Icodextrin is a starch-derived glucose polymer which is metabolised into maltose. Its use in peritoneal dialysis (PD) has been an alternative to glucose as an osmotic agent, obtaining greater ultrafiltration (UF) for long dwells.
Icodextrin is generally well tolerated by patients, but skin reactions associated with its use have been reported. We present our experience with two patients who had skin reactions to icodextrin.
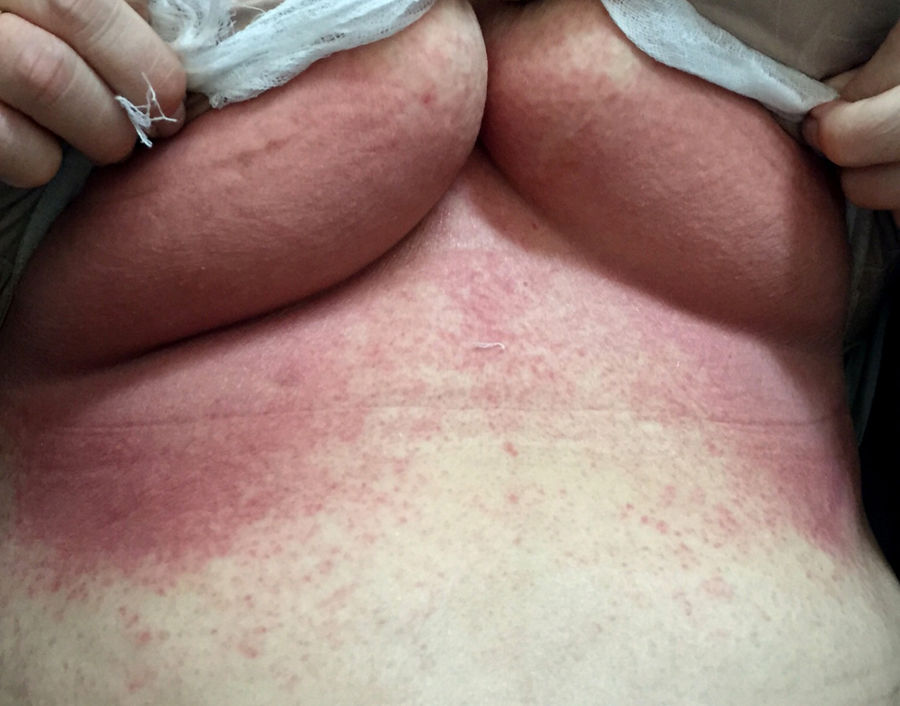
First case: a 73-year-old woman with chronic renal failure secondary to interstitial nephritis due to recurrent urinary tract infections. She had a history of severe toxicoderma caused by furosemide. She started PD in November 2013. After two years on PD, icodextrin was added to increase the UF. Seven days later, the patient consulted with generalised pruritus, poor general condition, sensation of dysthermia and the development of confluent desquamating maculopapular exanthema, mainly in inframammary folds (Fig. 1). She had no limb involvement or mucosal lesions. The patient was diagnosed with drug-induced toxicity and it was decided to withdraw the icodextrin, replacing it with 2.3% glucose. Dermatology performed epicutaneous allergy tests on icodextrin which came back negative. Seven days after the withdrawal, there was significant improvement of the lesions on her trunk, but still residual areas of desquamation on the palms of her hands. Fourteen days after discontinuing icodextrin the patient was asymptomatic.
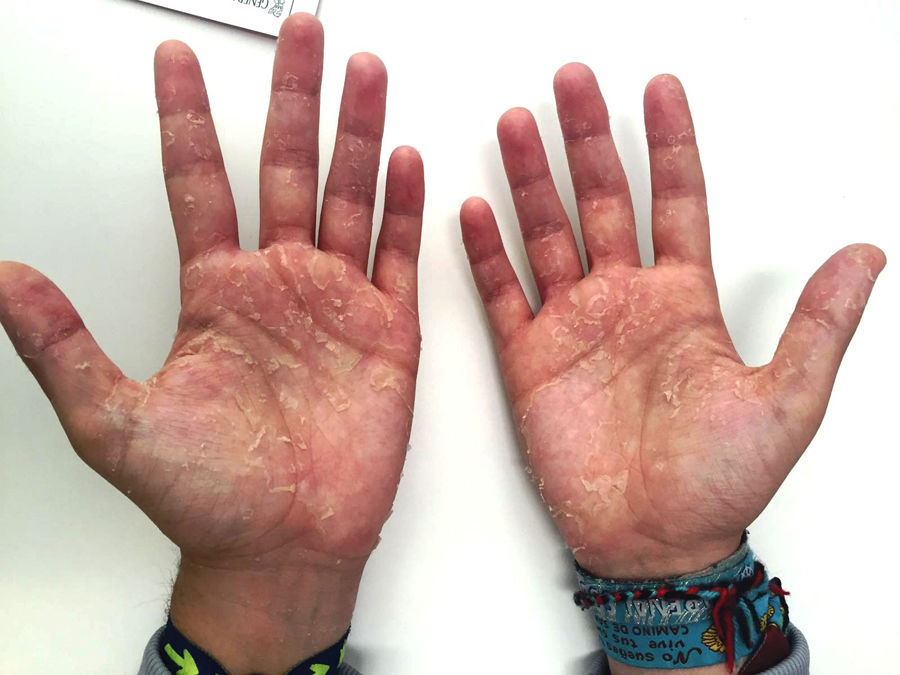
Second case: a 26-year-old man with no known allergies with renal failure due to episode of thrombotic microangiopathy secondary to methylmalonic acidaemia. In April 2016, he started PD from haemodialysis with three daytime 1.27% glucose exchanges and one nocturnal icodextrin exchange. After three weeks, the patient consulted with generalised pruritus, predominantly at night and the development of erythematous-desquamative lesions on the palms of his hands (Fig. 2) and soles of his feet, with no mucosal involvement. He was referred to dermatology and was diagnosed with drug-induced toxicoderma in the post-critical phase. In view of the possible relationship between the lesions and the use of icodextrin, it was discontinued. Fifteen days after withdrawal, the pruritus and skin lesions had disappeared.
Skin involvement associated with the use of icodextrin can present in many different ways; from localised pruritus and small desquamative areas on palms and soles1 to generalised pruritus and exfoliative lesions affecting the trunk and extremities, which can be potentially serious.2 As a result of the diverse forms of presentation, the incidence of icodextrin-induced skin lesions ranges from 7.8%3 to 17%4,5 according to the different series reported.
The mechanism by which icodextrin causes cutaneous toxicity is not fully understood. There are suggestions that it may be the result of a type iv hypersensitivity reaction, similar to that caused by the use of dextran solutions, given that the chemical composition of both polymers is very similar.1,4 The skin reaction usually occurs shortly after starting treatment with icodextrin, and it disappears rapidly after discontinuation.1,3,4 In most situations1,2,4 withdrawal of icodextrin is necessary for complete resolution of the condition. However, where the reaction was mild, the lesions are reported to have disappeared without the need to discontinue icodextrin.3
Despite the limitations in our two cases, as both lacked a skin biopsy, we think that nephrologists need to be aware of the risk of skin reactions associated with the use of icodextrin and their form of presentation in order to suspect them should they occur in PD patients.
Please cite this article as: Vizcaino Castillo B, Beltrán Catalán S, Molina Vila P, Gonzalez Moya M, Noguera-Morel L, Pallardó Mateu LM. Reacciones cutáneas debido al uso de icodextrina en pacientes en diálisis peritoneal. Nefrologia. 2019;39:211–213.