preserve the highest number of VA per patient. It is an acceptable option for patients in HD with CVC dysfunction or bacteraemia, provided that the appropriate antibiotic treatment has been established.10,792,793
CVC replacement obtains better patency results when it is associated with fibrin sheath treatment by balloon PTA before placing a new CVC. In the RCT of Oliver et al.,813 the median length of time before repeating the replacement procedure was clearly superior when PTA was performed than when only CVC was replaced (411 versus 198 days; p = 0.17). Lastly, the risk-benefit of placing the new CVC in another location to avoid fibrin sheath should be assessed as a last resort in the case of repeated dysfunction problems, given the risk of causing the loss of another central vascular territory. In fact, there are authors who even replace the CVC directly by putting it into a different location after failure of fibrinolytic treatment.801
Hereafter, among the studies reviewed, those requiring evidence analysis are appraised.
→ Clinical question XXVIII What is the best treatment for the persistent dysfunction of the tunnelled central venous catheter (stripping, fibrin sheath angioplasty, fibrinolytics or catheter replacement
(See fact sheet for Clinical question XXVIII in electronic appendices)
| Summary of evidence | |
| Percutaneous stripping of the fibrin sheath versus UK infusion. An RCT with 57 patients found no statistically significant differences in survival curves of the CVC | Moderate quality |
| Stripping versus replacement of CVC. An RCT of 15 percutaneous fibrin sheath stripping interventions versus 22 CVC exchanges using metallic guides in 30 adult patients presents a greater patency with replacement at lower cost, concluding that stripping should not be considered as normal therapy for CVC dysfunction | |
| Percutaneous stripping of the fibrin sheath versus replacing the CVC. An RCT with 30 patients found that CVC replacement was significantly better than stripping to achieve suitable patency for HD over four months and obtained more days of mean CVC patency | |
| Fibrin sheath balloon angioplasty versus non-intervention. An RCT with 30 patients found no statistically significant differences between the two options in relation to the median time to repeat CVC replacement and median time to repeat the intervention, but there was a significant difference in relation to increase in blood flow (340 versus 329 mL/min) | |
| UK at high dose (100,000 IU) versus lower dose (25,000 IU) for catheter thrombosis. An RCT with 65 patients found that the higher initial dose achieved better results in relation to providing adequate flow for HD and lower end consumption of UK. Both groups were receiving preventive treatment with warfarin | |
| Tenecteplase versus placebo. An RCT with 149 patients found differences in favour of tenecteplase in short term results in relation to the percentage of patent CVC at the end of 1 infusion hour and to the increase in blood flow | |
| Alteplase in short dwell time versus long in CVC. An RCT with 60 patients found no statistically significant differences in relation to CVC patency rate (in the following HD session and at 2 weeks) or in CVC survival, and considered that alteplase is a short-term option which allows a two-week window for more definitive treatments to be initiated | |
| Another RCT with 82 patients compared two administration options of alteplase, push versus dwell, and found no statistically significant differences in CVC patency or survival rates until the next necessary intervention, or in the post-thrombolysis blood flow or the litres processed per hour in the following HD session, finding that the push protocol is effective, safe and more practical than the longer permanence of infusion | |
| Values and preferences of patients. No relevant studies related to this aspect have been identified | |
| Use of resources and costs. No relevant studies related to this aspect have been identified | |
Evidence synthesis development
Fibrinolytics introduced via the CVC lumen have been used for years with good results. Along with these therapies, others have been used that are intended for mechanical withdrawal of the fibrin sheath.
In the literature, several systematic or narrative reviews have been found. The RCTs of these were located and the results are presented below.778,783,791,795,814
Percutaneous stripping of fibrin sheath versus urokinase infusion
In an RCT, Gray et al.810 compared 28 patients treated with percutaneous stripping with 29 treated with UK infusion (30,000 IU/h, for a total of 250,000 IU), in TCVC with flow rates < 250 mL/min and the reference flow established as ≥ 300 mL/min or flow rates 50 mL/min under the highest values laid down as baseline flows. Results were as follows:
- •
Initial clinical success: 89% (25 out of 28) for percutaneous stripping and 97% (28 of 29) for UK.
- •
Primary patency rates at 15, 30 and 45 days: 75% (n = 20), 52% (n = 13) and 35% (n = 8) for percutaneous stripping and 86% (n = 21), 63% (n = 13) and 48% (n = 9) for UK.
- •
The additional median duration of CVC function was 32 days (95% CI, 18-48 days) for percutaneous stripping and 42 days (95% CI, 22-153 days) for UK.
No statistically significant differences were found in survival curves of the CVC (p = 0.236).
Percutaneous stripping of the fibrin sheath versus replacing the central venous catheter
The RCT of Merport et al.811 compared the efficacy of 2 treatments for the poor functioning of the TCVC for HD: 15 percutaneous fibrin sheath stripping interventions versus 22 CVC replacements over guidewire in 30 adult patients with poorly functioning HD CVC (flow rates < 200 mL/min). The overall technical success rate was 97%.
The replaced CVC achieved significantly better adequate patency for dialysis over four months (23% versus 0%, p = 0.05), the main outcome measurement in this study.
The mean duration of the CVC was 52 days for CVC replacement versus 25 days for stripping (p < 0.001).
Mean CVC patency was 52.2 ± 43 days for the CVC replacement group and 24.5 ± 29.3 days for the group treated with percutaneous stripping (p < 0.0001). After CVC replacement, patency rates at 1, 2, 3, and 4 months were 71%, 33%, 27% and 27%, versus 31%, 16%, 7% and 0% after stripping (p = 0.04).
Costs were higher for stripping ($3022 versus $2586; p < 0.01).
The RCT concludes that stripping should not be considered as normal treatment for CVC dysfunction.
Fibrin sheath balloon angioplasty versus non-intervention
The RCT of Oliver et al.813 was a pilot study which analysed the effectiveness of fibrin sheath PTA on the patency and function of the CVC in 18 patients randomised to balloon PTA versus 12 patients randomised to no treatment. Results were as follows:
- •
Median time to repeat CVC replacement was 411 and 198 days, respectively (p = 0.17).
- •
Median time to repeat the intervention was 373 days and 97.5 days, respectively (p = 0.22).
- •
Blood flow, 340 versus 329 mL/min (p < 0.001) was statistically significant but clinically small (11 mL/min).
Central venous catheter replacement in the same access versus placement of a new one at another access
No study has been identified in the literature that would allow assessment of this aspect.
Fibrinolytic agents
No RCT has been found directly comparing different thrombolytics with each other.
UK at high dose (100,000 IU) versus lower dose (25,000 IU) for CVC thrombosis
In the RCT of Donati et al.,801 two initial doses of UK were compared for the treatment of CVC thrombosis. Results were as follows:
- •
Dose 25,000 IU (29 cases). Appropriate flow (≥ 250 mL/min): 4 cases (13.7%) and the remaining 25 (86.3%) required subsequent addition of 50,000 IU, and then a further 75,000 IU in the following HD session.
- •
Dose 100,000 IU (36 cases). Appropriate flow (≥ 250 mL/min): 36 cases (100%), and it was necessary to treat 12 cases (33.3%) with 100,000 IU in the following HD session.
Both groups were receiving preventive treatment with warfarin.
Tenecteplase versus placebo
In the RCT of Tumlin et al.794 with 149 patients, 74 treated with tenecteplase for 1 h, and 75 with placebo, results were as follows:
- •
Patent CVC at 1 h from infusion: 22% of patients in the tenecteplase group versus 5% in the placebo group (p = 0.004).
- •
Change in blood flow: increase of 47 mL/min in tenecteplase group versus 12 mL/min in the placebo group (p = 0.008).
Four bloodstream infections were observed related to the CVC (one with tenecteplase, three with placebo) and one thrombosis (with tenecteplase).
Alteplase in short dwell time versus long
An RCT798 with 60 patients assessed the optimal permanence of alteplase in CVC, comparing 1 h (26 patients) with more than 48 h before the subsequent HD session (34 subjects).
There are no statistically significant differences in any of the following outcome measures:
- •
Rate of catheter patency: in the following HD session, 76.9% versus 79.4; at 2 weeks, 42.3% versus 52.9%.
- •
CVC survival: median of 14 days for the short-term option and 18 for the long (p = 0.621).
They consider that alteplase is a short-term option, which allows a two-week window during which more final treatments should be initiated.
Another RCT799 with 82 patients compared two administration options of alteplase, push versus dwell (30 min versus 2 h).
There were no statistically significant differences in any of the following outcome measurements:
- •
Rate of CVC patency: 82% (32/39) in push protocol versus 65% (28/43) of CVC in dwell protocol to exceed 300 mL/min (p = 0.08).
- •
CVC survival until the next intervention needed on the CVC: mean of 65.5 versus 59.3 days; (p = 0.76).
- •
Post-thrombolysis blood flow: difference of means, –16.26 mL/min (95% CI, –44.68 to 14.16; p = 0.29).
- •
Litres processed per hour in the following HD session: difference of means 0.026 (95% CI, –1.302 to 1.353; p = 0.969).
They consider that the push protocol is effective, safe and more practical than infusion dwell over 2 h.
From evidence to recommendation
When faced with TCVC dysfunction, different strategies can be used including the use of fibrinolytic agents, interventional manoeuvres such as stripping or PTA of fibrin sheath and, finally, CVC replacement. Some studies are conducted in the form of RCTs although the sample sizes are limited.
Of the treatments assessed, the fibrinolytic-based approach can be reasonably considered as the first therapeutic approach, because it is non-invasive and survival results and flow recovery are acceptable. When UK is used, the higher doses obtain better results. There are no comparative studies with continuous infusion.
Of the interventional treatments, stripping has shown poorer results compared to catheter exchange. Although the differences are not significant, fibrin sheath balloon PTA can double the period without intervention on the CVC. Therefore, in the approach to the dysfunctioning CVC, it is suggested that the first step is the use of a non-invasive treatment such as fibrinolytic treatment and if this proves to be insufficient, the next step would be to consider catheter replacement with fibrin sheath PTA.
Persisting TCVC dysfunction would require proposing CVC replacement in another location if the patient in question preserves available sites.
Clinical question XXVIII. Recommendations
R 6.8.4) We suggest that, should the tunnelled central venous catheter need to be replaced due to failure of fibrinolytic treatment, it be replaced using a guidewire, provided there is no tunnel infection or catheter-related sepsis. We suggest fibrin sheath angioplasty be associated before placing a new catheter
R. 6.8.5) We suggest fibrin sheath not be treated by stripping where there is tunnelled central venous catheter dysfunction
→ Clinical question XXIX What influence do the different types of central venous catheter lumen lock have on its dysfunction and infection?
(See fact sheet for Clinical question XXIX in electronic appendices)
Rationale
The relevance of fibrin sheath and thrombosis due to the association they share with bacterial overgrowth, as well as CVC patency and longevity, has led to the search for multiple preventive measures.815 Lock solutions have been used since the introduction of TCVC in the 1980s to prevent the intraluminal component associated with dysfunction. Of these, the most commonly used in the interdialytic period has been heparin816 and subsequently, citrate.817
The standard procedure to maintain CVC patency is heparin lock. This is a polysaccharide anticoagulant that inactivates thrombin and activated factor X. It works by binding to antithrombin, forming a complex with thrombin. This heparin-antithrombin-thrombin complex turns thrombin inactive, thereby inhibiting its ability to convert fibrinogen to fibrin.818 The usual dose ranges from 1000 to 10,000 IU/mL, with sufficient volume to fill the lumen to the tip.778 Heparin concentration has been falling constantly in order to reduce the risk of systemic anticoagulation.819,820 The use of lower concentrations (2500 to 1000 IU/mL) has demonstrated an efficacy comparable to 5000 IU/Ml.808,816 Heparin leakage through the CVC lumen after lock or over the next hour (leak phenomenon) can have a systemic impact and explain bleeding episodes.821,822 Although less common, other complications associated with heparin include thrombocytopenia823 and allergic reactions.824
In this context, citrate has been proposed as an alternative, since it would not produce this systemic effect819,821,825 and because of its antithrombotic power and potential antibacterial properties.817,826 Citrate is an anticoagulant that blocks coagulation cascade by binding to calcium ions, preventing the activation of the cascade pathways, which are calcium-dependent. It was first used as an anticoagulant to preserve blood and also for many years as an anticoagulant in continuous HD techniques.827 The advantage of citrate use in lock when it leaves the CVC lumen is that it would be rapidly metabolised to bicarbonates without causing systemic bleeding.828 This, together with its antimicrobial effects, has renewed interest in its ability to prevent CVC-related infections. On the other hand, however, there has been some concern about citrate toxicity and the presence of arrhythmias in locks with excessive volume, especially at high concentrations.828 After a case of fatal cardiac arrest was reported following the use of trisodium citrate at 46.7%, the U.S. Food and Drug Administration withdrew TriCitrasol in 2000.829 However, lower concentrations with 4% have been widely used with comparable results to heparin.827
Other later solutions include trisodium citrate at different concentrations, antibiotics, anticoagulants such as alteplase, tenecteplase and dicumarinics, as well as different combinations of these.827,830 In relation to antibiotics, some lock solutions have been shown to reduce the risk of bacteraemias versus heparin831-832 but are not routinely recommended, both because of the lack of evidence relating to efficacy (see Clinical Question XXX) and risk of developing resistances.833 As a result of the occurrence of strains with reduced susceptibility or resistance to antibiotics, alternatives like taurolidine have been suggested. Taurolidine is a derivative of the amino acid taurine. This is a broad-spectrum antimicrobial against bacteria and fungi.834 Methylated derivatives are believed to interact with the components of the bacterial walls causing their destruction. They also appear to reduce the adhesion of bacteria to human epithelial cells in vitro835. Bacterial resistance has not been documented, since the mode of action of taurolidine resembles disinfectant more than antibiotic.836
The literature has been reviewed in search of controlled trials with different lock solutions versus heparin to compare efficacy in preventing dysfunction, thrombosis or CVC loss and infection prevention.
| Summary of evidence | |
| Citrate versus heparin lock. Meta-analyses of RCTs did not show any statistically significant differences between citrate and heparin lock in relation to mean CVC duration, CVC thrombosis, CVC removal, CVC-related readmissions, bacteraemias, exit-site infections, and mortality. Incidence of bleeding is lower in patients treated with citrate | Low quality |
| Citrate plus antimicrobial agents versus heparin lock. Meta-analyses of RCTs found that adding antimicrobial agents to the citrate in the lock is not associated with differences in CVC duration or withdrawal due to dysfunction | |
| Adding gentamicin to citrate is associated with a lower risk of bacteraemias and infections of the exit site | |
| Heparin versus tissue plasminogen activator lock. An RCT comparing heparin three days a week versus rt-PA one day per week and heparin another two days later in the week found that the option with r-TPA is associated with a lower risk of CVC dysfunction and CVC-related bacteraemia | |
| Use of resources and costs. The RCT of Hemmelgarn et al.246 estimates the average cost (in Canadian dollars) of rt-PA and heparin as $1794 and $195, respectively, and the management cost of complications associated with CVC dysfunction and CVC-related bacteraemia per patient was $156 with rt-PA and $582 with heparin. Therefore, the incremental cost of caring for patients with rt-PA versus heparin was $1173 per patient, or $13,956 per episode of CVC-related bacteraemia prevented | |
| Values and preferences of patients. No relevant studies related to this aspect have been identified | |
Evidence synthesis development
Citrate versus heparin lock
The systematic review with meta-analysis of Zhao et al.828 included 13 randomised controlled trials, comprising 1770 patients and 221,064 CVC days, and compared citrate (alone or with antimicrobial agents) with heparin to lock CVC. The results were as follows:
- •
CVC withdrawal due to dysfunction: no significant differences between them.
- –
Citrate alone versus heparin: 3 RCTs with 21,378 catheter days (RR: 0.94; 95% CI, 0.59-1.49; p = 0.78).
- –
Citrate + antimicrobial versus heparin: 3 RCT with 143,604 catheter days (RR: 1.06; 95% CI, 0.41-2.69; p = 0.91).
- –
- •
Mean CVC duration: no significant differences. Mean differences: 3 RCTs (–32.81 days; 95% CI, –82.91 to –17.29; p = 0.2).
- •
Incidence of bleeding: 2 RCTs (RR: 0.48; 95%CI, 0.30-0.76; p = 0.002—significantly lower in patients who received citrate lock).
- •
CVC thrombosis: no significant differences. 2 RCTs (RR: 1.04; 95% CI, 0.46-2.34; p = 0.9)
- •
Thrombolytic treatments:
- –
Citrate alone versus heparin: no significant differences. 3 RCTs with 55,851 catheter days (I2: 77%; RR: 1.25; 95% CI, 0.74-2.11; p = 0.41).
- –
Citrate plus gentamicin versus heparin: 2 RCTs with 76,496 CVC days (RR: 0.62; 95% CI, 0.38-1.00; p = 0.05)
- –
Citrate plus taurolidine versus heparin: 1 RCT with 150, 118 catheter days (RR: 2.47; 95% CI, 1.68-3.63; p < 0.00001).
- –
- •
All-cause mortality: no significant differences. 7 RCTs (RR: 0.81; 95% CI, 0.53-1.23; p = 0.3).
- •
CVC-related readmissions: no significant differences. 2 RCTs (RR: 0.61; 95% CI, 0.13-2.74; p = 0.5).
- •
CVC-related bacteraemias: The overall combined meta-analysis found locks with citrate were better than those with heparin: 11 RCTs with 217,128 catheter days (RR: 0.39; 95% CI, 0.27-0.56; p < 0.001).
However, the subgroup analysis showed that lock with different antimicrobial agents was better than heparin, but there was no significant difference with citrate alone.
- –
Citrate alone versus heparin: 3 RCTs with 56,746 catheter days (I2: 67%; RR: 0.54; 95% CI, 0.22-1.30; p = 0.17).
- –
Citrate plus gentamicin versus heparin: 4 RCTs with 85,343 catheter days (RR: 0.25; 95% CI, 0.13-0.47; p = 0.0001).
- –
Citrate plus taurolidine versus heparin: 3 RCTs with 25,370 catheter days (RR: 0.45; 95% CI, 0.27-0.77; p = 0.003).
- –
Citrate plus methylene blue plus methylparaben plus propylparaben versus heparin: 1 RCT with 49,669 catheter days (RR: 0.29; 95% CI, 0.12-0.72; p = 0.008).
The analysis disaggregated by citrate concentration levels showed that low (1.04%-4%) to moderate (4.6%-7%) concentrations of citrate lock were associated with a lower incidence of these infections (p < 0.001 and p = 0.003, respectively), but patients who received high concentrations (30%-46.7%) of citrate had no significant differences compared to heparin locks (p = 0.3).
- –
- •
Exit-site infections: no significant differences.
- –
Citrate alone versus heparin: 4 RCTs with 59,942 catheter days (I2: 60%; RR: 0.73; 95% CI, 0.35-1.53; p = 0.41).
- –
Citrate plus gentamicin versus heparin: 2 RCTs with 78,683 catheter days (RR: 0.57; 95% CI, 0.20-1.57; p = 0.28).
- –
Citrate plus taurolidine versus heparin: 2 RCTs with 21,175 catheter days (RR: 1.09; 95% CI, 0.44 -2.74; p = 0.85).
- –
Recently, two published systematic reviews analysed the effect of different types of locks versus heparin. In the first review, which includes 10 clinical trials and 2 controlled observational studies, heparin lock is compared with other methods, including citrate, but there are no significant differences in CVC patency and dysfunction (10 trials; RR: 0.89; 95% CI, 0.56-1.39).830
Another review837 showed similar results, while there was a significant reduction in the percentage of bleeding when locked with citrate (2 trials; RR: 0.48; 95% CI, 0.30-0.75), but no significant differences regarding mortality were found (3 trials; RR: 0.71; 95% CI, 0.42-1.24). Unlike Zhao et al.,828 this latest review did not show that citrate lock reduced the incidence of CVC-related bacteraemia (RR: 0.54; 95% CI, 0.23-1.29), although these results could be explained by the lower number of studies considered in this analysis (3 trials, versus the 7 trials that provided data on bacteraemia in the Zhao et al.837 review).
Finally, another systematic review838 analysed 6 clinical trials (3 on HD patients) comparing citrate lock combined with antimicrobial taurolidine versus heparin. As in the Zhao et al.828 review, the combination of citrate and taurolidine significantly reduced the risk of CVC-related bacteraemia (3 trials; RR: 0.46, 95% CI, 0.24-0.89), but showed no difference in the incidence of CVC thrombosis (3 trials; RR: 2.16; 95% CI, 0.72-6.74).838
Heparin lock versus tissue plasminogen activator
The published reviews on this subject839,840 located a parallel design RCT249 and two of cross-over design.841,842 In one of the reviews, Firwana et al.840 point out that the latter two cross-over studies reported the results at the end of both study phases; therefore, only the results of the RCT are analysed. It compares heparin three days a week versus rt-PA one day per week and heparin a further two days a week and finds that the option with rt-PA is associated with a lower risk of CVC malfunction and CVC-related bacteraemia.
From evidence to recommendation
After reviewing the available evidence, which uses heparin as a control group, no differences were found in comparison to other types of lock, such as citrate or rt-PA, so any of them can be recommended for dysfunction and infection prevention.
Different systematic reviews, based on the same studies as those included in the main assessed review used to develop the clinical question, have shown similar results. They mainly focus on reducing the risk of catheter-associated bacteraemia and the incidence of bleeding from citrate versus heparins.
6.9. Catheter-related infectionRecommendations
R 6.9.1) If central venous catheter bacteraemia is suspected and prior to the administration of empirical antibiotherapy, we recommend that 2 blood samples be extracted simultaneously for peripheral blood cultures. In addition, we recommend blood also be simultaneously extracted through the central venous catheter lumens and a peripheral vein if it is decided to preserve the catheter. For diagnostic purposes, they should be cultured by means of a quantitative technique or by calculating the difference of time in the positivity between the two
R 6.9.2) We recommend the central venous catheter be withdrawn in the case of catheter-related bacteraemia if it is a non-tunnelled central venous catheter, there is a complicated local infection (tunnellitis), a complicated systemic infection (septic shock, persistent fever or positive blood cultures within 72 h of initiating appropriate antibiotic treatment, metastatic infections such as endocarditis, thrombophlebitis or spondylodiscitis) or the patient has other intravascular prosthetic material (pacemakers, endovascular prostheses, valves, etc.)
R 6.9.3) We recommend that in cases of suspected catheter-related bacteraemia, broad spectrum systemic empirical antibiotic therapy be initiated while waiting for microbiological results
NEW R 6.9.4) We recommend that in case of uncomplicated catheter-related bacteraemia, treatment be initially and simultaneously performed with systemic antibiotics and antibiotic lock
NEW R 6.9.5) We suggest that after withdrawing an infected tunnelled central venous catheter, a new catheter be placed after appropriate antibiotic treatment has been established and negative control blood cultures obtained. If possible, the new catheter must be placed in a different anatomical site from the original location
NEW 6.9.6) We recommend antibiotic prophylaxis not be routinely administered before inserting or handling a central venous catheter
- (•)
NEW R 6.9.7) We recommend antibiotic prophylaxis not be routinely used in locking tunnelled central venous catheter for haemodialysis
- (•)
NEW R 6.9.8) We recommend the central venous catheter be withdrawn if there is catheter-related bacteraemia due to virulent micro-organisms such as Staphylococcus aureus, Pseudomonas species, Candida species or multi-resistant micro-organisms
- (•)
NEW R 6.9.9) We recommend that antimicrobial agents with activity against Gram-positive and Gram-negative micro-organisms be included in the empirical choice of antibiotics, depending on the epidemiology of each haemodialysis unit, sensitivity and resistance patterns of its usual micro-organisms, patient risk factors and severity of infection
- (•)
NEW R 6.9.10) We suggest vancomycin be used as the first choice for the empirical treatment of gram-positive micro-organisms in haemodialysis units
- (•)
NEW R 6.9.11) We suggest daptomycin be used for the empirical treatment of catheter-related bacteraemia in haemodialysis units in which methicillin-resistant Staphylococcus aureus cultures show values of minimum inhibitory concentration of vancomycin ≥1.5 μg/mL, in patients with septic shock or with a known allergy to vancomycin
- (•)
NEW R 6.9.12) We suggest detection and local or systemic antibiotic treatment not be performed routinely to eradicate Staphylococcus aureus in nasal carriers
NEW R 6.9.13) We suggest that 2 blood samples, taken 10 to 15 min apart, be extracted through the arterial line of the extracorporeal circuit without the need to interrupt the haemodialysis session, when suspected catheter-related bacteraemia occurs during the haemodialysis session (or if it is not possible to obtain blood cultures by needling a peripheral vein)
Rationale
CVC-related infection is the most common and severe CVC complication and is associated with increased morbidity and mortality.843 The incidence of CVC-related bacteraemia (CRB) is 2.5 to 5 events per 1000 days of catheter use, which corresponds to an incidence of 0.9 to 2 episodes of CRB per catheter per year.844-847 In CVC-bearing patients, a risk of developing bacteraemia is 10 times higher than in patients with nAVF.192,848-850 CRB is two to three times more common in NTCVC than in TCVC.192,673
A CVC-related infection usually results in withdrawal and serious complications, such as osteomyelitis, endocarditis, thrombophlebitis, and death in 5% to 10% of CVC-bearing patients.687,844,851,852 Serious metastatic infections occur most frequently in infections caused by Staphylococcus aureus, which is one of the most frequently isolated micro-organisms (10% to 40%).853 It is important to note that these metastatic complications may not be evident at first and appear weeks or even months after the initial CRB event.
There are three types of infections associated with CVC for HD747,833:
- •
Uncomplicated local infection, defined as the existence of signs of inflammation limited to 2 cm around the cutaneous exit site on the skin, without extension upwards towards the catheter cuff if it is tunnelled. It may or may not be associated with fever and bacteraemia, and accompanied by purulent exudate via the skin exit site.
- •
Complicated local infection, defined as the onset of signs of inflammation 2 cm beyond the exit site and in the subcutaneous tract of the catheter (tunnellitis). It may or may not be associated with fever and bacteraemia, and accompanied by purulent exudate via the skin exit site.
- •
Systemic infection or catheter-related bacteraemia. Defined as the isolation of the same micro-organism in blood and CVC in the absence of another focus of infection. It is considered a complicated systemic infection when there is septic shock, the fever continues and/or positive blood cultures are maintained 48-72 h after initiation of the appropriate antibiotic treatment, there are metastatic complications (endocarditis, thrombophlebitis or spondylodiscitis), or intravascular prosthetic material.
Diagnosis of catheter-related bacteraemia
The most sensitive clinical manifestations, although not highly specific for CRB diagnosis, are for fever and/or chills,854-856 while the presence of exudate or local signs of inflammation at the exit site of the CVC is more specific but much less sensitive. Indeed, in most cases of CRB there is no evidence of infection of the exit site.857 Other less common clinical manifestations are haemodynamic instability, alteration of the level of consciousness, CVC dysfunction and signs or symptoms related to sepsis. Sometimes, the complications of bacteraemia (endocarditis, septic arthritis, osteomyelitis or abscesses) may be the first manifestation of CRB.
CRB should be suspected clinically when a CVC carrier for HD presents with symptoms of fever or chills, and/or any suggestive clinical or haemodynamic abnormality; this suspicion is reinforced if this episode is associated with handling or local signs of inflammation at the insertion site or in the subcutaneous CVC tunnel. The episode should then be assessed through clinical history and basic physical examination in order to exclude possible sources of infection other than the CVC. Depending on results from the initial assessment, additional laboratory and radiological examinations should be performed.
The isolated clinical criterion is insufficient to establish CRB diagnosis. Therefore, this should be clinically assessed and microbiologically confirmed through blood and/or CVC cultures. Reference diagnostic techniques are based on culturing the tip of the catheter after CVC withdrawal858-862; thus, CRB diagnosis is established by the aforementioned blood culture positivity and isolation of this micro-organism in the blood culture. In recent years, new diagnostic tests have been developed in order to avoid unjustified catheter withdrawal and the potential risk associated with placing a new catheter in another location. Likewise, it is currently considered that CVC withdrawal is not always necessary for proper diagnosis and treatment.854,855,863-866
Quantitative blood cultures technique, obtained simultaneously through CVC and direct needling of a peripheral vein (ratio of the number of colony-forming units per millilitre [CFU/mL] of 3:1-10:1) is considered indicative of CRB with a sensitivity of 79-94% and specificity of 94-100%.867-871
Despite its high specificity, this technique is not routinely used in most local microbiology laboratories due to its complexity and cost. Since many hospitals have automatic devices for detecting microbial growth in blood samples, an alternative method to quantitative blood cultures has been proposed. This method measures the differential time to positivity of the blood cultures obtained simultaneously through the CVC and by direct venipuncture. The basis of this technique is that the positivity time of the blood samples is directly related to the number of micro-organisms initially present in the sample,872 so that when the blood cultures extracted through the CVC show positive at least 2 h earlier than those obtained after peripheral vein needling, a positive differential time is considered. The calculation of the differential time has a sensitivity of 94% and specificity of 91% for CRB diagnosis in patients with a CVC.873,874
If CRB is suspected and prior to the administration of antibiotics, two blood cultures should be extracted by venipuncture obtained from different locations or taken 10 to 15 min apart. After the CVC is removed, the distal segment should be cultured. When the decision to preserve the catheter is taken, a paired and simultaneous extraction of blood should be performed through all the CVC lumens and the peripheral vein.
CRB microbiological confirmation is established when:
- •
The same micro-organism is isolated at the tip of the CVC and in at least one blood culture obtained by peripheral venous needling.
- •
The same micro-organism is isolated in at least two blood cultures (one through the CVC lumens and the other through peripheral vein needling) and diagnostic criteria for quantitative blood cultures are met or a positive differential time is calculated.
In a significant number of HD patients, it is not possible to obtain peripheral vein blood samples because it is difficult to access the venous vascular bed due to previous thrombosed VA or the need to preserve them for VA creation.833,844,875 When blood cultures cannot be obtained by needling a peripheral vein, it is suggested two blood samples be extracted through both CVC lumens876-878 or from the extracorporeal circuit arterial line. CRB diagnosis is considered possible in symptomatic patients if there is no evidence of another focus of infection and blood cultures are positive. Although specificity and positive predictive value for CRB diagnosis is much greater in blood samples obtained by peripheral needling than in those obtained through the CVC, both have a high negative predictive value.879-881
If the micro-organism isolated in a single blood culture is a negative-coagulase staphylococcus, new blood samples will be needed to check if it is contamination or real CRB.
In cases where CVC is removed due to suspected CRB, the culture of the tip of the catheter should be performed by quantitative or semi-quantitative techniques. Colonisation should be considered when more than 15 CFU/mL (Maki technique) or more than 1000 CFU/mL (Cleri technique) are quantified in the growth.860-862,881a Removed catheter cultures should not be performed systematically unless there is suspected infection.882
Treatment of catheter-related infection
The most frequently isolated micro-organisms in CRB are gram-positive. Coagulase-negative staphylococci together with S. aureus make up 40-80% of cases, so initial treatment should be effective against these micro-organisms awaiting microbiological confirmation.844,863,883,884 Infection with Staphylococcus aureus has been associated with high level of morbidity and mortality.853,885-887
Non-staphylococcal CRB are predominantly due to enterococci, corynebacteria, and gram-negative bacilli. Gram-negative CRB have been increasing in recent years, and in some centres may represent up to 30%-40%.707,844,883
CRB treatment involves, on the one hand, initiating systemic antibiotic therapy and, on the other, CVC management regarding withdrawal or preservation. Therefore, once antibiotic treatment has started, one of the following options should be chosen:
Immediate withdrawal833
- •
All NTCVC.
- •
Complicated local infection.
- •
Presence of septic shock.
- •
Persistence of fever or bacteraemia 48-72 h after initiating antibiotic treatment appropriate to the sensitivity of the micro-organisms.
- •
Evidence of metastatic infection (endocarditis, suppurative thrombophlebitis, spondylodiscitis, etc.).
- •
Isolation of extremely virulent micro-organisms: S. aureus, Pseudomonas spp., Candida spp. or multi-resistant micro-organisms.
Once the infected CVC is removed, the best alternative is to place a new NTCVC, if possible in a different anatomical site. Although we do not have sufficient evidence at present, GEMAV suggests that a new CVT be implanted once the appropriate antibiotic treatment has been established and negative control blood cultures have been obtained. Also, if possible, it must be placed in a different location to the previous site occupied by the withdrawn CVC.
Catheter lumen lock using antibiotic solution
In uncomplicated CRB, conservative treatment, i.e. preserving a working catheter, may be tried. Previous experiences, in which the CVC was preserved and systemic antibiotic treatment was given intravenously (sometimes through the colonised CVC itself), showed cure percentages ranging from 32% to 74%, together with a high risk of recurrence when antibiotics are discontinued.845,888-891
The micro-organisms that develop and form biofilms spread universally on all endovascular CVC, both on the extra-luminal (primarily short-term catheters) and intraluminal surface (primarily long-term catheters).892 The micro-organisms that cause the infection are located within the biofilm on the inner CVC surface, and this gives them resistance to the action of antibiotics and would explain the difficulty in eradicating infection from CVC treated with intravenous antibiotics alone.893
It has been confirmed that prolonged contact of the intraluminal CVC surface with antibiotic solution at high concentrations, the micro-organisms can be eradicated using antibiotic concentrations at least 1000 times higher than the minimum inhibitory concentration (MIC).894-897
Thus, the means of treatment by locking the CVC lumen with a highly concentrated antibiotic solution is known as antibiotic lock therapy865 and is considered a good option for treating a CVC infection.
In the systematic review of Aslam et al.898 with meta-analysis of observational studies of treatment of CRB of TCVC in HD, a similar cure proportion was obtained between the antibiotic lock therapy and CVC replacement over a guidewire (alternative treatment that is explained below), although in Staphylococcus aureus CRB there was greater success with CVC replacement. Published studies on HD patients treated with antibiotic lock are mostly descriptive and show success percentages ranging from 44% and 100%. Degree of success has been found to be related to micro-organism type, with cures being described of 87-100% of patients with infections by gram-negative micro-organism, 75-84% for Staphylococcus epidermidis, 61% for Enterococcus and between 40% and 55% for S. aureus.853,854-856,899-901
Antibiotic lock consists of placing a concentrated solution of antimicrobial, usually with heparin, inside the catheter. Other anticoagulants have been used, such as sodium citrate and EDTA (ethylenediaminetetraacetic acid) in antibiotic lock solutions in order to prevent CRB.902 The antibiotics used for CVC lock must be chemically stable, with prolonged antimicrobial activity (approximately 1 week) and without precipitating inside. Concentrations tend to range from 1 and 5 mg/mL, usually mixed with 1% or 5% heparin, with enough volume to fill the catheter lumen.741
Antibiotic lock is administered by filling both CVC lumens at the end of each HD session, in strict aseptic conditions, using a different syringe and needle for each lumen of the catheter. Depending on the organisation of the centre, the antibiotic lock solution can be prepared in the pharmacy or in the dialysis unit. Antibiotic lock treatment should be carried out simultaneously with systemic antibiotic therapy, preferably using the same antimicrobial agent. Treatment should last as long as systemic antibiotics (usually 2-3 weeks depending on aetiology). Patients should be closely monitored in order to detect the persistence of fever, positive blood cultures within 48-72 h of initiating appropriate antibiotic treatment adapted to microbial sensitivity, onset of septic complications or recurrence of CRB. In these cases, CVC withdrawal is recommended.
There is a considerable diversity in the antimicrobials chosen and their concentrations when preparing the lock solution (Table 30).
Lock solutions described in the literature and with potential use in clinical practice
| Micro-organism | Antimicrobial | Concentration | Observations |
|---|---|---|---|
| Staphylococcia | Daptomycin | 5 mg/mL | Dilute in Ringer’s lactate solution (calcium) |
| Vancomycin | 2-5 mg/mL | Incompatible with heparin at concentrations > 5 mg/mL | |
| Enterococcib | Vancomycin + gentamicin | Both at 2 mg/mL | |
| Gram-negative bacillic | Levofloxacin | 5 mg/mL | Precipitates with heparin |
| Amikacin | 10 mg/mL | ||
| Piperacillin-tazobactam | 10 mg/mL | ||
| Candida spp.d | Echinocandin | 5 mg/mL | |
| Anphotericin B liposomal | 1-5 mg/mL | ||
| Others | Ethanol | 70% |
The aim of this table is not to provide an exhaustive compendium, nor are there clinical trials that provide the level of evidence needed for its use. For this reason, it only reflects expert opinions. Antibiotic catheter lock is a necessary but insufficient part of treatment. All antibiotic lock regimens must be accompanied by systemic antibiotic treatment which may be extended depending on the pathogen involved.
Only conservative treatment is recommended in the case of negative-coagulase staphylococci. In the case of catheter-associated Staphylococcus aureus bacteraemia, catheter withdrawal is recommended.
There is insufficient experience to recommend conservative treatment. However, if the patient is stable and the bacteraemia uncomplicated, conservative treatment can be considered.
CVC lock with antiseptics, such as taurolidine, ethanol or the combination of citrate with methylene blue-para-bens or with taurolidine and heparin, have shown efficacy against the bacterial biofilm and in CRB prophylaxis.903-907 These substances would have the advantage of preventing possible induction of resistance to antibiotics, although clinical experience in CRB treatment is extremely limited to be able to make recommendations.
CRB treatment by systemic antibiotics alone, preserving the CVC and without antibiotic lock, is insufficient to eradicate the micro-organisms in the biofilm and cure in most cases of CRB.898
Replacement of the infected central venous catheter over a guidewire
Delay in withdrawing an infected CVC (when there is no indication for immediate withdrawal or withdrawal was not possible at the time) and replacement with a new CVC over a metal guidewire is considered an acceptable alternative to antibiotic lock. This therapeutic option aims to definitively eradicate the biofilm causing the infection from the interior of the CVC and would be highly effective in cases where dysfunction of the infected CVC is observed.
Replacement of the infected catheter over a metallic guidewire has obtained similar cure results when compared to immediate withdrawal in different non-randomised studies.793,883,888,908
The substitution of the infected CVC over a metallic guidewire should be considered only if symptoms have disappeared rapidly. Though no time limit has been determined, GEMAV considered replacing the CVC at least 48-72 h after initiating antibiotic treatment, when the patient is clinically stable and there is no evidence of infection in the subcutaneous tunnel.
If the CVC is replaced after clinical improvement through antibiotic treatment, and positive blood cultures are later confirmed, it seems prudent to perform new blood cultures to confirm the resolution of the bacteraemia. If this has not occurred, the new CVC must also be removed.
Empirical treatment in catheter-related infections747,833,875
Initial empirical treatment in patients in HD with suspected CRB should include intravenously administered broad-spectrum antibiotics for gram-negative and gram-positive micro-organisms. Vancomycin (or teicoplanin) is suggested as first choice against gram-positive micro-organisms, due to the high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in haemodialysis units. Daptomycin is recommended as first choice when there is a high prevalence of MRSA with a vancomycin MIC ≥ 1.5 µg/mL or in severe cases with septic shock or metastatic complications. Aminoglycosides or third-generation cephalosporins should be combined to cover gram-negative micro-organisms. Depending on the seriousness of the patient’s illness and resistance rates in the unit, the administration of piperacillin-tazobactam or carbapenems should be considered to extend coverage against gram-negatives.
The combination of vancomycin or daptomycin and gentamicin or ceftazidime may be appropriate on most occasions, as their pharmacokinetic characteristics enable convenient application in HD. The usual doses are:
- •
Vancomycin. Initial 20 mg/kg dose administered during the last hour of the HD session. Initially, subsequent sessions will continue with 500 mg, adjusting later by plasma levels.909,910
- •
Gentamicin. 1.5-2 mg/kg dose (it is recommended 100 mg not be exceeded) administered after the HD session, adjusting later by plasma levels.
- •
Cefazolin. 1000-2000 mg dose administered after the HD session.
- •
Ceftazidime. 2000 mg dose administered after the HD session.
- •
Daptomycin. Dose of 8 to 10 mg/kg/48 h. It has been suggested dispensing 6 mg/kg after HD or 7 to 9 mg/kg administered over the last 30 min of the HD session (three times per week) depending on the permeability of the dialyser.911
Aetiological treatment of catheter-related infections
The choice of the specific systemic antibiotic treatment is developed in Table 31.
Recommendations for aetiological antibiotic treatment of catheter-related infection (at same time as lock treatment if central venous catheter is preserved)
| Regimen of choice | Alternative regimen | Comments | |
|---|---|---|---|
| Empirical treatment | |||
| Gram-positives: vancomycin + gram-negatives: gentamicin or third-generation cephalosporin | Gram-positives: daptomycin if: septic shock; metastatic complication; prosthesis; previous MRSA with MIC for vancomycin ≥ 1.5; previously vancomycin-resistant enterococciGram-negatives: if allergy or depending on according to severity: piperacillin-tazobactam/carbapenem | Assess catheter withdrawal | |
| Aetiological antibiotic treatment | |||
| Methicillin sensitive Staphylococcus aureus | Cloxacillin or cefazolin | Daptomycin | Catheter withdrawal recommendable |
| Duration: 3 weeks, 6-8 weeks if metastatic complications | |||
| Methicillin resistant Staphylococcus aureus | Vancomycin if MIC < 1.5 | Daptomycin: previous MRSA with MIC for vancomycin ≥ 1,5; isolation of previously vancomycin-resistant enterococci; septic shock; metastatic complications; endovascular devices | Catheter withdrawal recommendable |
| Duration: 4 weeks, 6-8 weeks if metastatic complications | |||
| Methicillin-sensitive negative-coagulase Staphylococcus | Cloxacillin or cefazolin | Daptomycin | Duration: 3-5 days if stable patient and catheter is withdrawn, 10-14 days if catheter is preserved |
| Methicillin-resistant negative-coagulase Staphylococcus | Vancomycin if MIC < 1.5 | Daptomycin: previous MRSA with MIC for vancomycin ≥ 1,5; isolation of previously vancomycin-resistant enterococci; septic shock; metastatic complications; endovascular devices | Duration: 3-5 days if patient is stable and catheter is withdrawn, 10-14 days if catheter is preserved |
| Enterobacterias | Ceftriaxone or levofloxacin | If allergy or severity: aminoglycoside or piperacillin-tazobactam or carbapenem | Duration: 4 weeks, 6-8 weeks if metastatic complications |
| Pseudomonas aeruginosa | Carbapenem or piperacillin-tazobactam or cefepime ± gentamicin | Ceftazidim or levofloxacin + gentamicin | Catheter withdrawal recommendable |
| Duration: 4-6 weeks, 6-8 weeks if metastatic complications | |||
| Candida spp. | Echinocandin until strain is known De-escalate to fluconazole if Candida albicans or Candida parapsilosis | Anphotericin B liposomal or voriconazole | Catheter withdrawal recommendable |
| Duration: 2 weeks after negative blood cultures, 6-8 weeks if metastatic complications | |||
MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus.
In the case of Staphylococcus lugdunensis, same action to be taken as in S. aureus.
For those patients with methicillin-sensitive Staphylococcus aureus isolated in blood cultures and empirically treated with vancomycin, this antibiotic should be replaced with cloxacillin or cefazolin. Cefazolin is a good option in CRB patients sensitive to this antibiotic, as it is easy to administer in HD.912,913 The use of vancomycin to treat infections due to methicillin-susceptible S. aureus can lead to therapeutic failures.850,914 In bacteraemias caused by MRSA, MICs must be determined for vancomycin. If it is equal to or greater than 1.5 μg/mL, it should be replaced by an alternative antibiotic such as daptomycin.
The duration of antibiotic treatment will depend on the causative agent and presence or absence of CRB complication. Usually, antibiotic therapy should be maintained for 2 to 3 weeks in the absence of CRB-related complications and extended depending on the causative agent or occurrence of complications (persistence of positive blood cultures, septic metastases, endocarditis etc.)
Treatment of local infection associated with central venous catheter833
Uncomplicated local infection should be treated by topical cleansing methods that include antimicrobial agents, based on the result of cultures of the exit site (mupirocin if Staphylococcus aureus or topical azoles if Candida spp.) and, if there is no improvement, systemic antibiotic therapy should be initiated, removing the CVC in the case of persistent infection.
The treatment of complicated local infection (tunnellitis) always includes CVC withdrawal and treatment with systemic antibiotic therapy for 7-10 days.
Complications of catheter-related bacteraemia
The complications that can most frequently be caused by a CVC infection are infective endocarditis, spondylodiscitis and septic thrombosis and the catheter must be immediately removed if one is present. They should be suspected when bacteraemia persists 72 h after the initiation of appropriate antibiotic treatment, and must be extended up to 6-8 weeks.
- •
If endocarditis is suspected, a transoesophageal echocardiography should be performed and repeated if there is high suspicion, even if the initial study is negative. Transthoracic ultrasound is less sensitive in the detection of small valvular vegetations.915
- •
In the event of clinical and analytical suspicion of spondylodiscitis, magnetic resonance imaging of the spine should be initially used because of its high sensitivity and specificity.851
- •
To diagnose septic thrombophlebitis, radiological study (computed tomography, ultrasound or other) must be performed to prove the existence of the thrombus. Systemic anticoagulation with heparin may be indicated to treat septic thrombosis when progression of the thrombus is evident, although it is a controversial option and there are no controlled studies which would allow for its recommendation.833 Thrombolytic agents are not indicated as adjuvant treatment for septic thrombosis.
General recommendations for the prevention of catheter-related infection
Different strategies have been developed to reduce the incidence of infection related to the CVC for HD. These include strict asepsis to handle the CVC during connection and disconnection manoeuvres from the HD circuit, as well as the care of the skin at the CVC exit site (see section 6.5 “Catheter handling”). Others, such as coating the CVC surface with anticoagulant, antiseptic or antibiotic products, aim to minimise the risk of thrombosis and of infection. Experiences have been reported which show the effectiveness of this strategy, but only in NTCVC used in critical patients and for limited periods of time. There is no evidence to support its routine use in HD populations with long-term TCVC.688,916,917
Antibiotic prophylaxis before inserting the CVC has been tested in oncological patients and in populations using CVC for parenteral nutrition. In two RCTs, teicoplanin was used as prophylaxis and in one of them involving 88 oncohaematological patients, a decrease in insertion site infections, tunnellitis, and gram-positive bacteraemia was observed918; while in the other with 65 patients, CVC-related infection rates were not reduced.919 Vancomycin was administered in two other RCTs prior to CVC insertion, without finding a decrease in bacteraemia rate in 55 non-oncological patients with CVC for parenteral nutrition920 and in 98 oncohaematological patients.921 A meta-analysis published in 2013 and reviewed in 2015, which includes 11 studies with 828 patients, analysed the efficacy of the prophylactic use of antibiotics before insertion or use of an intravascular CVC to prevent gram-positive bacteraemia.922 In 5 studies of this meta-analysis no difference was found in the number of associated cases of bacteraemia between one group of patients in which systemic vancomycin, teicoplanin or ceftazidime were administered versus another group where prophylaxis was not administered. Moreover, prophylactic administration of glycopeptides has been associated with the emergence of resistant micro-organisms, thus its use in prophylaxis is not recommended in many clinical guidelines.923
Therefore, GEMAV recommends antibiotic prophylaxis not be routinely administered before inserting or handling a CVC.
Routine administration of intranasal antimicrobial agents to decolonise S. aureus carriers and the routine use of antibiotic CVC lock as prophylaxis for CRB, are developed in the following clinical questions, but have not been considered for recommendation, either.924
→ Clinical question XXX Is the use of antibiotic prophylaxis justified to lock a tunnelled central venous catheter for haemodialysis?
(See fact sheet for Clinical question XXX in electronic appendices)
| Summary of evidence | |
| Evidence from low quality RCTs with a risk of bias showed that catheter lock using antibiotics and heparin solutions or using antibiotics and citrate are more effective than heparin alone to prevent catheter-related bacteraemia | Low quality |
| The authors of the review point out that there is a risk of publication bias, because the funnel plot shows an underrepresentation of studies with no or negative effect. They also point out that none of these studies were double blind, which poses a risk of performance bias by professionals, and that the concealment of treatment was adequate only in four of the eight studies and only one of them conducted intention to treat analysis | |
Evidence synthesis development
Several systematic reviews that analyse this topic have been found.831,832,925-928 These reviews only analyse the risk of bacteraemia but do not gather information on other possible outcomes of interest, such as mortality, catheter survival rates or hospitalisation episodes.
The following sections are based on the review of Snaterse et al.925 as it is the most recent, gives separate information for TCVC and provides a risk analysis of bias in the available evidence. The systematic review of Snaterse et al. located 8 RCTs (123,300 catheter-days) that analysed the use of antibiotic solutions versus solutions with heparin for prophylactic TCVC lock.
In relation to the risk of bacteraemia, when comparing the use of heparin-only lock solution, they found statistically significant differences favouring both antibiotic prophylaxis combined with heparin and that combined with citrate, but not for antibiotic plus EDTA.
Bacteraemia risk difference per 1000 catheter days:
- •
Antibiotics plus heparin versus heparin: –2.08 (95% CI, –2.64 to –1.53) (five studies with 108,313 catheter-days; I2: 0%. Note: one of the studies was with 4503 catheter-days with NTCVC).
- •
Antibiotics plus citrate versus heparin: –2.88 (95% CI, –4.34 to –1.41) (three studies with 15,036 catheter-days; I2: 0%).
- •
Antibiotics plus EDTA versus heparin: –0.47 (95% CI, –1.40 to 0.45; one study with 4454 catheter-days).
As regards the antibiotic regimen that may be the best, they report that only two studies with few patients were published, and that they did not find statistically significant differences between solutions that included different antibiotics (citrate-gentamicin, minocycline-EDTA, vancomycin-heparin, vancomycin-ciprofloxacin-heparin).
They further point out that potential negative effects, including side effects, development of bacterial resistance or cost-effectiveness of interventions, should be taken into account, as well as the potential benefits in relation to bacteraemia prevention.
They also point out that their review supports the position of the Centers for Disease Control and Prevention (CDC) not to recommend the systematic use of antibiotics in catheter lock solutions.
From evidence to recommendation
The available evidence on CRB prevention using antimicrobial lock comes from low quality RCTs with risk of bias. This, together with possible side effects and resistance to antibiotics, lead us not to recommend the routine use of antibiotic prophylaxis in locking TCVC for HD.
→ Clinical question XXXI Does catheter-related bacteraemia secondary to infection with Staphylococcus aureus, Pseudomonas spp. and Candida spp. force catheter withdrawal and therefore contraindicate antibiotic lock treatment to attempt to preserve the catheter?
(See fact sheet for Clinical question XXXI in electronic appendices)
| Summary of evidence | |
| The evidence available comes from clinical series, with a limited number of patients who have been treated by antimicrobial lock and systemic antibiotic therapy. Cure rates are around 50% for bacteraemia by S. aureus, with serious complications being described in almost 10%. There is a small number of cases described in the literature for Pseudomonas spp., with varying results but there is no experience for Candida spp. | Low quality |
| In patients with CVC-related bacteraemia secondary to infection with S. aureus, Pseudomonas spp. or Candida spp., treated with antimicrobial lock, no published study has been found directly comparing the results of immediate CVC withdrawal versus its preservation | |
| Therefore, authors of the largest clinical series and the guidelines of the Infectious Disease Society of America (IDSA) recommend CVC withdrawal in these cases | |
Evidence synthesis development
Saxena and Panhotra’s review929 showed that the aetiology of CVC-related bacteraemias in HD ranged from 21.9% and 60% for S. aureus and between 2.3% and 15.2% for Pseudomonas aeruginosa, without providing data on Candida.
At present, it is considered that withdrawal of infected CVC is not always necessary for proper diagnosis and treatment. CVC lumen lock with a highly concentrated antibiotic solution is known as antimicrobial or antibiotic lock therapy and is considered a good therapeutic option for the treatment of CVC infection, although it is unknown whether its usefulness and safety depend on the isolated micro-organism.
Maya930 points out that most treated cases of CVC-associated bacteraemia are resolved without major complications, but depending on the patient’s clinical situation and the micro-organism involved, there is a high risk that up to 20% of infected CVC embolise micro-organisms to remote sites, including heart valves, bones, joints, epidural space, subcutaneous tissue amongst others.
This review930 has located an RCT comparing antimicrobial lock with placebo, both associated with parenteral antimicrobial therapy, in the treatment of long-term CVC-related bacteraemia. It includes 46 patients, of which only one had an infection with S. aureus and none with Pseudomonas spp. or Candida spp. Although those treated with antimicrobial lock had better results than those treated with placebo, the differences were not statistically significant in relation to treatment failure: failure to cure bacteraemia in 33% (7 of 21) of the antimicrobial lock group versus 57% (13 of 23) in the placebo group (p = 0.10); recurring bacteraemia with the same strain in 3 of 31 of the antimicrobial lock group versus 9 of 23 of those treated with placebo (p = 0.06).
The guide made by the IDSA833 indicates that CVC for HD must be removed if there is a bacteraemia complicated by severe sepsis (haemodynamic instability), osteomyelitis, endocarditis, suppurative thrombophlebitis or persistent positive blood cultures 72 h after appropriate antibiotic therapy. In uncomplicated bacteraemia caused by coagulase-negative staphylococci or gram-negative bacilli, they suggest that an attempt can be made to treat with intravenous antibiotics and antimicrobial lock for 2 weeks without CVC withdrawal. However, if the micro-organism involved is S. aureus, P. aeruginosa or fungi, they do not recommend antibiotic lock.
Bacteraemia secondary to infection with Staphylococcus aureus
The review of Fitzgibbons et al.893 indicates that the incidence of CVC bacteraemia in patients on HD would be in the range of 7.6 to 14.4 cases per 100 days of CVC use, with S. aureus being the pathogen responsible for 56% of cases. This review gathers the data of three observational studies853,854,893 that show that the combined use of systemic antibiotic therapy and antibiotic lock achieved CVC preservation rates of between 40% and 55% for infections with S. aureus.
Fitzgibbons et al.893 consider that CVC withdrawal is the best strategy for dealing with Staphylococcus aureus bacteraemia in patients on HD dialysed by CVC.
The case series of Fernandez-Hidalgo et al.899 included 115 patients with long-term CVC-related bacteraemia, of which only 37 were patients in HD. In 20 patients bacteraemia was secondary to S. aureus, and combined treatment with systemic therapy and antibiotic lock failed in 9 cases (7 with catheter for HD and 2 for chemotherapy).
The prospective case series of Maya et al.853 analysed 113 patients with bacteraemia by S. aureus secondary to the CVC for HD. All were treated by systemic antibiotic therapy and antibiotic lock. 40.7% of patients (46 of 113) were cured and the CVC was removed in 67 patients, in 40 because of persistent fever and in 27 because of recurrent bacteraemia. In 9.7% of patients (11 of 113) there were serious complications of bacteraemia. They conclude that systemic therapy with antibiotic lock is not appropriate if the micro-organism involved is S. aureus.
The case series of Poole et al.854 included 10 cases of S. aureus bacteraemia, and all were treated systemically and with antibiotic lock. Treatment only was successful in 4 patients (40%).
The study of Joshi and Hart900 included 7 cases of S. aureus bacteraemia, and all were treated systemically and by antibiotic lock. In most cases the catheter had to be removed to resolve the infection regardless of whether it was MRSA or MSSA.
The case series of Krishnasami et al.855 included 2 cases of bacteraemia by Staphylococcus aureus, treated systemically and with antibiotic lock. Treatment failed to eradicate the infection in all cases and required the withdrawal of the CVC.
The study of Capdevila et al.864 included 2 cases of bacteraemia by Staphylococcus aureus, sensitive to antibiotics, treated systemically and with antibiotic lock. In both cases the infection was controlled and CVC preserved.
Bacteraemia secondary to infection with Pseudomonas
The case series of Fernandez-Hidalgo et al.899 included 115 patients with long-term CVC-related bacteraemia, of which only 37 were HD patients.
In 5 of the cases bacteraemia was due to Pseudomonas, but they do not detail how many were patients on HD. Combined treatment with systemic therapy and antibiotic lock failed in one case.
The case series of Capdevila et al.864 included 5 cases of P. aeruginosa bacteraemia sensitive to antibiotics, treated systemically and with antibiotic lock. In all cases the infection was controlled and the CVC preserved.
The clinical series of Joshi and Hart900 included 2 cases of bacteraemia by Pseudomonas, treated systemically and with antibiotic lock. In both cases the treatment failed to eradicate the infection and required withdrawal of the CVC.
Bacteraemia secondary to infection with Candida
There are no published studies that provide results of antibiotic lock in cases of CVC-related candidemia. In the case of candidemia in CVC for HD, it seems reasonable to remove the CVC.833,899,931
From evidence to recommendation
No published study has been found directly comparing the results of immediate CVC withdrawal versus antibiotic lock in patients with catheter-related bacteraemia secondary to infection with S. aureus, Pseudomonas spp. or Candida spp.
Clinical studies with a limited number of patients, treated with antimicrobial lock in an attempt to preserve a functioning CVC, have shown low cure rates for CRB secondary to S. aureus, also associated with severe complications. These findings prompt the idea that attempting to recover CRB-infected catheter, can lead to significant rates of treatment failure and severe complications, depending on aetiology (difficult-to-treat micro-organisms like S. aureus, Pseudomonas spp. and Candida spp.).
It would therefore be recommendable to withdraw TCVC in cases where these germs are isolated.
→ Clinical question XXXII Should empirical antibiotic treatment to cover gram-positive bacteraemia in haemodialysis patients who are tunnelled central venous catheter carriers initially be started with cefazolin (vancomycin if MRSA level > 10%) or daptomycin, associated with the treatment for gram-negatives, when the catheter is preserved?
(See fact sheet for Clinical question XXXII in electronic appendices)
| Summary of evidence | |
| No comparative studies have been identified comparing these empirical antibiotic treatment strategies for CRB in HD | |
| The evidence comes from guidelines of professional organisations, which take into account the experience in different healthcare centres, and show how important it is to adapt empirical treatment to the epidemiological conditions of the bacteraemias in each specific HD unit, as well as the sensitivity and resistance of its usual germs | |
| Empirical treatment would involve acting before knowing which micro-organisms are involved, choosing the antibiotic depending on the epidemiology in each unit, taking into account the sensitivity and resistance of its habitual micro-organisms, the patient’s risk factors (previous colonisation of the patient by some micro-organism and/or their state of immunity) and the severity of the infection | |
Evidence synthesis development
The guide of the SEIMC (Spanish Society of Infectious Diseases and Clinical Microbiology)932 indicates that the aetiology and sensitivity pattern of nosocomial bacteraemias shows large differences between centres, so that knowledge of local epidemiology is essential for the selection of empirical antimicrobial treatment. Because aetiology of methicillin-sensitive S. aureus and MRSA CRB in HD patients is highly prevalent, the guide considers that vancomycin is the empirical treatment of choice. In severe sepsis or septic shock, it suggests replacing vancomycin with daptomycin and extending the coverage against gram-negative bacilli, including P. aeruginosa.
In 2008, a guideline for the management of MRSA infection was published,933 prepared by representatives of the SEQ (Spanish Society of Chemotherapy), SEMI (Spanish Society of Internal Medicine), SEMICYUC (Spanish Society of Intensive Care Medicine, Critical and Coronary Units), AEC (Spanish Association of Surgeons) and SEHH (Spanish Society of Haematology and Haemotherapy). In this guideline it is proposed that the use of vancomycin as an initial empirical therapy for a severe infection is not advisable when MIC for vancomycin is ≥ 1.5 μg/mL. Risk situations could be patients who have received vancomycin during the previous month or in-centre nosocomial infection where the prevalence of such strains is greater than 10% of those isolated.
The European Renal Best Practice (ERBP)741 recommends that:
- •
HD units must record all the details of the epidemiology of bacteraemias related to catheter use, as well as all episodes of bacteraemia (events, causative organisms with their susceptibility and the evolution in response to treatment).
- •
In general, antibiotics only requiring post-dialysis administration (vancomycin, teicoplanin, cefazolin, ceftazidime, daptomycin) should be preferred.
- •
Vancomycin or teicoplanin as first choice for the empirical treatment of gram-positives where MRSA is highly prevalent.
The NHS (Nottingham University Hospitals) guide934 recommends the use of vancomycin and gentamicin as empirical antibiotics.
The IDSA guide833 recommends vancomycin for the empirical treatment of bacteraemias in healthcare settings with a high prevalence of MRSA infections. For the departments in which MRSA cultures show mostly MIC values of vancomycin > 2 μg/mL or there is an allergy to vancomycin, they propose the use of daptomycin.
Lock and Mokrzycki670 propose using vancomycin or teicoplanin for empirical treatment due to the high prevalence of MRSA in HD units. In cases where MIC of vancomycin is > 2 μg/mL, they propose the use of daptomycin.
From evidence to recommendation
As there are no studies that compare different strategies in the empirical treatment of CRB in HD, evidence comes from clinical guidelines following the adaptation of the empirical treatment to the epidemiology of each HD unit and, in particular to the sensitivity and resistance of the usual germs.
In our setting, SEIMC considers that the prevalence of MRSA and methicillin-sensitive staphylococcus is high in the HD patient, which is why vancomycin should be considered the treatment of choice, suggesting that, in case of severe sepsis, it be replaced with daptomycin and extended to gram-negative bacilli, including pseudomone. At the same time, the multidisciplinary guide of MRSA treatment advises against vancomycin in severe cases when MIC is ≥ 1.5 μg/mL.
In the same environment, in the case of high MRSA prevalence for empiric treatment of gram positives, the European Guidelines (ERBP) and the IDSA guide also recommend vancomycin as the first option. The latter also proposes daptomycin in case of MRSA with vancomycin MIC > 2 μg/mL or in case of allergy.
Therefore, GEMAV recommends first empirically covering gram-positives and gram-negatives based on the epidemiology of each HD unit, suggesting vancomycin as first choice for the empirical treatment of gram-positive micro-organisms and using daptomycin for MRSA with MIC ≥ 1.5 μg/mL, in patients with septic shock or with a known allergy to vancomycin.
Clinical question XXXII. Recommendations
R 6.9.9) We recommend that antimicrobial agents with activity against gram-positive and gram-negative micro-organisms be included in the empirical choice of antibiotics, depending on the epidemiology of each dialysis unit, sensitivity and resistance patterns of their usual micro-organisms, patient risk factors and severity of infection
R 6.9.10) We suggest vancomycin be used as the first choice for the empirical treatment of gram-positive micro-organisms in haemodialysis units
R 6.9.11) We suggest daptomycin be used for the empirical treatment of catheter-related bacteraemia in haemodialysis units in which methicillin-resistant Staphylococcus aureus cultures show values of minimum inhibitory concentration of vancomycin ≥ 1.5 µg/mL, in patients with septic shock or with a known allergy to vancomycin
→ Clinical question XXXIII Does the detection and eradication of Staphylococcus aureus in nasal carriers reduce episodes of catheter-related bacteraemia? Is it cost-effective?
(See fact sheet for Clinical question XXXIII in electronic appendices)
| Summary of evidence | |
| A meta-analysis of 8 RCTs, with only one of them in HD patients, find that treating S. aureus carriers with intranasal mupirocin is associated with a lower rate of nosocomial infection by S. aureus, but is accompanied by an increase in the rate of infection caused by organisms other than S. aureus | Moderate quality |
| An RCT that analysed treatment with oral rifampicin versus no treatment found a lower rate of infection, but also a high rate of colonisation recurrence by S. aureus at three months and the development of strains resistant to rifampicin | Moderate quality |
Evidence synthesis development
The available evidence from RCTs only addresses findings related to bacteraemias, without providing information on mortality, hospital admissions or antibacterial resistances. A Cochrane review that analyses the significance of local treatment with mupirocin in nasal carriers935 to prevent S. aureus bacteraemia has been found.
Nasal mupirocin
The Cochrane review of Van Rijen et al.935 in 2008 analysed the effectiveness of nasal mupirocin in the prevention of Staphylococcus aureus infections in nasal carriers. Nine RCTs were found with 3396 patients with a high clinical heterogeneity among patients in the different studies: patients on HD, peritoneal dialysis, surgical and non-surgical patients.
Rate of infection by Staphylococcus aureus
The meta-analysis of the 8 studies, with 3374 participants, which compared mupirocin with placebo or with no treatment, found a statistically significant reduction of S. aureus infection in those treated with intranasal mupirocin (RR: 0.55; 95% CI, 0.43-0.70).
Rate of infection caused by organisms other than Staphylococcus aureus
The meta-analysis of 3 studies, with 1393 patients, finds a significantly higher rate of infection by microorganisms other than Staphylococcus aureus in patients treated with mupirocin than in the placebo group (RR: 1.38; 95% CI, 1.12-1.72).
Mortality
The meta-analysis of 5 studies, with 2161 patients, found no statistically significant differences between those treated with mupirocin or placebo (RR: 0.91; 95% CI, 0.64-1.31).
The only RCT in HD patients in this review, with 17 patients treated with nasal mupirocin and 18 with placebo treated three times a week for 9 months, found fewer infections in patients treated with mupirocin, but the difference was not statistically significant (RR: 0.18; 95% CI, 0.02-1.32). Analysis of the incidence of bacteraemia did not show any differences.
Oral rifampicin plus intranasal bacitracin
Several published reviews locate a single RCT on HD patients who are nasal carriers of S. aureus that compared non-treated versus twice-weekly treatment with 600 mg of oral rifampicin, plus intranasal bacitracin four times a day for one week, repeated every three months.936 They found a lower rate of infections in those treated actively: 2 of 18 (11%) versus 12 of 26 (46%) (RR: 0.24; 95% CI, 0.06-0.95; p = 0.02).
Barraclough et al.937 mention that rifampicin-resistant strains were identified in this study, thereby limiting the application of this intervention. They commented that the study also analysed whether rifampicin use for a week was effective in eradicating carrier state and indicate that they found a high recurrence rate of Staphylococcus aureus colonisation at 3 months.
From evidence to recommendation
There is not enough evidence to recommend the systemic detection and treatment with local or systemic antibiotic to eradicate Staphylococcus aureus in patients undergoing HD.
Difficulties of diagnosing central venous catheter-related bacteraemia in the dialysis population
Alternative method for diagnosing central venous catheter-related bacteraemia in patients undergoing haemodialysis: extraction of blood cultures through the central venous catheter lumens or through the extracorporeal haemodialysis circuit line
CRB must be suspected when a HD patient with a CVC presents signs or symptoms of bacteraemia, particularly in the absence of another focus of infection. As previously mentioned, in the face of this suspicion, blood cultures should be drawn through a peripheral vein and through the CVC lumens, or 2 peripheral blood cultures should be taken at two different locations or two separate samples taken 10 to 15 min apart.
The definitive diagnosis of bacteraemia requires one of the following criteria:
- •
Positive blood cultures with the same micro-organism in the peripheral samples and CVC, with a colony count 3:1 times higher in the CVC or a differential time growth greater than 120 min.
- •
Culture of the same micro-organism in the tip of the CVC and in at least one peripheral blood culture.
- •
Cultures of the same micro-organism from two peripheral blood samples and absence of another focus of infection.
In the population of HD patients, the diagnosis criteria for CRB are limited, for a variety of reasons. In over 40% of patients, it is not possible to obtain blood samples for culture, either due to the difficulty in accessing peripheral veins or due to the need to preserve them for nAVF or pAVF creation.833,844,875 Likewise, dialysis is carried out on an outpatient basis and, in this context, it is more difficult to achieve an absence of significant variations in transport times or blood sample temperature until incubation in the microbiology laboratory.844 Very often, symptoms or signs of CRB occur during the HD session, when blood from the patient has already circulated through the extracorporeal circuit lines and CVC lumens. In this scenario, it is highly likely that the quantitative differential of colonies between the samples obtained through a peripheral vein and those extracted from the CVC or extracorporeal circuit line will not be maintained. Therefore, the interruption of the HD session, with line disconnection manoeuvres to obtain blood cultures through the CVC, may not be justified due to manipulation and coagulation risks in the dialysis circuit. There may also be the loss of differential in the number of colonies in relation to the peripheral blood that indicated whether the origin of the bacteraemia is the CVC.938
The recommended protocol for obtaining blood culture samples in HD patients with CVC should be applied when the suspicion appears in the interdialytic period. However, when it occurs during the HD session, given the difficulty in extracting samples, GEMAV considers the extraction of two blood samples acceptable, taken 10-15 min apart, through the arterial line of the extracorporeal circuit without the need to interrupt the dialysis session. In these cases and when it is not possible to obtain blood cultures by peripheral vein needling, CRB diagnosis should be considered when blood cultures are positive, in symptomatic patients and without evidence of an alternative source of infection.
7Quality indicatorsIntroduction
Although many aspects of renal replacement therapy for stage 5 chronic kidney disease (CKD) have been standardised in internationally accepted guidelines,6,10,13-15,30,939,940 the literature shows that there are significant differences in the degree of compliance with the standards proposed, both among centres and among different countries.941-943
One of the current challenges is to reduce variability in the assistance offered. Although some differences could be justified by the different demographic characteristics of the patients dialysed at the centres, there are others that are related to the different means of action taken.944
Systematic and planned measurement of quality indicators has demonstrated that it helps improve control over patients and outcomes of the treatments applied, as it enables professionals to be aware of their situation, introduce improvement activities and check effectiveness in a systematic and continued way.945 The real rationale for trying to ensure compliance with these indicators is the recent evidence proving that if a combination of them is attained (anaemia, dialysis dose, calcium-phosphorus metabolism, albumin or type of vascular access), it has an impact on patient survival, morbidity and costs.946,947
As discussed throughout the Guide, the presence of a central venous catheter (CVC) for haemodialysis (HD) compared to a native arteriovenous fistula (nAVF) is associated with higher morbimortality and cost.95,630,843,948 For this reason, one quality objective is to restrict the existing CVC rate as far as possible.216
One of the factors that is certainly influencing this increase in the percentage of CVC in HD patients is patient related factor (older and higher prevalence of diabetes mellitus and cardiovascular comorbidities), causing medical and surgical contraindications for nAVF creation,216,667,948 but this is not the only one. Provider related factor is another of the aspects that influence these results and where work can be done to make improvements.216 Several studies have shown differences between different HD units in the distribution of vascular access (VA) type in incident and prevalent patients.278,664,949 These differences are dependent on a variety of factors: existence of a structured advanced chronic kidney disease (ACKD) outpatient clinic, deployment of VA monitoring programmes, attitude of the different departments involved, extent of involvement and coordination between them, nephrological follow-up time and existence of multidisciplinary team, among others.216
There are two key points for quality monitoring: the selection and construction of good indicators and the design of adequate and efficient control plans. The definition of quality indicators and monitoring system in this section aims to identify the existence of problematic situations which need to be assessed or on which work needs to be done.
Indicators by sections
1Procedures prior to vascular access creationIndicator 1.1
Percentage of patients followed up in the advanced chronic kidney disease outpatient clinic who fulfil the established criteria for referral to arteriovenous fistula (AVF) creation and are referred to surgery
Type of indicator
PROCESS indicator.
Definition of terms Criteria for AVF referral.
We suggest that referral time for AVF creation, provided the patient does not refuse, is when the glomerular filtrate rate (GFR) is less than 15 mL/min/1.73 m2 (validated standard by gender, age and body surface area) and/or a progressive decrease is observed in this rate and entry into HD is predicted within 6 months. Applicable to patients with no contraindication to AVF creation (see section 1: “Procedures prior to vascular access creation”).
Rationale
- •
The indicator would express the quality of patient assessment when followed up in the ACKD outpatient clinic to determine whether they are referred for AVF creation in accordance with the agreed criteria.
- •
This indicator assumes AVF is the first option for these patients.
Population to which it applies
All patients in pre-dialysis stage seen in the ACKD outpatient clinic.
Data sources
Patient clinical records.
Formula
- •
Numerator: patients followed up in the ACKD clinic who meet criteria for AVF creation referred to surgery during a 1-year period × 100.
- •
Denominator: patients followed up in the ACKD outpatient clinic who meet criteria for referral for AVF creation during a 1-year period.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: 90%.
Checklist. Percentage of patients followed up in the ACKD clinic who meet the referral criteria and referred to surgery for assessment.
If the patient meets criteria 1, 2, 3 and does not meet criteria 4 and 5, they should be referred for assessment by surgery.
If the patient does not meet any of criteria 1, 2 and 3 and fulfils either criteria 4 and 5, they should not be referred to surgery.
Exceptions
Patients with a theoretical indication for AVF referral, who were given a better VA indication for other reasons. These patients should be excluded both from the numerator and the denominator; for example: a patient who is going to receive a living donor transplant.
Comment
As there is no evidence on this indicator, the standard has been established by consensus within Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Indicator 1.2
Percentage of patients with indication for arteriovenous fistula creation referred to surgical outpatient clinic, whose intervention is performed within the recommended time
Type of indicator
PROCESS indicator.
Period recommended for access creation
- •
Scheduled referral: within 3 months.
- •
Preferential referral: within 6 weeks after consultation.
- •
Priority referral: within 2 weeks.
- •
Urgent referral: within 48 h.
Rationale
To assess the response of the surgery department in AVF creation.
Population to which it applies
Patients referred from ACKD outpatient clinic to surgical outpatient clinic to perform AVF and considered candidates for AVF creation.
Sources of data
Patient clinical records.
Formula
1. Scheduled referral.
- •
Numerator: number of patients referred to surgical outpatient clinic for scheduled AVF surgery performed within 3 months × 100.
- •
Denominator: total number of patients referred to surgical outpatient clinic for scheduled AVF creation.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: 90%.
Checklist. Continuous measurement. Percentage of patients referred for scheduled assessment, who fulfil the established criteria for AVF creation and have surgery within 3 months.
If the patient meets any of criteria 1 to 3, the surgeon should decide if they are considered suitable for access creation, taking the necessary precautions in each case.
2. Preferred referral.
- •
Numerator: number of patients referred to surgical outpatient clinic to perform AVF on a preferential basis on whom AVF is performed within 6 weeks × 100.
- •
Denominator: total number of patients referred to surgical outpatient clinic for AVF creation on a preferential basis.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: 90%.
Checklist. Percentage of patients referred for preferred assessment, who fulfil the established criteria for AVF creation and have surgery within 6 weeks.
If the patient meets any of criteria 1 to 3, the surgeon should decide if they are considered suitable for access creation, taking the necessary precautions in each case.
3. Priority referral.
- •
Numerator: number of patients referred to surgical outpatient clinic for priority AVF creation on which surgery is performed within 2 weeks × 100.
- •
Denominator: total number of patients referred to surgical outpatient clinic for priority AVF creation.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: 90%.
Checklist. Percentage of patients with priority referral for assessment, who fulfil all the established criteria for AVF creation and have surgery within 2 weeks.
If the patient meets any of criteria 1 to 3, the surgeon should decide if they are considered suitable for access creation, taking the necessary precautions in each case.
4. Urgent referral
- •
Numerator: number of patients referred to surgical outpatient clinic for AVF creation and accepted for AVF creation urgently, on whom surgery is performed within 48 h × 100.
- •
Denominator: total number of patients referred urgently to the surgical outpatient clinic for AVF creation.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: 90%.
Checklist. Percentage of patients urgently referred for assessment, who fulfil the established criteria for AVF creation and have surgery within 48 h.
If the patient meets any of criteria 1 to 3, the surgeon should decide if they are considered suitable for access creation, taking the necessary precautions in each case.
Comment
As there is no evidence on this indicator, the standard has been established by consensus within GEMAV.
2Vascular access creationIndicator 2.1
Patients with early fistula failure within the first 30 days following the procedure
Type of indicator
OUTCOME indicator.
Definition of terms
Early failure. Early VA failure is considered when patency fails within 30 days following creation.
Rationale
It is assumed that early failure (first 30 days) occurs due to technical problems from surgery or the selection of inappropriate vessels. Intimal hyperplasia is not considered to have any significant impact in terms of failure of the surgical technique.10
Population to which it applies
Patients with CKD who have undergone surgery to create AVF.
Sources of data
Patient clinical records.
Formula
- 1.
Radiocephalic nAVF
- •
Numerator: procedures with radiocephalic nAVF thrombosed at 30 days × 100.
- •
Denominator: total number of patients who undergo radiocephalic nAVF creation.
- •
Units: percentage
- •
Periodicity: annual.
- •
Standard: < 35%.
- •
- 2.
Proximal nAVF using brachial artery (brachiomedian, brachiocephalic or brachiobasilic and brachiobrachial).
- •
Numerator: procedures with proximal nAVF thrombosed at 30 days × 100.
- •
Denominator: total number of patients who undergo proximal nAVF creation.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: < 25%.
- •
- 3.
Prosthetic AVF (pAVF)
- •
Numerator: procedures with pAVF thrombosed at 30 days × 100.
- •
Denominator: total number of patients who undergo pAVF creation.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: < 15%.
- •
Comments
A recent meta-analysis94 has reported an overall frequency of primary failure of 23%, with a distribution of 28% in radiocephalic nAVF and 20% in nAVF at the proximal level in the elbow. The percentage of primary failure described in pAVF ranges from 5% to 15% depending on the type and location of the prosthesis in forearm and arm, respectively.10 However, there is no uniformity in the studies included with respect to the variables studied, with the delay in maturation often included in the concept of primary failure.
For this reason, GEMAV has decided to propose standards using the aforementioned meta-analysis as a basis, adding a “correction factor” in the case of nAVF, in order to promote their use versus other vascular accesses, coinciding with the lines of action proposed in other best practice guidelines.10
Indicator 2.2
Percentage of patients being followed up in the advanced chronic kidney disease outpatient clinic for at least 6 months and who start haemodialysis with mature arteriovenous fistula
Type of indicator
OUTCOME indicator.
Definition of terms
- •
Pre-dialysis follow-up in the ACKD outpatient clinic. Patients assessed by the multidisciplinary team.
- •
Incident patients. Type of VA used to start first HD session.
- •
Mature fistula. Developed AVF, nAVF, pAVF, suitable for cannulation with 2 needles and initiation of HD treatment.
Rationale
It is assumed that the VA of choice is AVF and that adequate clinical assessment allows AVF candidate patients to be selected. Although not all patients will be able to undergo AVF creation, it is important that no patient is indicated for a vascular access alternative without having had prior multidisciplinary follow-up.
Population to which it applies
Patients with CKD who are assessed in the ACKD outpatient clinic for at least 6 months.
Sources of data
Patient clinical records.
Formula
- •
Numerator: patients followed up in ACKD outpatient clinic for a minimum of 6 months starting HD with a mature AVF × 100.
- •
Denominator: patients being followed up at ACKD outpatient clinic for a minimum of 6 months.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: ≥ 75%.
Comments
Although other guidelines such as the UK Association13 or NICE guidelines939 indicate an objective of 65%, these guides either do not establish a minimum for pre-dialysis follow-up or the period they establish is lower (3 months).
GEMAV has agreed by consensus to establish a higher percentage for this standard and a longer follow-up period. Six months allows sufficient time for the incident patient to carry out the first HD session through nAVF or pAVF, bearing in mind that, during this pre-dialysis period, a salvage intervention may be needed in the event of thrombosis, or an elective intervention in the case of insufficient maturation. Moreover, GEMAV considers that, in addition to the current CKD patient’s demographic and clinical profile, organisational factors greatly affect the percentage of these patients who start HD using AVF. In this sense, a recent national epidemiological study of almost 10,000 incident HD patients using data from the Renal Disease Registry of Catalonia (RMRC) showed that approximately 50% of patients with CKD started HD treatment through an AVF each year in Catalonia during the period 2000-2011.667 According to the authors of this study, this rate may increase and have an impact on various organisational aspects because: a) if the increase in CKD patients’ age and comorbidity observed in recent years were decisive, the percentage of AVF as first VA should have gradually decreased in Catalonia but, in contrast, it has remained steady over time, and b) during the first year of follow-up in HD programme, most patients who started HD using a CVC were already being dialysed using an AVF; in other words, it would have been technically possible to create an AVF in these patients in the pre-dialysis period.667
Indicator 2.3
Percentage of arteriovenous fistulae with early clinical monitoring (4 weeks after creation)
Type of indicator
PROCESS indicator.
Definition of terms
Non-mature nAVF. An nAVF which fails to meet the criteria for maturation 4 weeks after creation (see section 3 “Arteriovenous fistula care”).
Rationale
It is estimated that, once created, between 28% and 53% of AVF do not mature enough for use in HD. Different studies have shown the usefulness of elective treatment in cases with alteration in access maturation. The identification of candidates for non-mature fistula is important to ensure VA patency. Therefore, as outlined in section 5, a clinical check-up is recommended 4 weeks after creating any AVF to detect delayed or non-mature fistulae and suggest early treatment if necessary. It is recommended that suspected lesions be confirmed by Doppler ultrasound (DU).
Population to which it applies
Newly created AVF.
Sources of data
Patient clinical records.
Formula
- •
Numerator: AVF created in the HD unit or the ACKD outpatient clinic, reviewed 4 weeks after creation × 100.
- •
Denominator: All nAVF created in the HD unit or ACKD outpatient clinic.
- •
Units: Percentage.
- •
Periodicity: annual.
- •
Standard: 100%.
Comments
Most of the haemodynamic and morphological changes following AVF creation occur during the first 2-4 weeks. After this time, in cases of non-mature AVF, there is a gradual reduction in flow, so a clinical check-up is recommended within a maximum of 4-6 weeks, to detect cases with access maturation changes. Early treatment of underlying lesions may increase the probability of the access maturing by 47%. An action is recommended to be taken where indicated.520
A late review (2-3 months) prevents early diagnosis and treatment. Therefore, the review 4 weeks after the surgical intervention is considered to be an indicator of quality of the VA care process.
In the absence of bibliographical references, the standard has been established by consensus within GEMAV.
3Arteriovenous fistula careIndicator 3.1
Percentage of patients with a graphic record of cannulation areas during haemodialysis sessions
Type of indicator
PROCESS indicator
Definition of terms
Graphic record of cannulation areas. This is a diagram of the AVF limb with a picture of the AVF and the cannulation areas.
Rationale
A full and detailed AVF examination and a record of the cannulation areas are required in each HD session. For this purpose, an AVF map with the cannulation areas in the patient’s medical record is extremely useful.
Population to which it applies
Prevalent HD patients and functioning AVF.
Sources of data
Patient clinical records.
Formula
- •
Numerator: Number of HD patients with recorded cannulation areas × 100.
- •
Denominator: total number of HD patients undergoing AVF cannulation.
- •
Units: percentage.
- •
Periodicity: quarterly.
- •
Standard: 100%.
Comments
The correct technique for cannulating the AVF prolongs its average life. The cannulation technique used, recent cannulation areas and problems encountered should be known for each patient as a control system for complications.
- •
The existence of a record of this information will help to understand the situation and improve the care provided to patients.
- •
The existence of a record per patient is a quality indicator of the care provided.
In the absence of evidence, the standard has been established by consensus within GEMAV.
4Monitoring and surveillance of arteriovenous fistulaIndicator 4.1
Percentage of prevalent patients on HD programme for more than 3 months who, on 31 December of the study year, are being dialysed through a native arteriovenous fistula
Type of indicator
OUTCOME indicator.
Rationale
This indicates the degree of implementation of structured AVF follow-up programmes in each HD unit (see section 4: “Monitoring and surveillance of arteriovenous fistula”).
Population to which it applies
Prevalent patients who are being dialysed in the HD unit on 31 December of the study year.
Sources of data
Patient clinical records
Formula
- •
Numerator: Number of patients in HD programme being dialysed through a functioning nAVF on 31 December of the study year × 100.
- •
Denominator: Number of patients in HD programme with more than 3 months on 31 December of the year in progress.
- •
Units: Percentage.
- •
Periodicity: annual.
- •
Standard: ≥ 75%.
Comment
The different published guidelines to date establish a variable percentage of between 60% and 85% for prevalent patients dialysed through an nAVF as an objective.6,10,13,15,939
In Spain, as an orientation, the results of the multicentre study carried out by the S.E.N (Spanish Society of Nephrology) published in 2008 show that the median percentage of nAVF in HD centres is 50% (25th percentile: 34.5% and 75th percentile: 61.2%).664 In other studies like those conducted by the Community of Madrid, Canary Islands and Catalonia, nAVF rate in prevalent patients is 58.6%, 64% and 73.3%, respectively.278,950,951 According to the DOPPS 5 study (2013-2014), this percentage is 65% for the overall population of Spain.32 Considering this background, the standard has been established by GEMAV at a minimum of 75%.
Indicator 4.2
Percentage of prevalent patients in HD programme for more than 3 months who, on 31 December of the study year, are being dialysed through a tunnelled central venous catheter
Type of indicator
OUTCOME indicator.
Rationale
This indicates the degree of implementation of structured VA follow-up programmes in HD units (see section 4: “Monitoring and surveillance of arteriovenous fistula”).
Population to which it applies
Prevalent patients who are being dialysed in the HD unit on 31 December of the study year.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients in HD programme being dialysed through a Tunnelled CVC (TCVT) on 31 December of the study year × 100.
- •
Denominator: number of patients in a HD programme for more than 3 months on 31 December of the year in progress.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: ≤ 20%.
Comment
Most guidelines published to date do not establish, as an objective, a maximum percentage of patients who must be dialysed through TCVC,13-15,30,939,940 with the exception of
the KDOQI 2006 guidelines and the S.E.N 2005 guidelines, which establish that a maximum of 10% of patients should be dialysed through TCVC.6,10 However, the number of patients who are dialysed through TCVC is much higher in many centres. For example, in Spain the Madrid Nephrology Society reports that among prevalent patients, 29.5% of patients are dialysed through TCVC278; a study published in the Canary Islands shows 33% with TCVC,950 and the latest results provided by the DOPPS 5 study show 29% of patients with TCVC.32 However, data from Catalonia put the percentage of prevalent patients being dialysed through TCVC at 14.3%.216 The standard has been established by consensus within GEMAV at ≤ 20%.
Indicator 4.3
Percentage of prevalent patients in HD programme for more than 3 months who, on 31 December of the study year, are being dialysed through a prosthetic arteriovenous fistula
Type of indicator
OUTCOME indicator.
Rationale
This indicates the degree of implementation of structured VA follow-up programmes in each HD unit (see section 4: “Monitoring and surveillance of arteriovenous fistula”).
Population to which it applies
All prevalent patients who are being dialysed in the HD unit on 31 December of the study year.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients in HD programme being dialysed through a pAVF on 31 December of the study year × 100.
- •
Denominator: number of patients in HD programme for more than 3 months on 31 December of the year in progress.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: none established (as it is complementary).
Comment
The clinical guidelines published to date do not establish any objective for pAVF in HD patients.6,10,13-15,30,939,940 As an orientation, the study by the Madrid Nephrology Society shows that in prevalent patients 11.9% are dialysed through pAVF278; the RMRC 2012, 4.8% and the DOPPS 5 study, 6%.32,951 In accordance with the recommendations made previously in this Guide, when nAVF cannot be performed in a patient, an attempt should be made to create pAVF before placing TCVC. With the same percentage of HD patients with nAVF, units with a higher percentage of pAVF than CVC are considered to have better clinical practice. This indicator is complementary to the previous two in order to reduce TCVC rate in prevalent HD patients. In the light of these facts, GEMAV has decided against establishing a standard for pAVF.
Indicator 4.4
Percentage of patients who are dialysed through a non-tunnelled central venous catheter for more than 2 weeks consecutively
Type of indicator
OUTCOME indicator.
Definition of terms
Non-tunnelled central venous catheter (NTCVC). Type of CVC for HD that is not located in a tunnel within subcutaneous tissue nor has an anchor (cuff) in it.
Rationale
A patient should not be dialysed for more than 2 weeks through NTCVC due to the increased risk of infection, venous thrombosis and central venous stenosis.
Population to which it applies
Prevalent patients who are dialysed in the HD unit through NTCVC.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients in HD programme being dialysed through NTCVC for more than 2 weeks × 100.
- •
Denominator: number of patients in HD programme being dialysed through NTCVC.
- •
Units: percentage.
- •
Periodicity: monthly.
- •
Standard: < 5%.
Comment
In the absence of evidence, the standard has been established by consensus within GEMAV.
Indicator 4.5
Annual rate of thrombosis in native arteriovenous fistula
Type of indicator
OUTCOME indicator.
Definition of terms
- •
Thrombosis. This is the functional loss of the nAVF, i.e. blood flow (QA) is 0 mL/min, which is reflected by the disappearance of the thrill and bruit in the physical examination (see section 4: “Monitoring and surveillance of arteriovenous fistula”).
- •
Patients/year at risk. Number of days that each patient is on a certain type of VA in the course of 1 year (maximum 365) divided by 365. Example: Patients with nAVF and number of dialysis days: patient A, 365 days, patient B, 200 days and patient C, 165 days; the sum is 730 days. If we divide 730 by 365, the number of patients/year at risk with nAVF is 2.
Rationale
This indicates the degree of implementation of structured VA follow-up programmes in each HD unit (see section 4: “Monitoring and surveillance of arteriovenous fistula”).
Population to which it applies
Prevalent nAVF carriers who are dialysed in the HD unit over the study year.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of nAVF thromboses during the study year.
- •
Denominator: total number of patients/year at risk with nAVF during the study year.
- •
Units: rate.
- •
Periodicity: annual.
- •
Standard: < 0.15 thrombosis patient/year.
Comment
Although clinical guidelines generally set out a maximum rate of nAVF thrombosis of 0.25,6,10,13,15,939 various publications in Spain show a much lower rate of thrombosis: between 0.03 and 0.10.227,272,278,952 For this reason, GEMAV has considered that this standard is not correctly proportioned and has decided to establish it at 0.15.
Indicator 4.6
Annual rate of thrombosis in prosthetic arteriovenous fistulae
Type of indicator
OUTCOME indicator.
Definition of terms
- •
Thrombosis. Same as indicator 4.5.
- •
Patients/year at risk. Same as indicator 4.5, applied to pAVF.
Rationale
This indicates the degree of implementation of structured VA follow-up programmes in each HD unit (see section 4: “Monitoring and surveillance of arteriovenous fistula”).
Population to which it applies
Prevalent pAVF patients who are dialysed in the HD unit over the study year.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of pAVF thromboses during the study year.
- •
Denominator: total number of patients/year at risk with pAVF during the study year.
- •
Units: rate.
- •
Periodicity: annual.
- •
Standard: < 0.50 thromboses/patient/year.
Comment
As indicated in section 4, second-generation surveillance methods are not predictive of pAVF thrombosis. Nonetheless, in a prospective study relating to AVF surveillance by periodic determinations of QA, pAVF thrombosis rate was 0.42 events per year.227 Previous publications in Spain, such as those of the Nephrology Society of Madrid, show similar data.278 Therefore, GEMAV has agreed that this standard is correctly proportioned and must remain at < 0.50, in accordance with other clinical practice guidelines.6,10,13,15,939
Indicator 4.7
Percentage of arteriovenous fistulae which have a record of regular assessments
Type of indicator
PROCESS indicator.
Definition of terms
- •
Thrombosed AVF. see previous definition.
- •
Regular assessments. Criteria for monitoring and surveillance have been followed in accordance with the methodology reported in section 4, for nAVF and pAVF.
Rationale
Systematic monitoring and surveillance of certain parameters which are indicators of AVF function, regular assessment and records allow early detection of their dysfunction. This early detection allows identification and elective correction of lesions, allowing the AVF to be repaired and the risk of thrombosis to be decreased.
Population to which it applies
All HD patients at the time of measurement.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of AVF which have a record of regular monitoring and surveillance throughout the year × 100.
- •
Denominator: all prevalent AVF (nAVF and pAVF) throughout 1 year.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 90%.
Comments
The analysis of use of monitoring and surveillance in patients on periodic HD allows the prevention of thrombosis related to the lack of control of the evolution of the AVF stenosis.
In the absence of bibliographical references, the standard has been established by consensus within GEMAV, following monitoring and surveillance criteria set out in the methodology described in section 4 for nAVF and pAVF.
Indicator 4.8
Percentage of incident patients with indwelling central venous catheter, with no contraindication for an arteriovenous fistula, who undergo arteriovenous fistula creation within 6 weeks following catheter placement
Type of indicator
SENTINEL indicator.
Rationale
This indicator assesses the efficacy of the multidisciplinary team in reducing the exposure time of the HD patient to CVC.
Population to which it applies
CKD patients starting HD through CVC.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of incident patients with no contraindication for AVF who start HD through CVC and have an AVF constructed within 6 weeks × 100.
- •
Denominator: number of incident patients with no contraindication for AVF who start HD treatment by CVC.
- •
Units: percentage.
- •
Periodicity: monthly.
- •
Standard: > 90%.
Exceptions
Patients with pathologies which suggest the possibility of renal function recovery and decide to continue with CVC, or who refuse to undergo AVF creation.
Comment
In the absence of bibliographical references, the standard has been established by consensus within GEMAV. It is considered that a patient starting HD with a CVC should have an AVF created at the earliest possible moment to minimise exposure time to CVC, for which a maximum of 6 weeks has been established.
5Complications of arteriovenous fistulaIndicator 5.1
Percentage of potentially recoverable thrombosed arteriovenous fistulae that are rescued after a year
Type of indicator
OUTCOME indicator.
Definition of terms
- •
AVF salvage. Flow restoration, following thrombosis, in an AVF which is potentially recoverable by thrombectomy and/or pharmaco-mechanical thrombolysis, and the performance of at least one HD session after the procedure if they are on renal replacement therapy (RRT).
- •
Potentially recoverable thrombosed AVF. Thrombosed AVF which merits salvage, given the characteristics of the patient and/or the AVF itself.
Rationale
To assess the outcome of surgery and/or vascular radiology in the event of thrombosis.
Population to which it applies
All prevalent patients with AVF who are dialysed in the HD unit over the study year or are in the ACKD outpatient clinic.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with potentially recoverable thrombosed AVF which are salvaged and have enough flow restored for them to be used in at least one HD session if the patient is in the RRT programme × 100.
- •
Denominator: number of patients with potentially recoverable thrombosed AVF.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 70%.
Comment
A study published in 2013 in Spain shows a percentage of salvaged pAVF of 80%.167 The standard has been established by consensus within GEMAV.
Indicator 5.2
Percentage of native arteriovenous fistulae with significant stenosis, non-thrombosed, surgically repaired, which remain patent after one year
Type of indicator
OUTCOME indicator.
Definition of terms
Significant stenosis. Reduction in vascular lumen > 50% shown by Doppler ultrasound in nAVF or pAVF with high risk of thrombosis according to the criteria set out in section 4, i.e. deserving elective or preventive treatment.
Rationale
To assess the efficacy of surgery in the treatment of significant stenosis in non-thrombosed nAVF, in the context of multidisciplinary management.
Population to which it applies
Prevalent nAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require surgical repair due to significant stenosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with nAVF which is still patent after surgical repair due to significant stenosis after 1 year follow-up × 100.
- •
Denominator: number of patients with nAVF who have a surgically repaired significant stenosis and 1-year follow-up.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 1 year.
Comment
Based on the traditional criterion of significant stenosis, KDOQI-2006 and Canadian guidelines suggest a standard > 50% at 1 year.10,15 Despite using a more restrictive criterion of stenosis, GEMAV has by consensus established the same standard.
Indicator 5.3
Percentage of native arteriovenous fistulae with significant stenosis, non-thrombosed, repaired endovascularly, which remain patent after 6 months.
Type of indicator
OUTCOME indicator.
Definition of terms
Significant stenosis. Similar to indicator 5.2.
Rationale
To assess the efficacy of endovascular treatment in the repair of significant stenosis in non-thrombosed nAVF, in the context of multidisciplinary management.
Population to which it applies
Prevalent nAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require endovascular repair due to significant stenosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with nAVF which is still patent after endovascular repair due to significant stenosis after 6 months of follow-up × 100.
- •
Denominator: number of patients with nAVF with significant stenosis repaired using interventional radiology techniques and who are followed up for 6 months.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 6 months.
Comment
Based on the traditional criterion of significant stenosis, KDOQI-2006 and Canadian guidelines suggest a standard > 50% at 6 months.10,15 Despite using a more restrictive criterion of stenosis, GEMAV has by consensus established the same standard.
Indicator 5.4
Percentage of native arteriovenous fistulae, thrombosed, surgically repaired, which remain patent after the year
Type of indicator
OUTCOME indicator.
Definition of terms
Thrombosis. Similar to previous indicators.
Rationale
To assess the efficacy of surgery in the treatment of thrombosed nAVF, in the context of multidisciplinary management.
Population to which it applies
Prevalent nAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require surgical salvage after thrombosis.
Sources of data
Patient clinical records
Formula
- •
Numerator: number of patients with nAVF that remains patent after surgery to repair due to thrombosis at 1 year of follow-up × 100.
- •
Denominator: number of patients with nAVF who have a surgically repaired thrombosis and have been followed up for 1 year.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 1 year.
Comments
KDOQI-2006 guidelines suggest a patency standard > 50% at 1 year; 2006 Canadian guidelines, 40% at 1 year; and the 2007 European guidelines, 80% at 1 year.10,14,15
GEMAV has decided by consensus to establish a patency rate > 50% at 1 year. However, there are studies showing that elective intervention on dysfunctional AVF increases length of patency in comparison to repair after thrombosis, as seen in section 5.272,273 Therefore, although GEMAV has decided by consensus to establish a patency rate similar to that of other guidelines, > 50% at one year, it is likely that this indicator is not adequately proportioned.
Indicator 5.5
Percentage of native arteriovenous fistulae, thrombosed, repaired endovascularly, which remain patent after 6 months
Type of indicator
OUTCOME indicator.
Definition of terms
Thrombosis. Similar to previous indicators.
Rationale
To assess the efficacy of endovascular procedures in the treatment of thrombosed nAVF, in the context of multidisciplinary management.
Population to which it applies
Prevalent nAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require endovascular repair after thrombosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with nAVF which remains patent after endovascular repair due to thrombosis after 6 months’ follow-up × 100.
- •
Denominator: number of patients with nAVF who have thrombosis repaired with interventional radiology techniques and have been followed up for 6 months.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 6 months.
Comments
KDOQI-2006 guidelines suggest a patency standard > 50% at 6 months; 2006 Canadian guidelines, 40% at 3 months; and the European guidelines, 50% at 1 year.10,14,15
GEMAV has decided by consensus to establish a patency rate of > 50% at 6 months.
Indicator 5.6
Percentage of prosthetic arteriovenous fistulae with significant stenosis, non-thrombosed, surgically repaired, which remain patent after a year
Type of indicator
OUTCOME indicator.
Definition of terms
Significant stenosis. Similar to indicator 5.2.
Rationale
To assess the efficacy of surgery in the treatment of significant stenosis in non-thrombosed pAVF, in the context of multidisciplinary management.
Population to which it applies
Prevalent pAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require surgical repair due to significant stenosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with pAVF which is still patent after surgical repair due to significant stenosis at 1 year of follow-up × 100.
- •
Denominator: number of patients with pAVF who present a surgically repaired significant stenosis and have been followed up for 1 year.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 1 year.
Comments
Based on the traditional criterion of significant stenosis, KDOQI-2006 and Canadian guidelines suggest a standard > 50% at 1 year.10,15
Despite using a more restrictive criterion of stenosis, GEMAV has by consensus established the same standard.
Indicator 5.7
Percentage of prosthetic arteriovenous fistulae with significant stenosis, non-thrombosed, repaired endovascularly, which remain patent at 6 months
Type of indicator
OUTCOME indicator.
Definition of terms
Significant stenosis. Similar to indicator 5.2.
Rationale
To assess the efficacy of endovascular treatment in the repair of significant stenosis in non-thrombosed pAVF, in the context of multidisciplinary management.
Population to which it applies
Prevalent pAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require endovascular repair due to significant stenosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with pAVF which is still patent after endovascular repair due to significant stenosis at 6 months of follow-up × 100.
- •
Denominator: number of patients with pAVF with significant stenosis repaired with interventional radiology techniques and who have been followed up for 6 months.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 6 months.
Comments
Based on the traditional criterion of significant stenosis, KDOQI-2006 and Canadian guidelines suggest a standard > 50% at 6 months.10,15
Despite using a more restrictive criterion of stenosis, GEMAV has by consensus established the same standard.
Indicator 5.8
Percentage of prosthetic arteriovenous fistulae, thrombosed, repaired surgically, which remain patent after 6 months
Type of indicator
OUTCOME indicator.
Definition of terms
Thrombosis. Similar to previous indicators.
Rationale
To assess the efficacy of surgery in the treatment of thrombosed pAVF.
Population to which it applies
Prevalent pAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require surgical rescue after a thrombosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with pAVF which remains patent after repair surgery due to thrombosis at 6 months of follow-up × 100.
- •
Denominator: number of patients with pAVF who have a surgically repaired thrombosis and have been followed up for at least 6 months.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 40% at 1 year or 50% at 6 months.
Comments
KDOQI-2006 and 2006 Canadian guidelines suggest a patency standard > 40% at 1 year or 50% at 6 months.10,15
GEMAV has decided by consensus to establish an indicator similar to these guidelines.
Indicator 5.9
Percentage of prosthetic arteriovenous fistulae, thrombosed, repaired endovascularly, which remain patent at 6 months
Type of indicator
OUTCOME indicator.
Definition of terms
Thrombosis. Similar to previous indicators.
Rationale
To assess the efficacy of endovascular procedures in the treatment of thrombosed pAVF.
Population to which it applies
Prevalent pAVF patients who are dialysed in the HD unit or are in the ACKD outpatient clinic over the study year and require endovascular repair after their thrombosis.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with pAVF which remains patent after endovascular repair due to thrombosis at 6 months of follow-up × 100.
- •
Denominator: number of patients with pAVF who have thrombosis repaired with interventional radiology techniques and have been followed up for at least 6 months.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: > 50% at 6 months.
Comments
KDOQI-2006 guidelines suggest a patency standard > 40% at 3 months; 2006 Canadian guidelines, 40% and the European guidelines, > 30%.10,14,15
GEMAV has decided by consensus to establish a patency rate > 50% at 6 months.
Indicator 5.10
Infection rate of native arteriovenous fistula
Type of indicator
OUTCOME indicator.
Definition of terms
- •
AVF infection. Presence of local inflammatory signs or suppuration at the AVF cannulation site, alone or associated with general symptomatology or fever, and blood cultures which are positive for the same micro-organism isolated from the exudate collected at the cannulated site.
- •
Patient/year at risk. Similar to indicator 4.5.
Rationale
This indicates the efficacy of the multidisciplinary team, especially nursing staff, in preventing and managing nAVF infection.
Population to which it applies
Prevalent patients with nAVF who are dialysed at the HD unit or are in the ACKD outpatient clinic throughout the year.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with nAVF who have an AVF infection during the study year.
- •
Denominator: total number of patients/year at risk with nAVF during the study year.
- •
Units: rate.
- •
Periodicity: annual.
- •
Standard: < 0.01 patient/year at risk.
Comments
The nAVF infection presents with a variety of objectives and forms of expression. KDOQI-2006 guidelines establish an infection objective < 1% in nAVF and < 10% in pAVF.10
Gruss et al. provide values between 0.86% and 8.13%, respectively.272 Other publications like that by Stevenson show a joint rate of infection for nAVF and pAVF of 2.53 infection episodes/1000 days.953 The Canadian guidelines15 establish an infection rate objective of 0.01 events per patient/year at risk for nAVF and 0.1 episode per patient/year for pAVF.
GEMAV considered the latter standard as it is the most suitable for assessing infections in nAVF.
Indicator 5.11
Prosthetic arteriovenous fistula infection rate
Type of indicator
OUTCOME indicator.
Definition of terms
Infection and patient/year at risk. Similar to previous indicators
Rationale
This indicates the efficacy of the multidisciplinary team, especially nursing staff, in preventing and managing pAVF infection.
Population to which it applies
Prevalent patients with pAVF who are dialysed in the HD unit or are in the ACKD outpatient clinic throughout the year.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of patients with pAVF who have an infection during the study year.
- •
Denominator: total number of patients/year at risk with pAVF during the study year.
- •
Units: rate.
- •
Periodicity: annual.
- •
Standard. < 0.1 patient/year at risk.
Comment
Comment similar to indicator 5.10.
6Central venous cathetersIndicator 6.1
Incidence density of tunnelled central venous catheter-related bacteraemia (number of tunnelled central venous catheter-related bacteraemias/1000 days of tunnelled central venous catheter use)
Type of indicator
OUTCOME indicator
Definition of terms
Bacteraemia. Isolation of the same micro-organism in peripheral blood and at the tip of the withdrawn catheter. If the catheter is not removed, isolation of the same micro-organism in at least 2 blood cultures (one through the CVC lumens and the other taken from a peripheral vein) and the diagnostic criteria are met for quantitative blood cultures or a positive differential time is calculated.
Rationale
A high rate of TCVC-related bacteraemia indicates poor adherence to universal asepsis measures.
Population to which it applies
HD unit patients with TCVC during the study period.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of catheter-related bacteraemias in TCVC patients in one year × 1000.
- •
Denominator: total number of days with TCVC in 1 year.
- •
Units: incidence density.
- •
Periodicity: annual.
- •
Standard:
- –
Excellent: < 1/1000 catheter-days.
- –
Good: 1-2/1000 catheter-days.
- –
Fair: 3-5/1000 catheter-days.
- –
Poor: 6-7/1000 catheter-days.
- –
Really bad: > 7/1000 catheter-days.
- –
Comment
GEMAV considered this standard the most ideal for classifying TCVC-related bacteraemias.954
Exclusions
Those bacteraemias which have not been shown to be CVC-related or do not meet catheter-related bacteraemia criteria.
Indicator 6.2
Percentage of tunnelled central venous catheters with immediate dysfunction
Type of indicator
OUTCOME indicator.
Definition of terms
Dysfunction of the TCVC. Average flow < 300 mL/min or inability to start a HD session due to insufficient flow after having attempted to restore TCVC patency.
Rationale
A high incidence of malfunctioning TCVC in the first HD after placement indicates poor compliance with TCVC placement protocol. Even so, the decision to replace a catheter will be made on a case-by-case basis depending on the ability to obtain an adequate Kt/V index (quantity of plasma cleared of urea [K] during the course of a HD session [t] divided by the distribution volume of urea [V]) for the patient’s age and gender.
Population to which it applies
HD Unit patients with TCVC during the study period.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of TCVC with immediate dysfunction throughout the study period × 100.
- •
Denominator: number of incident TCVC that the HD unit may have throughout the study period.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: < 2%.
Comment
In the absence of bibliographical references, the standard has been established by consensus within GEMAV.
Indicator 6.3
Percentage of tunnelled central venous catheters with dysfunction
Type of indicator
OUTCOME indicator.
Definition of terms
Dysfunction of the TCVC. Average flow < 300 mL/min or inability to start a HD session due to insufficient flow after having attempted to restore TCVC patency.
Rationale
Although a TCVC dysfunction is mostly due to thrombosis, it may also indicate a shifted tip or poorly placed TCVC. A high incidence of dysfunction requires investigation of the possible related causes.
Population to which it applies
TCVC patients in the HD unit during the study period.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of malfunctioning TCVC during the study period × 100.
- •
Denominator: number of TCVC in the unit in the study period.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: < 5%.
Comments
Although they use a different definition of dysfunction, KDOQI-2006 guidelines suggest a percentage of dysfunction of 5%.
In the absence of other recommendations, GEMAV considered this standard the most suitable in terms of the percentage of TCVC dysfunction.
Indicator 6.4
Percentage of major complications during placement of a central venous catheter
Type of indicator
OUTCOME indicator
Definition of terms
Major complications. Occurrence of pneumothorax, haemothorax, haematoma requiring drainage, perforation, haemomediastinum, air embolism or sepsis following the CVC insertion procedure.
Rationale
A high incidence of major complications related with placing a CVC indicates poor compliance with the CVC placement technique.
Population to which it applies
All patients who have had a CVC placed.
Sources of data
Patient clinical records.
Formula
- •
Numerator: number of complications in the study period × 100.
- •
Denominator: number of CVC placed in the study period.
- •
Units: percentage.
- •
Periodicity: annual.
- •
Standard: < 3%.
Comments
The overall percentage of major complications which is accepted in image-guided implantation of a CVC is 3%, with 7% of total complications being accepted (major and minor).955
In the absence of other recommendations, GEMAV considered this standard the most suitable in terms of the percentage of major complications during TCVC placement.
ACKNOWLEDGEMENTSWe would especially like to thank the Presidents of the five Scientific Societies involved in the elaboration of this GUIDE, i.e. Sociedad Española de Nefrología (Spanish Socie ty of Nephrology [S.E.N.]), Sociedad Española de Angiología y Cirugía Vascular (Spanish Society of Angiology and Vascular Surgery [SEACV]), Sociedad Española de Radiología Médica (Spanish Society of Medical Radiology [SERAM]) and its Vascular and Interventional Radiology Department (SERAM-SERVEI), Sociedad Española de Enfermería Nefrológica (Spanish Society of Nephrology Nursing [SEDEN]) and, finally, the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (Spanish Society of Infectious Diseases and Clinical Microbiology [SEIMC]) and its Study Group of Healthcare-related Infection/Study Group of Hospital Infection (GEIRAS/GEIH-SEIMC) for their unconditional support. In particular, we would like to thank Dr. Alberto Martínez-Castelao and Dr. M. Dolores del Pino (S.E.N.), Francisco S. Lozano Sánchez and Dr. Luis Javier Álvarez Fernández (SEACV), Dr. José Luis del Cura (SERAM), Dr. Teresa Moreno (SERVEI), Ms. María Jesús Rollán (SEDEN) and Dr. Miguel Salavert (GEIRAS/GEIH-SEIMC).
We are indebted to the members of the Grupo Español Multidisciplinar de Acceso Vascular (Spanish Multidisciplinary Group on Vascular Access [GEMAV]) involved in this guide for the time end effort they have generously put into reviewing the literature, participating in GEMAV meetings, writing the sections and having a rational discussion of the most controversial aspects of the GUIDE. We believe that the multidisciplinarity of these professionals has served to enrich the guide. Many controversial issues have been approached from different viewpoints.
Nor must we forget the work of the external reviewers, whose experience has provided us with invaluable assistance in the elaboration of this guide.
The methodological support provided by the researchers from the Iberoamerican Cochrane Centre must also be acknowledged. Their contribution was critical in the correct formulation of the clinical questions, the synthesis of scientific evidence and the formulation of recommendations according to the guidelines of the GRADE system.
There is no doubt that the experience provided by Dr. Carlos Quereda Rodriguez-Navarro, coordinator of the Clinical Practice Guides of the S.E.N., has been extremely helpful during the final steps in the edition of the guide. His valuable advice has also been an incalculable help.
Finally, we sincerely appreciate the help we have always received from the Director and the Editor in Chief of the journal Nefrología, Dr. Mariano Rodríguez Portillo and Dr. Roberto Alcázar Arroyo, respectively, which made the publication of this guide possible.
Jose Ibeas López, MD, PhD
Ramon Roca-Tey, MD, PhD
Coordinators of the guide
GLOSSARY| CONCEPT | TERM | ACRONYM | SYNONYMS | DEFINITION |
|---|---|---|---|---|
| Advanced chronic kidney disease | ||||
| Advanced chronic kidney disease | ACKD | Phase of chronic kidney disease in which the glomerular filtration rate is lower than 30 mL/min/1.73 m2 | ||
| Allen test | ||||
| Allen test | Manoeuvre performed to know the patency of the palmar arch, with a first phase of induced ischaemia and a second phase of reperfusion of the hand | |||
| Aneurysm | ||||
| Aneurysm | True aneurysm | Segmentary dilation of a vessel above its normal size | ||
| Angioplasty balloon | ||||
| Angioplasty balloon | Catheter with a balloon incorporated in the distal end. The balloon can be inflated to a determined pressure, increasing its volume and reaching a specific diameter. The pressure at which the diameter is reached is called working pressure. The pressure needed to produce rupture of the balloon is called burst pressure | |||
| Compliance or semicompliance angioplasty balloon | A type of balloon whose diameter is determined by the pressure to which it is submitted, which can increase the aforesaid diameter as the inflated pressure increases (till a maximum pressure or burst pressure is reached) | |||
| High-pressure angioplasty balloon | A type of balloon whose maximum diameter, once reached (working pressure), does not increase beyond its maximum limit even if the inflated pressure increases. The materials with which these balloons are made allow high burst pressures (> 24 atmospheres) to be reached | |||
| Antibiotic lock therapy | ||||
| Antibiotic lock therapy | Exposure of the endoluminal surface of the central venous catheter, between haemodialysis sessions, on contact with a high-concentration of antibiotic solution, usually associated to an anticoagulant, and for a prolonged period of time | |||
| Arteriovenous fistula | ||||
| Arteriovenous fistula | AVF | Arteriovenous vascular access | Circuit created through the connection of an artery and a vein so that it can be used to make the connection for haemodialysis through its cannulation | |
| Native arteriovenous fistula | nAVF | Arteriovenous fistula (AVF) | Arteriovenous fistula where the vein is used as an access conduct for cannulation and connection for haemodialysis | |
| Prosthetic arteriovenous fistula | pAVF | Arteriovenous graft (AVG) | Arteriovenous fistula where a synthetic graft or prosthesis, usually made of a plastic derived from polytetrafluoroethylene, is interposed between the artery and the vein, where the body of the graft is used as an access conduct for cannulation and connection for haemodialysis | |
| Inflow | Segment of the native arteriovenous fistula that includes the feeding artery, the anastomosis itself and the initial segment of the arterialised vein up to 5 cm post-anastomosis | |||
| Outflow | Segment of the arteriovenous fistula at the arterialised vein that includes the needling area and the posterior venous segment up to the outflow into the right atrium | |||
| Arteriovenous fistula bruit | ||||
| Arteriovenous fistula bruit | It is a sound detectable by auscultation which constitutes the auditory manifestation of thrill | |||
| Arteriovenous fistula follow-up programme | ||||
| Arteriovenous fistula follow-up programme | Prevalent patient care in chronic haemodialysis that is based on 2 complementary aspects: a) early diagnosis of significant stenosis in the arteriovenous fistula using different first- and/or second-generation screening methods or techniques, and b) corrective treatment of this using preventive percutaneous transluminal angioplasty and/or surgery to avoid thrombosis of the arteriovenous fistula | |||
| AVF screening technique | ||||
| First-generation screening techniques | Techniques used to provide early diagnosis of significant stenosis in the arteriovenous fistula that include clinical monitoring, pressure monitoring, determination of recirculation percentage and unexplainable reduction of haemodialysis adequacy | |||
| Second-generation screening techniques | Techniques used to provide early diagnosis of significant stenosis in the arteriovenous fistula that allow for the estimation of blood flow directly in the arteriovenous fistula (Doppler ultrasound) or indirectly (dilution methods) | |||
| Arteriovenous fistula monitoring methods | Monitoring techniques | Screening techniques used to provide early diagnosis of significant stenosis that do not require special instruments and include all first-generation methods except determination of static venous pressure | ||
| Arteriovenous fistula surveillance methods | Surveillance techniques | Screening techniques that provide early diagnosis of significant stenosis which may require special instruments and include the two second-generation methods and determination of static venous pressure | ||
| Intra-access pressure | IAP | Static venous pressure | Estimation of the pressure inside the arteriovenous fistula with the blood flow pump of the haemodialysis monitor on OFF (pump flow = 0 mL/min) | |
| Equivalent static intra-access pressure | IAP/MAP | Normalised static intra-access pressure | Adjustment of the intra-access pressure (IAP) by the mean arterial pressure (MAP), so that the value of IAP is expressed in an equivalent or normalised way using the quotient IAP/MAP | |
| Dynamic venous pressure | DVP | Pressure needed to return the dialysed blood to the interior of the vascular access through the venous needle. It corresponds to the sum of the pressure needed to overcome the resistance exercised by the venous needle and the existing pressure inside the arteriovenous fistula | ||
| Arteriovenous fistula recirculation | Percentage of blood already dialysed which, after entering the vein through the venous needle, re-enters the dialyser of the haemodialysis machine through the arterial needle | |||
| Bio-synthetic prosthesis | ||||
| Bio-synthetic prosthesis | Vascular prosthesis composed of the combination of a synthetic material component and another of biological origin | |||
| Central venous catheter | ||||
| Central venous catheter | CVC | A type of vascular access composed of synthetic material of one or two lumens that allows the central veins or right atrium to be reached from its insertion into a vein, usually the internal jugular or common femoral vein | ||
| Non-tunnelled central venous catheter | NTCVC | A type of central venous catheter which is not located in a subcutaneous tunnel until it enters the vein | ||
| Tunnelled central venous catheter | TCVC | A type of central venous catheter located in a subcutaneous tunnel until it enters the vein, usually provided with an anchorage system through a cuff which allows it to be fixed to the subcutaneous tissue through a fibrotic reaction | ||
| Central venous catheter lock | Catheter lock | Filling the lumen or lumens of the central venous catheter with a solution of anticoagulant and/or antimicrobial in the interdialytic period in order to prevent thrombosis and/or infection | ||
| Central venous catheter infection | ||||
| Exit site infection of the central venous catheter | Signs of swelling limited to 2 cm around the cutaneous exit site, without extension of the central venous catheter cuff if tunnelled. It may or may not be associated with fever and bacteraemia, and be accompanied by purulent exudate through the cutaneous exit site. Isolation of the germ in culture will provide the definitive diagnosis; if there is no germ isolation, it will be probability diagnosis | |||
| Infection of subcutaneous tunnel of tunnelled central venous catheter | Tunellitis | Signs of swelling that spread over 2cm beyond the exit site and in the subcutaneous path of the tunnelled central venous catheter. It may or may not be accompanied by purulent exudate through the cutaneous exit site. Isolation of the germ in culture will provide the definitive diagnosis; if there is no germ isolation, it will be probability diagnosis | ||
| Central venous catheter-related bacteraemia | CRB/CRBSI | Catheter-related bloodstream infection | Isolation of the same microorganism in blood and catheter in the absence of any other focus of infection | |
| Cephalic vein arch | ||||
| Cephalic vein arch | CVA | End segment of the cephalic vein, corresponding to the trajectory from its superficial position in the deltopectoral groove to the confluence in the deep venous system (axillar vein/subclavian) | ||
| Digital pressure index | ||||
| Digital pressure index | Ratio between digital pressure at the studied limb (usually measured by photoplethysmography) and pressure of the brachial artery in the contralateral limb | |||
| Distal hypoperfusion syndrome | ||||
| Distal hypoperfusion syndrome | DHS/HAIDI | Steal sindrome, Haemodialysis access-induced distal ischaemia | Development of ischaemia symptoms in the distal territory of the limb after creation of an arteriovenous fistula | |
| Banding | Surgical technique consisting of the reduction in the outflow vein diameter of the arteriovenous fistula by banding the said vein using an external device, in order to reduce flow at the vascular access level | |||
| Distal revascularisation interval ligation | DRIL | Surgical technique consisting of ligating the artery distal to the anastomosis of the arteriovenous fistula with interposition of a bypass from the proximal artery to the distal to the vascular access | ||
| Distal radial artery ligation | DRAL | Surgical technique consisting of disconnecting the radial artery distal to the arteriovenous anastomosis in order to prevent retrograde flow through this | ||
| Proximal radial artery ligation | PRAL | Surgical technique consisting of ligating the radial artery proximally adjacent to the anastomosis as a method of reducing flow in the radiocephalic arteriovenous fistula | ||
| Minimally invasive limited ligation endoluminal-assisted revision | MILLER | Surgical technique consisting of minimally invasive banding, assisted by the percutaneous introduction of an angioplasty balloon in the arteriovenous anastomosis | ||
| Proximalisation of arterial inflow | PAI | Surgical technique consisting of the ligation of the arteriovenous fistula at the anastomosis and vascularising the said fistula by means of a prosthetic bypass between the axillar or proximal brachial artery and the outflow vein of the arteriovenous fistula | ||
| Revascularisation using distal inflow | RUDI | Surgical technique consisting of surgically disconnecting the arteriovenous anastomosis and making it more distal, by means of a retrograde bypass—prosthetic or autologous—from a distal arterial trunk (radial or ulnar arteries) to the outflow vein of the arteriovenous fistula | ||
| Expanded polytetrafluoroethylene | ||||
| Expanded polytetrafluoroethylene | PTFE | Teflon | Synthetic fluoropolymer, the main component of certain prostheses for vascular access in haemodialysis | |
| Failure | ||||
| Early failure | Absence of patency of the arteriovenous fistula in the first 30 days after its creation | |||
| Immediate failure | Absence of patency of the arteriovenous fistula in the first 72 h after its creation | |||
| Primary failure | Absence of patency of the arteriovenous fistula that includes immediate and early failure | |||
| Fibrinolysis | ||||
| Fibrinolysis | Treatment consisting of intravenous administration of fibrinolytics in order to achieve lysis of the intravascular thrombotic material | |||
| Fistulography | ||||
| Fistulography | Radiological exploration carried out by intravenously administering iodinated contrast in order to explore patency and adequacy of the vascular territory related to the arteriovenous fistula | |||
| Flow | ||||
| Arteriovenous fistula flow | QA | Volume of blood per unit of time that circulates through the arteriovenous fistula (expressed in mL/min) | ||
| Haemodialysis circuit pump flow | QB | Volume of blood per unit of time extracted from the patient, which is joined to the extracorporeal circuit of haemodialysis, expressed in mL/min | ||
| Glomerular filtration rate | ||||
| Glomerular filtration rate | GFR | Measurement index of renal function. It measures the volume filtered by the renal glomerulus per unit of time | ||
| Grupo Español Multidisciplinar del Acceso Vascular | ||||
| Grupo Español Multidisciplinar del Acceso Vascular | GEMAV | Spanish Multidisciplinary Group on Vascular Access, formed by representatives of the Spanish Society of Nephrology (Sociedad Española de Nefrología [S.E.N.]), Spanish Angiology and Vascular Surgery Society (Sociedad Española de Angiología y Cirugía Vascular [SEACV]), Spanish Society of Interventional and Vascular Radiology-Spanish Society of Medical Radiology (Sociedad Española de Radiología Vascular e Intervencionista-Sociedad Española de Radiología Médica [SERVEI-SERAM]), Spanish Society of Nephrological Nursing (Sociedad Española de Enfermería Nefrológica [SEDEN]) and Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [SEIMC]) | ||
| Haemodialysis | ||||
| Haemodialysis | HD | Type of renal replacement therapy that allows the uraemic toxins and accumulated excess fluid to be eliminated through the processes of diffusion and ultrafiltration of blood in the dialyser (semipermeable membrane) of the haemodialysis machine | ||
| Haemodialysis adequacy | ||||
| Kt index | Kt | Parameter of equivalent haemodialysis adequacy of urea clearance (K) provided by biosensors incorporated into some haemodialysis monitors adjusted to the time of the session (t) | ||
| Kt/V index | Kt/V | Parameter of haemodialysis adequacy that determines the amount of cleared urea plasma (K) over the time of the session (t) in relation to the distribution volume of urea (V) | ||
| Urea reduction rate | URR | Parameter of haemodialysis adequacy that determines the amount of plasma which is cleared of urea by directly comparing prior concentrations of urea and those following haemodialysis measuring its reduction rate as a percentage | ||
| Maturation | ||||
| Arteriovenous fistula maturation | Process through which the vein or the prosthesis/graft acquires the morphological and haemodynamic characteristics needed to allow for cannulation and use for haemodialysis | |||
| Non-mature arteriovenous fistula | Arteriovenous fistula that does not fulfil maturation criteria between 4 and 6 weeks after creation | |||
| Arteriovenous fistula used successfully for chronic haemodialysis | FUSH (fistula used successfully for haemodialysis) | Arteriovenous fistula that allows cannulation via 2 needles in at least two-thirds of the haemodialysis sessions for 1 month and that allows the programmed haemodialysis routine to be completed | ||
| Needling | ||||
| Area needling method | Needling carried out in an extremely delimited area of the arterialised vein in the native arteriovenous fistula or the prosthesis in the prosthetic arteriovenous fistula | |||
| Buttonhole needling method | Needling carried out though the same hole in all haemodialysis sessions, after the creation of a subcutaneous tunnel with a standard needle and the later use of a blunt needle | |||
| Rope ladder needling method | Rotating needling method | Needling distributed regularly along the whole length of the arterialised vein in the native arteriovenous fistula or the prosthesis in the prosthetic arteriovenous fistula | ||
| Percutaneous transluminal angioplasty | ||||
| Percutaneous transluminal angioplasty | PTA | Intravascular dilation technique with a balloon inserted by a needle used to treat vascular stenosis | ||
| Phlebography | ||||
| Phlebography | Exploration carried out by intravenously administering iodinated contrast through cannulation of a peripheral vein that allows the veins to be studied in the upper and inferior limbs and their drainage to cardiac cavities | |||
| Pseudoaneurysm | ||||
| Pseudoaneurysm | Expandable dilation caused by persistent bleeding through a loss of continuity in the arteriovenous fistula wall | |||
| Reactive hyperaemia test | ||||
| Reactive hyperaemia test | Study of the changes produced in the Doppler wave in an artery after causing ischaemia in the territory distal to it | |||
| Renal replacement therapy | ||||
| Renal replacement therapy | RRT | Therapy used in end stage chronic renal disease that substitutes the renal function by means of different modalities of dialysis or kidney transplant | ||
| Stenosis | ||||
| Stenosis | Reduction in the diameter of the normal lumen of the vessel | |||
| Central vein stenosis | Stenosis localised in the venous sector from the axillar vein to drainage in the right atrium, and which comprises the axillar and subclavian veins, the brachiocephalic trunk and the superior vena cava. In inferior cava territory, it would comprise the iliac veins in addition to this | |||
| Juxta-anastomotic stenosis of the arteriovenous fistula | Perianastomotic stenosis of the native arteriovenous fistula | Stenosis localised in an area which comprises the area immediately adjacent to the anastomosis to 5 cm post-anastomosis | ||
| Significant stenosis. GEMAV criteria | Reduction in the vascular lumen of the native or prosthetic arteriovenous fistula shown by Doppler ultrasound with high risk of thrombosis, that is to say, all reduction of the vascular lumen that fulfils 2 main criteria (percentage of reduction in vascular lumen > 50% + ratio peak systolic velocity > 2) and at least one of the following additional criteria: morphological criterion (residual diameter < 2 mm) or functional criterion (blood flow of the vascular access [mL/min] < 500 [native arteriovenous fistula], 600 [prosthetic arteriovenous fistula] or decrease in the blood flow of the vascular access > 25% if the flow is < 1,000 mL/min) | |||
| Significant stenosis. KDOQI criteria | Reduction > 50 % of the vascular lumen of a native or prosthetic arteriovenous fistula shown by Doppler ultrasound or fistulography, associated with a repetitive alteration of any parameter obtained using first- and/or second-generation screening methods | |||
| Anatomical success of treatment of a stenosis of arteriovenous fistula | Disappearance of stenosis or persistence of a residual stenosis lower than 30% after intervention | |||
| Stent | ||||
| Stent | Metallic endovascular device placed to maintain vessel patency | |||
| Endovascular stent graft | Stent graft, covered stent | Prosthetic endovascular conduct with the external support of a stent | ||
| Thrill | ||||
| Thrill | Palpable vibration of the vessel due to the presence of turbulent flow | |||
| Thrombectomy | ||||
| Thrombectomy | Therapeutic procedure carried out to extract intravascular thrombotic material | |||
| Thrombolysis | ||||
| Thrombolysis | Therapeutic procedure carried out to achieve lysis of intravascular thrombotic material | |||
| Complete arteriovenous fistula thrombosis | ||||
| Complete arteriovenous fistula thrombosis | AVF thrombosis, AVF occlusion | Occupation of the whole arteriovenous fistula lumen by thrombotic material, which impedes blood circulation in its interior and makes it impossible to use in haemodialysis treatment | ||
| Ultrasound | ||||
| Ultrasound | US | Non-invasive imaging technique that allows organs and tissues to be explored using the emission and reception of ultrasound waves and their transformation into images | ||
| Doppler ultrasound | DU | Modality of ultrasound that uses the Doppler effect to assess the direction and measure the velocity and flow volume of fluids in certain structures, especially in blood vessels | ||
| Colour Doppler ultrasound | DDU | Duplex Doppler ultrasonography | Ultrasound mode which combines bi-dimensional ultrasound and the Doppler effect to offer information on speed in the colour range | |
| Peak systolic velocity | PSV | Ultrasound parameter that corresponds to the value of the maximum velocity detected by the Doppler curve of the vessel being studied. Measurement is given in cm/s | ||
| Urokinase | ||||
| Urokinase | UK | Drug with fibrinolytic activity that is used to treat intraluminal thrombosis in central venous catheters | ||
| Vascular access | ||||
| Vascular access | VA | Access to blood circulation to perform renal replacement therapy through haemodialysis. It can be a native arteriovenous fistula, a prosthetic arteriovenous fistula or a central venous catheter | ||
| Fall-back vascular access | Vascular access performed in absence of suitable venous drainage to the right atrium (subclavian vein, brachiocephalic trunk and superior vena cava) | |||
| Vascular access patency | ||||
| Primary patency (arteriovenous fistula) | Primary unassisted patency | Period elapsed since the creation of the arteriovenous fistula (or since the performed therapeutic procedure if the procedure is assessed for level of success) till the first elective intervention (endovascular or surgical) in order to maintain or restore blood flow, or to the first episode of thrombosis, or until there is loss due to follow-up censoring (death, transferral to another haemodialysis unit, change of renal replacement therapy—peritoneal dialysis, renal transplant—) or end of study period | ||
| Primary assisted patency (arteriovenous fistula) | Post-intervention primary patency | Period elapsed since the creation of the arteriovenous fistula (or since the performed therapeutic procedure if the procedure is assessed for level of success) till the first episode of thrombosis, or until there is loss due to follow-up censoring (death, transferral to another haemodialysis unit, change of renal replacement therapy—peritoneal dialysis, renal transplant—) or end of study period | ||
| Secondary patency (arteriovenous fistula) | Cumulative survival | Period elapsed since the creation of the arteriovenous fistula (or since the performed therapeutic procedure if the procedure is assessed for level of success) till the definitive abandonment of the fistula, or until there is loss due to follow-up censoring (death, transferral to another haemodialysis unit, change of renal replacement therapy—peritoneal dialysis, renal transplant—) or end of study period | ||
| Primary patency (central venous catheter) | Period between catheter placement and the moment when the first intervention is required to maintain patency (this includes fibrinolytic treatment, mechanical thrombectomy and interventional treatment of the fibrin sheath without catheter withdrawal), or until there is loss due to follow-up censoring (death, transferral to another haemodialysis unit, change of renal replacement therapy—peritoneal dialysis, renal transplant—) or end of study period | |||
| Secondary patency (central venous catheter) | Period between catheter placement and the moment it is withdrawn for any reason, including the time after any intervention to maintain catheter function, or until there is loss due to follow-up censoring (death, transferral to another haemodialysis unit, change of renal replacement therapy—peritoneal dialysis, renal transplant—) or end of study period | |||
| Vascular mapping | ||||
| Vascular mapping | Exploration carried out using an imaging technique to assess the anatomical and/or functional characteristics of the blood vessels in order to create an arteriovenous fistula | |||
1. Professional profile of the coordinators of the guide
JOSE IBEAS LÓPEZ, MD, PhD
Graduate in Medicine and Surgery from the Faculty of Medicine, University of Alicante and Doctor of Medicine, Universitat Autònoma de Barcelona. Nephrologist after his training as resident at the Fundación Puigvert-Hospital de San Pablo, Barcelona. Master’s Degree in Evidence-based Medicine from Universitat Autònoma de Barcelona-Universitat Rovira i Virgili-The Cochrane Collaboration, Barcelona. Currently Consultant at the Nephrology Department and Coordinator of the Vascular Access Programme of the Hospital Universitari Parc Taulí, Sabadell, Barcelona. He has a great deal of experience in vascular access in the field of care, teaching and research, with more than 400 communications and lectures at national and international congresses and several national and international publications, direction of Doctoral Theses and recipient of 10 awards. Member of the Clinical Research Ethics Committee of the Hospital Universitari Parc Taulí. He has been Director of numerous national and international courses and symposia, in particular the Co-chair of the 9th Congress of the Vascular Access Society
He is the Secretary of the Vascular Access Working Group that belongs to the Sociedad Española de Nefrología (Spanish Society of Nephrology). Member of the Vascular Access Working Group of the Sociedad Catalana de Nefrología (Catalan Society of Nephrology). Member of the Grupo Español Multidisciplinar del Acceso Vascular (Spanish Multidisciplinary Group on Vascular Access [GEMAV]). Member of the Interventional Nephrology Working Group of the Sociedad Española de Nefrología (promoting group). Member of the Board of the Vascular Access Society. Member of the International Committee of the American Society of Diagnostic and Interventional Nephrology. Member of the European Vascular Access Guidelines Group of the ERA-EDTA and member of the Clinical Practice Guide on Chronic Renal Disease Group of the Spanish National Health System-GuíaSalud.
RAMON ROCA-TEY, MD, PhD
Graduate in Medicine and Surgery from the Faculty of Medicine, Universidad Central de Barcelona and Doctor of Medicine, Universitat Autònoma de Barcelona (Extraordinary Doctorate Award). He is a specialist in nephrology through his training as resident at the Hospital General Universitari of the Ciutat Sanitària Vall d’Hebron, Barcelona. Currently, he is a consultant at the Nephrology Department of the Hospital de Mollet del Vallès, Barcelona. His research activity is centred on the field of vascular access for haemodialysis, with a doctoral thesis, 6 awards, 200 lectures and communications in national and international congresses and several articles in national and international journals and chapters of books.
Coordinator of the vascular access working groups of the Sociedad Catalana de Nefrología (Catalan Society of Nephrology) and Sociedad Española de Nefrología (Spanish Society of Nephrology) and coordinator of the Grupo Español Multidisciplinar de Acceso Vascular (Spanish Multidisciplinary Group on Vascular Access [GEMAV]). He is also a member of the board of the Vascular Access Society, member of the Interventional Nephrology Working Group of the Sociedad Española de Nefrología (promoting group) and member of the European Vascular Access Guidelines Group of the ERA-EDTA. He has participated as external reviewer in the “Vascular Access Clinical Practice Guidelines of the European Society of Surgery” (2018). He has been member of the council of the Sociedad Catalana de Nefrología (2010-2014), Chairman of the Commission for Research and Innovation at the Hospital of Mollet del Vallès, Barcelona, and he has participated in the organisation of various courses and congresses, in particular the “ Jornada sobre el Acceso Vascular para Hemodiálisis en el Vallès Oriental “ and the “9th Congress of the Vascular Access Society” (Chair).
2. Professional profile of the authors of the guide
Joaquín Vallespín
Department of Angiology and Vascular Surgery, Parc Taulí Hospital Universitari, Sabadell, Barcelona. Surgical Coordinator of the Vascular Access Programme. Secretary of the Section of Vascular Access of the Spanish Society of Angiology and Vascular Surgery. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Teresa Moreno
Interventional Vascular Radiology Unit, Radiology Department, Hospital Juan Ramón Jiménez, Huelva. Coordinator of the Working Group on Vascular Accesses of the Spanish Society of Interventional and Vascular Radiology (SERVEI). Member of the Council—President and Vice-President—(2011 to 2015) and of the Scientific Committee of SERVEI (2009 to 2015). Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV) and the Vascular Access Society (VAS).
Guillermo Moñux
Department of Angiology and Vascular Surgery, Hospital Clínico Universitario San Carlos, Madrid. Associate Professor of Surgery, Universidad Complutense de Madrid. Associate Professor at the Universidad Europea de Madrid. Coordinator of the Vascular Access Section of the Spanish Society of Angiology and Vascular Surgery (SEACV). Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Anna Martí-Monrós
Nursing Supervisor of the Nephrology Department of the Consortium Hospital General of Valencia. President of EDTNA/ERCA 1994-1995, Editor of the International Journal EDTNA/ERCA 1996-2006. Member of the European Renal Best Practice Group. Co-chair European Vascular Access Guidelines, Professor of the Master of Nephrology at the Catholic University of Valencia. European Product Manager DOPPS Study. Founding Member of SEDEN. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
José Luis del Pozo
Director of the Infectious Diseases and Microbiology Area of the University of Navarra Clinic. Professor of Medicine at the University of Navarra. Member of council of GEIRAS/GEIH-SEIMC (Study Group on Nosocomial Infection of the Spanish Society of Infectious Diseases and Clinical Microbiology). Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Enrique Gruss
Unit of Nephrology, Hospital Universitario Fundación Alcorcón, Alcorcón, Madrid. Honorary Professor of the Faculty of Medicine of the Universidad Rey Juan Carlos, Madrid. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
Manel Ramírez de Arellano
Chief of the Nephrology Department at the Hospital de Terrassa, Consorci Sanitari de Terrassa, Barcelona and Chairman of its Multidisciplinary Committee on Vascular Access for Haemodialysis. Member of the Working Group on Vascular Accesses of the Catalan Society of Nephrology. Member of the council of the Catalan Society of Nephrology. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
Néstor Fontseré
Nephrology and Renal Transplant Department, Dialysis Section, Hospital Clínic, Barcelona. Medical Coordinator of the Functional Unit of Vascular Access (UFAV). Consultant 1. Collaborating professor at the University of Barcelona. Collaborating Member of the Research Group on Nephro-urological Diseases and Renal Transplantation (Area 2), Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). Member of the Working Group on Vascular Accesses of the Catalan Society of Nephrology. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Vascular Access Society (VAS). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
Dolores Arenas
Chief of the Nephrology-Haemodialysis Department at Vithas International Perpetuo Hospital, Alicante. Coordinator of the Quality Management Group of the Spanish Society of Nephrology. Member of the Committee of Experts of the Manual of Accreditation for the Haemodialysis Units of the Conselleria de Sanitat de la Comunitat Valenciana (Health Council of Valencia). Member of the Committee of Experts on Standards and Recommendations for the Haemodialysis Unit of the Quality Agency of the National Health System, Ministry of Health and Social Policy. Collaborating Professor in the Master’s Degree in Haemodialysis at the Universidad Complutense de Madrid. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
José Luis Merino
Department of Nephrology, Hospital Universitario de Henares, Coslada, Madrid. Member of the Vascular Access Group of the Madrid Society of Nephrology. Collaborating Professor of the Faculty of Medicine of the University Francisco de Vitoria. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
José García-Revillo
Unit of Vascular and Interventional Radiology, Radiology Department, Hospital Universitario Reina Sofía of Cordoba. Associate Professor of Radiology at the Faculty of Medicine of Córdoba. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Pilar Caro
Nephrology Department, Haemodialysis Unit, Hospital Ruber. Expert in Haemodialysis in Nephrology and Coordinator of the Vascular Access. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
Cristina López-Espada
Faculty Specialist in the Angiology and Vascular Surgery Area of the Hospital Universitario of Granada. Coordinator of the Quality Group of the Spanish Society of Angiology and Vascular Surgery (SEACV). Member of the Vascular Access Group of the SEACV. Member of the VASCUNET Group of European Registries of the European Society of Vascular Surgery (ESVS). Expert in Quality Assurance and Patient Safety. Master in Clinical Management and Medical Management. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Antonio Giménez
Director of the Department of Angiology and Vascular Surgery, Parc Taulí Hospital Universitari, Sabadell, Barcelona. Clinical Associate of the Autonomous University of Barcelona. Member of the Council of the European Society of Vascular Surgery. Past-President of the Catalan Society of Angiology, Vascular and Endovascular Surgery, Past-Vicepresident of the Spanish Society of Angiology and Vascular Surgery. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Milagros Fernández-Lucas
Department of Nephrology, Haemodialysis Section, Hospital Universitario Ramón y Cajal and Coordinator of its Unit for Vascular Access. Associate Professor at the University of Alcalá. Secretary of the Madrid Society of Nephrology (SOMANE). Coordinator of the SOMANE Vascular Access Group. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
Pablo Valdés
Director of Radiodiagnosis area, Costa del Sol Health Agency. Vice-president of the Spanish Society of Medical Radiology (SERAM). Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Fidel Fernández-Quesada
Specialist in the Angiology and Vascular Surgery Area of the Hospital Universitario of Granada. President of the Spanish Chapter of Phlebology and Lymphology (CEFyL) of the Spanish Society of Angiology and Vascular Surgery (SEACV). Master’s Degree in Public Health and Health Management. Professor of Surgery at the University of Granada. Representative for Spain in the International Union of Angiology (UIA). President of the Ethics Committee of the Provincial Bioethics Research of Granada. Vice-President of the Official College of Physicians of Granada. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Natalia de la Fuente
Department of Angiology and Vascular Surgery, Hospital Galdakao-Usansolo, Bizkaia. Consultant of Vascular Accesses for Haemodialysis. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
David Hernán
Director of Nursing of the Fundación Renal Íñigo Álvarez de Toledo. Collaborating Professor of the Expert Course in Dialysis at the Universidad Europea de Madrid. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Patricia Arribas
Hospital Universitario Infanta Leonor. Nursing Supervisor of the Dialysis Unit and Coordinator of the Working Group on Vascular Accesses of the Unit. Teacher of the Spanish Society of Nephrology Nursing (SEDEN). Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
María Dolores Sánchez de la Nieta
Department of Nephrology, Hospital General Universitario of Ciudad Real and Coordinator of Vascular Access until 2009 and Responsible for the Protocol of Bacteraemia by catheter. Associate Professor at the Medical School of Ciudad Real. Responsible for training and Residents’ supervisor. Expert in Haemodialysis in Nephrology, Universidad Complutense de Madrid. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV). Member of the Working Group on Vascular Access of the Spanish Society of Nephrology (S.E.N.).
María Teresa Martínez
Hospital General Universitario Gregorio Marañón, Madrid. Nephrology Care Nurse in the Haemodialysis, Renal Transplant and Therapeutic Apheresis Units and Coordinator of its Vascular Access Unit. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Ángel Barba
Specialist in Angiology and Vascular Surgery. Chief of the Angiology and Vascular Surgery Department of the Hospital Galdakao-Usansolo, Bizkaia. Reference Surgeon for Vascular Accesses for Haemodialysis of the Igualatorio Médico Quirúrgico (IMQ) (Equality of care in surgery) of Bizkaia. Medal awarded by the SEDyT. Director of the Courses of Vascular Accesses for Haemodialysis in Bilbao. Member of the Spanish Multidisciplinary Group on Vascular Access (GEMAV).
Ángel Barba
None.
Anna Martí Monrós
- •
Fees received for participation in conferences:
- –
NIPRO: Buttonhole course Congress SEDEN Valencia 2015.
- –
OTSUKA: Nursing Advisory Board London 2015.
- –
- •
Honorarium as speaker:
- –
NIPRO: MADIALISIS 2014.
- –
- •
Financial support of educational programmes:
- –
RUIBO 2013, 2014, 2015.
- –
- •
Advisor of pharmaceutical company:
- –
OTSUKA.
- –
RUIBO.
- –
Antonio Giménez
- •
Financial support of educational programmes:
- –
11/2012-11/2013: Covidien Proctor of Dispositive.
- –
12/2013-(current): Medtronic Proctor of Dispositive and Head of the Peripheral Arterial Disease Studies Board.
- –
04/2014-(current): Abbot Supera Stent Proctor.
- –
4 09/2014-(current): Cardiva Proctor of the Aortic Fenestrated Endoprosthesis of Vascutek.
- –
09/2015-(current): Jotec. Proctor of the Aortic Fenestrated Endoprosthesis of Jotec.
- –
- •
Financial support for research work:
- –
03/2011-05/2014: Grifols Institute, S.A. Human plasma-derived fibrin sealant Grifols Haemostasia. Fibrinogen sealant.
- –
10/2012-(current): Astra Zeneca Ticagrelor Peripheral Arterial Disease (EUCLID).
- –
06/2014-09/2014: BARD Drug eluting balloon Revascularisation of limb ischaemia.
- –
03/2014-01/2015: SERVIER Daflon 500 Chronic venous insufficiency.
- –
11/2014-(current): Ivascular Luminor Angioplasty Balloon. Revascularisation of limb ischaemia.
- –
11/2015-(current): BAYER Rivaroxaban in symptomatic artery disease undergoing lower extremity revascularisation procedures.
- –
05/2016-(current): BARD Drug eluting Balloon Revascularisation of limb ischaemia below the knee arteries.
- –
Cristina López Espada
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
National Congress of the SEACV for 3 consecutive years in different locations, registration and accommodation financed by the company Rovi, S.A.
- –
National Congress of Quality in Gijón (October 2015), registration financed by Bama-Geve and accommodation by Medtronic, S.A.
- –
VEITH symposium 2014 in New York, registration and accommodation funded by the company GORE, S.A.
- –
- •
Honorarium as speaker:
- –
Preparation Course for the “European Board of Vascular Surgery”, June 2014, during the National Congress of the SEACV by the Jotec company. GMbH.
- –
David Hernán
None.
Dolores Arenas
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
2013: National Congress S.E.N.-Amgen.
- –
2014: National Congress S.E.N.-Fresenius.
- –
2015: National Congress S.E.N.-Shire.
- –
- •
Fees for writing articles:
- –
2014: Abbot Nutrition-Abbot Experiences.
- –
2015: Clinical Findings Book-Experiences in Nutrition. Shire Pharma.
- –
- •
Honorarium as speaker:
- –
2014: Scientific talk. Amgen
- –
2014/2015: Scientific talk. Haemodialysis Master for Nephrology Specialists. The Complutense University.
- –
2015: Scientific talk on quality management in haemodialysis. Palex.
- –
- •
Advisor of pharmaceutical company:
- –
2015: Advisory Board Velphoro®. Fresenius.
- –
Enrique Gruss
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
October 2015: S.E.N. Congress. Gambro (travel, hotel and registration).
- –
May 2015: EDTA Congress. Fresenius (travel, hotel and registration).
- –
October 2014: S.E.N. Congress. Genzyme (hotel and registration). Gambro (Travel).
- –
September 2013: Argentine Congress. Abbvie (inscription). Gambro (hotel).
- –
- •
Honorarium as speaker:
- –
February 2014: Coslada Hospital. Fresenius.
- –
February 2015: Coslada Hospital. Fresenius.
- –
Fidel Fernández
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
During the last 5 years support from FAES Pharma, as President of the CEFyL of the SEAC to attend the National Congress of the Society and of the SEACV (this company covers the expenses of the CEFyL board of directors).
- –
- •
Honorarium as speaker:
- –
Speaker in several congresses. Servier.
- –
Articles in a journal magazine edited by Tedec-Meiji and by Servier.
- –
Speaker at an international conference on vascular access sponsored by BARD.
- –
Teacher and co-director SEACV Courses sponsored by Servier, Leo Pharma and Andaru Pharma.
- –
- •
Financial support of educational programmes:
- –
Six years ago, participation in the course on Endovascular procedure using porcine models. Cáceres, sponsored by Gore.
- –
- •
Financial support for research work:
- –
Participation in a multicentre clinical trial as a researcher, sponsored by Servier.
- –
- •
Advisor of Pharmaceutical company:
- –
Consultant for Tedec Meiji, Servier and Cinfa.
- –
Consultant in research topics for a bioceramic textile material company.
- –
Guillermo Moñux
None.
Joaquin Vallespín
None.
José García Revillo
None.
José Ibeas
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
Congress of the Vascular Access Society of the Americas. Houston, 2013. Sanofi.
- –
- •
Honorarium as speaker:
- –
Continuing education course of the Catalan Society of Nephrology on Vascular Access. Barcelona 2013. Financed by the Society.
- –
Dialysis Meeting of the Madrid Society of Nephrology, 2013 and 2014. Financed by the Society.
- –
Vascular Access Symposium, Alcorcón University Hospital, 2014. Financed by the organisation.
- –
Course on Ultrasound in Vascular Access. University Hospital Río Hortega, Valladolid, 2014. Amgen.
- –
Course on Vascular Access. Reina Sofía University Hospital, Madrid 2014. Financed by organisation.
- –
Course, Ultrasound for Vascular Access. Hospital Son Espases, Mallorca 2014. Sanofi.
- –
I Multidisciplinary Day of Vascular Access Vithas, Alicante 2015. Financed by the organisation.
- –
Course on Ultrasound for Nursing, Mallorca 2015. Hospital Son Espases. Rubio.
- –
Workshop on Ultrasound of Vascular Access in Haemodialysis -Nephrology, Valladolid 2014. Amgen.
- –
Meeting of the Castellano Astur-Leonesa Society of Nephrology, Burgos 2014. Financed by the Society.
- –
Theoretical and Practical Workshop on Ultrasound for Vascular Access in Nephrology, Burgos 2015. Amgen.
- –
National Congress of the Italian Vascular Access Group, Italian Society of Nephrology, Trento 2014. Financed by the Society.
- –
Nephrology: Meeting of Experts. Ultrasound for vascular access in haemodialysis, Alicante 2015. Amgen.
- –
Continuing education course of the Catalan Society of Nephrology on Vascular Access, 2015. Financed by the Society.
- –
European Vascular Course, Maastricht 2015. Financed by Congress Organisation.
- –
11th Annual Scientific Meeting of the American Society of Diagnostic and Interventional Nephrology, Orlando 2015. Financed by the Society.
- –
Echography courses Hospital del Mar. Solving problems by Echography “Eco-soluciones”, Barcelona. Esteve.
- –
Congress of the Spanish Society of Infectious Diseases and Clinical Microbiology, 2015. Financed by the Society.
- –
Congress of the Spanish Society of Interventional Radiology, 2015. Financed by the Society.
- –
Courses on Echography, Parc Taulí Sabadell University Hospital, Barcelona. Financed by the organisation.
- –
- •
Advisor of Pharmaceutical company:
- –
2013: Vascular Access Advisory Board meeting, Paris, Covidien.
- –
2014: Vascular Access Advisory Board meeting, Milan, Covidien.
- –
2016: Vascular Access Advisory Board Meeting. London, Medtronic.
- –
- •
Strategies or commercial interest in health-related companies:
- –
Author of the NephroCloud® Software. Co-owner together with the Parc Taulí Foundation and the Seys company.
- –
- •
Sponsor of educational programmes. Financial support for the following courses and symposium in the Parc Taulí University Hospital of Sabadell, Barcelona:
- –
2013: BARD, Covidien, General Electric.
- –
2014: General Electric, BARD, Covidien, Gore, Mindray, Rubió, Sonosite.
- –
2015: BARD, Covidien, General Electric.
- –
2016: BARD, Covidien, General Electric, Medtronic, Cardiva-anigodynamics, Gore, Toshiba, Mindray.
- –
José Luis del Pozo
- •
Honorarium as speaker:
- –
Scientific sessions organised by Pfizer, MSD, Novartis and Gilead.
- –
José Luis Merino
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
MaDiálisis 2013 Meeting. Pfizer.
- –
VAS Congress 2013. Palex.
- –
European Congress ERA-EDTA, 2013. Gambro.
- –
SOMANE Meeting 2013. Shire.
- –
Congress of the S.E.N. 2013. Gambro.
- –
Renal Week. 2013. Fresenius.
- –
MaDiálisis Meeting 2014. Gambro.
- –
HTA and Kidney Conference. 2014. Menarini.
- –
European Congress ERA-EDTA, 2014. Gambro.
- –
SOMANE Meeting 2014. Sanofi.
- –
Congress of the S.E.N. 2014. Palex.
- –
MaDiálisis Meeting 2015. Shire.
- –
VAS Congress 2015. Vifor.
- –
European Congress ERA-EDTA 2015. Fresenius.
- –
SOMANE 2015 Meeting. Shire.
- –
- •
Honorarium as speaker:
- –
I Conference on Haemodialysis Techniques. February 2014. Nipro.
- –
II Conference on Haemodialysis Techniques. February 2015. Palex.
- –
Clinical experience with Ferinject. Conference. February 2015. Fresenius.
- –
Dyslipidemia and chronic kidney disease. December 2015. Rovi.
- –
- •
Provision of material to clinical facilities:
- –
II Conference on Haemodialysis Techniques, February 2015. Anatomical trunk-neck model for central veins canalisation. Nipro.
- –
María Teresa Martínez
- •
Financial support for congress registration, trips or accommodation:
- –
2013. Bilbao. National Congress SEDEN. Nipro Europe.
- –
- •
Honorarium as speaker:
- –
2015. Valencia. National Congress SEDEN. 3M.
- –
Manel Ramírez de Arellano
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
Scientific Conference (Home HD). Madrid. Palex.
- –
European Congress EDTA. Istanbul, 2013. Amgen.
- –
National Congress S.E.N. Bilbao, 2013. Fresenius.
- –
International Congress ISPD. Madrid, 2014. Baxter.
- –
National Congress S.E.N. Barcelona, 2014. Sanofi.
- –
European Congress EDTA. London, 2015. Amgen
- –
National Congress S.E.N. Valencia, 2015. Otsuka.
- –
- •
Honorarium as speaker:
- –
VIII Mediterranean Conference on Vascular Access for HD. Murcia, 2013. Financed by the organisation.
- –
III Course of Chronic Haemodialysis. Barcelona, 2013. Financed by the organisation.
- –
1st Multidisciplinary Conference on Vascular Access Vithas. Alicante, 2015. Financed by the organisation.
- –
IV Course of Chronic Haemodialysis. Barcelona, 2015. Financed by the organisation.
- –
Scientific Conference HD and ACKD. Sitges, 2015. Palex.
- –
María Dolores Sánchez de la Nieta
None.
Milagros Fernández-Lucas
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
XLV National Congress of Nephrology, Valencia 2015. Fresenius.
- –
XLIV National Congress of Nephrology, Barcelona 2014. Fresenius.
- –
Natalia de la Fuente
- •
Honorarium as speaker:
- –
2014. First meeting Vascular-Nephrology. 60th Congress de la SEACV. Madrid.
- –
Néstor Fontseré
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
National congresses (Spanish Society of Nephrology-S.E.N.), regional congresses (Catalan Society of Nephrology-SCN) and international congresses (ERA-EDTA, VAS). Fresenius Medical Care and Amgen.
- –
- •
Honorarium as speaker:
- –
Second Congress of the Spanish Society of Vascular Access, Madrid 2015. Boston.
- –
Continuing education programme for physicians of Fresenius Medical Care (Nephrocare). Hospital Clínic. Barcelona 2015. Fresenius Medical Care.
- –
Boston theoretical and practical thrombectomy workshop in animal model using Angiojet device. Service for research in animals. Vall d’Hebron Institute of Research (VHIR). Barcelona 2015. Boston.
- –
Master: Care of the critical patient in emergencies. “Vascular access in renal substitution techniques”, 2014. University of Barcelona and Autonomous University of Barcelona. Hospital Clínic Barcelona. University of Barcelona.
- –
Practical of Clinical Applications of l’Enginyeria-II. P10: “The use of biosensors of volume overload and follow-up of the dysfunction of vascular access”. University of Barcelona. Applied Research Centre Manso. Barcelona 2013
- –
Pablo Valdés
None.
Patricia Arribas
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
2013: National Congress SEDEN, Bilbao. Fresenius Medical Care.
- –
- •
Honorarium as speaker:
- –
2015: National Congress SEDEN. 3M, Valencia.
- –
- •
Financial support of educational programmes:
- –
2015. Baxter.
- –
Pilar Caro
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
XLIII Nephrology Congress in Bilbao, 2013. ABBOTT.
- –
8th International Congress of vascular access, Prague 2013. ABBOTT and Fresenius Medical Care.
- –
XLIV Nephrology Congress, Barcelona 2014. Fresenius Medical Care.
- –
XLV Congress of the Spanish Society of Nephrology, Valencia 2015. SANOFI.
- –
Ramon Roca-Tey
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
50th Congress of the European Renal Association-European Dialysis and Transplant Association. Istanbul, Turkey, 2013: Fresenius Medical Care.
- –
XXXI Annual Meeting of the Catalan Society of Nephrology. 2015. Sant Joan Despí Moisès Broggi Hospital (Sant Joan Despí). AMGEN
- –
XXXII Annual Meeting of the Catalan Society of Nephrology. Castelldefels 2016. AMGEN.
- –
- •
Honorarium as speaker:
- –
VII Theoretical and Practical Course of Ultrasound for Vascular Access in Nephrology. Health Corporation Parc Taulí de Sabadell, 2013. Financed by the organisation.
- –
III Theoretical and Practical Course on Update in Ultrasound and Multidisciplinary Management of Vascular Access. Health Corporation Parc Taulí, Sabadell 2013. Financed by the organisation.
- –
III Course of Chronic Haemodialysis. Puigvert Foundation, Barcelona 2013. Financed by the organisation.
- –
Conference on Vascular Access for Haemodialysis: Update on Vascular Access dysfunction. Bellvitge Hospital, Barcelona 2013. Covidien.
- –
VIII Theoretical and Practical Course on Ultrasound for Vascular Access in Nephrology. Health Corporation Parc Taulí de Sabadell, 2014. Financed by the organisation.
- –
IV Edition of the Theoretical and Practical Workshop; update on Ultrasound and Multidisciplinary Approach for Vascular Access. Health Corporation Parc Taulí de Sabadell, 2014. Financed by the organisation.
- –
I Theoretical and Practical Course on Vascular Access Ultrasound for Nephrological Nursing. Health Corporation Parc Taulí de Sabadell, 2014. Financed by the organisation.
- –
IX Theoretical and Practical Course on Vascular Access Ultrasound in Haemodialysis. Health Corporation Parc Taulí de Sabadell, 2015. Financed by the organisation.
- –
26 Continuing Training Course (Vascular Access) of the Catalan Society of Nephrology (SCN). 2015. Financed by the SCN.
- –
X Theoretical and Practical Course on Vascular Access Ultrasound in Haemodialysis. Health Corporation Parc Taulí de Sabadell, 2015. Financed by the organisation.
- –
52st European Renal Association-European Dialysis and Transplant Association Congress (London), Lecture. 2015. Financed by the Society.
- –
20è Cours Congrès de La Société Française by L’Abord Vasculaire. Montpellier, France, 2015. Financed by the Society.
- –
Course “Aula d’Hemodiàlisi 2015” of the SCN, Faculty of Medicine (Campus Casanova) of the University of Barcelona, 2015. Financed by the Society.
- –
II Theoretical and Practical Course on Vascular Access Ultrasound for Nephrology Nursing. Health Corporation Parc Taulí de Sabadell, 2015. Financed by the organisation.
- –
XI Theoretical and Practical Course on Ultrasound for Vascular Access in Haemodialysis. Health Corporation Parc Taulí de Sabadell, 2016. Financed by the organisation.
- –
20th European Vascular Access Course. 2016, MECC. Maastricht, The Netherlands. Financed by the organisation.
- –
III Theoretical and Practical Course on Ultrasound for Vascular Access for Nephrology Nursing. Health Corporation Parc Taulí de Sabadell, 2015. Financed by the organisation.
- –
V Edition of the Theoretical and Practical Workshop on Updates on Ultrasound and Multidisciplinary Approach for Vascular Access. Health Corporation Parc Taulí, 2016. Financed by the organisation.
- –
Teaching Project “Image in Nephrology”. Promoted by S.E.N. I Course of University Expert in Diagnosis and Interventional Nephrology (Online mode). Module 6: Ultrasound of Vascular Access; ultrasound of the dysfunctional AVF. University of Alcalá and Ramón y Cajal University Hospital, Madrid 2012.
- –
Teaching Project “Image in Nephrology”. Promoted by S.E.N. II Course of University Expert in Diagnosis and Interventional Nephrology (Online mode). Module 6: Ultrasound of Vascular Access; ultrasound of the dysfunctional AVF. University of Alcalá and Ramón y Cajal University Hospital, Madrid 2013.
- –
I Master’s Degree in Diagnostic and Interventional Nephrology. Ultrasound of Vascular Access: ultrasound of the dysfunctional AVF. University of Alcalá and Ramón y Cajal University Hospital, Madrid 2014.
- –
Teresa Moreno
- •
Financial support to attend congresses. Registrations, trips or accommodation:
- –
Congress CIRSE 2016. Lisbon. Cook.
- –
SEDAV Congress. Madrid 2015. BARD.
- –
I Meeting of women radiologists. Madrid 2016. Iberoinvesa, Pharma.
- –
SERVEI Congress 2015. Córdoba. Financed by the Society.
- –
SERAM Congress. Madrid 2014. Financed by the Society.
- –
Theoretical and practical course of embolisation. Madrid 2014. Covidien.
- –
XII Update in embolisation materials. 2014. Terumo.
- –
XIII SERVEI Congress. Santander 2013. Financed by the Society.
- –
- •
Honorarium as speaker:
- –
VII Interventional Vascular Endo-school. Madrid 2014. Puerta de Hierro Hospital. Biotronik.
- –
VIII Interventional Vascular Endo-school. Madrid 2015. Hospital Puerta de Hierro. Biotronik.
- –
I Theoretical and practical course on Doppler ultrasound. Madrid 2013. By the Spanish Society of Ultrasound and the Spanish Society of Medical Radiology (SEUS and SERAM).
- –
II Theoretical and practical course on Doppler ultrasound. Madrid 2015. By the Spanish Society of Ultrasound and the Spanish Society of Medical Radiology (SEUS and SERAM).
- –
32 Congress of the SERAM, Oviedo. Financed by the Society.
- –
9th Congress of the Vascular Access Society, Barcelona 2015. Financed by Society.
- –
1st Multidisciplinary Conference on Vascular Access VITHAS. Alicante 2015. Financed by the organisation.
- –
XVI Course of Vascular and Interventional Radiology in Animal Models. León 2014. Financed by the SERVEI.
- –
I Course of Duplex Doppler Ultrasound for vascular access in haemodialysis. Madrid 2013. Financed by SERVEI
- –
XV Congress of the Association of Radiologists of the South of Spain. Cádiz 2013. Financed by the SERAM.
- –
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access